Abstract
Poloxamer 188 NF (national formulary (NF) grade of P-188) improves cardiac muscle function in the mdx mouse and golden retriever muscular dystrophy models. However in vivo effects on skeletal muscle have not been reported. We postulated that P-188 NF might protect diaphragm muscle membranes from contraction-induced injury in mdx and mdx/utrophin-/- (dko) muscular dystrophy models. In the first study 7-month old mdx mice were treated for 22 weeks with subcutaneous (s.c.) injections of saline or P-188 NF at 3 mg/Kg. In the second, dkos were treated with saline or P-188 NF (1 mg/Kg) for 8 weeks beginning at age 3 weeks. Prednisone was the positive control in both studies. Respiratory function was monitored using unrestrained whole body plethysmography. P-188 NF treatment affected several respiratory parameters including tidal volume/BW and minute volume/BW in mdx mice. In the more severe dko model, P-188 NF (1 mg/Kg) significantly slowed the decline in multiple respiratory parameters compared with saline-treated dko mice. Prednisone’s effects were similar to those seen with P-188 NF. Diaphragms from P-188 NF or prednisone treated mdx and dko mice showed signs of muscle fiber protection including less centralized nuclei, less variation in fiber size, greater fiber density, and exhibited a decreased amount of collagen deposition. P-188 NF at 3 mg/Kg s.c. also improved parameters of systolic and diastolic function in mdx mouse hearts. These results suggest that P-188 NF may be useful in treating respiratory and cardiac dysfunction, the leading causes of death in Duchenne muscular dystrophy patients.
Introduction
Duchenne muscular dystrophy (DMD) is a genetic disorder that occurs with a frequency of approximately 1 in every 3500 live male births [1] resulting from mutations in the dystrophin gene, located on the short arm of the X chromosome [2–4]. Affected boys are usually diagnosed at 3–5 years of age with symptoms of delayed walking or gait disturbances progressing to general muscle weakness and eventually death [5]. Genetic testing for mutations in the gene encoding dystrophin is used to confirm the diagnosis. In the later stages of the disease, a network of fibrous connective tissue and adipose tissue replaces muscle fiber lost due to necrosis, leaving only small islands of intact muscle fibers. Over time the muscles become progressively weaker, and the use of a wheelchair becomes necessary at a mean age of 9.5 years [5, 6]. Weakness in the diaphragm and intercostal muscles impairs respiratory function, which is the major cause of death in this patient population. The second most frequent cause of death is heart failure due to dilated cardiomyopathy. [7–11].
Dystrophin has been shown to protect the sarcolemma from contraction-induced damage. Evidence for this comes from the mdx mouse model, where muscle cells show increased permeability, increased intracellular calcium, and increased susceptibility to osmotic shock when stretched [12–14]. In cardiomyocytes from mdx mice, the membrane sealant P-188 NF has been shown to interact with exposed hydrophobic regions of fragile membranes and prevent unregulated entry of extracellular Ca2+ into the muscle cell, thereby restoring normal tension development in mdx cardiomyocytes [14]. The exact nature of the exposed hydrophobic regions was not identified but could occur from either direct microscopic tears in the lipid portion of the membrane [14] or from alterations in membrane protein composition, or activity, that has been shown to occur in this dystrophic model [15–19]. In any case, the resulting dysregulation of calcium in heart muscle resulted in abnormal heart structure and function [14]. The authors suggested that P-188 NF acts as a molecular “Band-aid” to seal the tears and permit intracellular calcium levels to return to normal [14]. The ability of P-188 NF to improve intracellular calcium concentration also translated into improved heart structure and function in the GRMD dog model of muscular dystrophy [20].
While P-188 NF has been shown to improve cardiac muscle function, its effects on skeletal muscle have been more difficult to demonstrate with some apparently contradictory results. P-188 NF did not prevent uptake of Evan’s Blue dye in rectus femoris muscle in exercised mdx mice [21] but did prevent uptake in Tibialis Anterior (TA) muscle [22]. While it was effective in preventing force decline during isometric contractions of isolated mdx mouse lumbrical muscle [23], it was not found to be effective, in situ, in Tibialis Anterior muscle, when administered intraperitoneally (i.p.) and in combination with anesthesia [24], although P-188 NF did protect muscle cells in the latter model. Further it did not reduce plasma creatine kinase levels in the GRMD dog model of muscular dystrophy [20] when dosed chronically. Regardless of whether or not P-188 NF can protect dystrophic limb muscles from contraction-induced damage, diaphragm muscle is arguably the most important skeletal muscle to protect in muscular dystrophy because respiratory failure is the leading cause of death for patients with DMD (6). The diaphragm, which is one of the first skeletal muscles to deteriorate in mdx mice [25] is also highly vascularized [26] which should permit high exposure to P-188 NF.
The studies reported here were undertaken to determine if P-188 NF could protect diaphragm muscle and prevent further respiratory dysfunction. The effect of P-188 NF on respiratory deficits in unanesthetized mdx and mdx/utrophin-/- (dko) mice was monitored in vivo using unrestrained whole body plethysmography (WBP). The treatment regimen for the mdx mice was initiated at 7 months ± 2 weeks of age, a time when respiratory deficits and diaphragm damage are already present in the mdx mouse model [27–32], and continued for 22 weeks until the mice were 1 year of age. The dosing regimen used in the dkos was initiated at 3 weeks ± 3 days of age and continued for 8 weeks. Subsequently, diaphragm muscles were evaluated histologically. We show here that P-188 NF treatment had an impact on several respiratory parameters in the mdx and dko mice with respect to baseline and/or the saline-treated control group. Further, P-188 NF treatment significantly reduced the number of centralized nuclei, variance in the minimal Feret’s diameter [33, 34], and collagen deposition suggesting that P-188 NF slows the degeneration process in dystrophic diaphragm muscle. These results indicate that P-188 NF might delay the loss of respiratory activity in DMD patients.
Materials and Methods
Animals
Male C57BL/10SnJ, hereafter referred to as wild type, and male mdx C57BL/10ScSn-Dmdmdx mice, were purchased from Jackson Laboratory, and aged in University of Michigan (UM) facilities. Heterozygous mdx/utrophin+/- mice were purchased from Jackson Laboratory (STOCK Utrntm1Jrs Dmdmdx/J; stock number 016622) and bred at the breeding colony facility at the UM. All studies were performed in facilities run by the UM Unit for Laboratory Animal Medicine (ULAM). Mice were housed on a 12 hr dark-light cycle and provided water and chow ad libitum. All ages for mdx mice are ± 2 weeks and dko mice are ± 3 days.
All animal work was approved by the University of Michigan University Committee on the Use and Care of Animals (PRO00005303) and all studies were performed in facilities run by the UM ULAM. The facility holds an Office of Laboratory Animal Welfare-approved assurance from the NIH. All studies conformed to the Guide for the Care and Use of Laboratory Animals by the National research council and the International Guiding Principles for Biomedical Research Involving Animals by the International Council for Laboratory Animal Sciences. Euthanasia was performed as recommended by the AVMA Guidelines for the Euthanasia of Animals. Finally 3 of the 5 authors hold DVM degrees. Isoflurane was used as an anesthetic for performance of echocardiography. No other studies with the animals required anesthesia.
Dko mice were housed in cages with a heat strip running across the bottom of the cage which was adjusted such that the temperature at the front of the cage was 37° ± 3° while the back of the cage was at room temperature. These mice were fed DietGel (Clear H2O; Portland, ME) in containers placed on the cage bottom, in addition to access to normal chow, and provided with nesting material (Envirodry). In spite of these extra measures, attrition was high in the dko groups.
At 7 months of age for mdx mice, treatment with P-188 NF, saline or prednisone was initiated. All compounds were administered subcutaneously (s.c.). Animals were dosed once daily (QD) and received saline, P-188 NF at 3 mg/Kg or prednisone at 1 mg/Kg in a volume of 0.1 ml. For dko mice, P-188 NF (1 mg/Kg), prednisone (1 mg/Kg) or saline were administered QD, s.c. in a volume of 0.1 ml.
Respiratory measurements
Respiration was monitored using a Buxco Whole Body Plethysmography apparatus (Buxco, Troy, NY) according to the manufacturers instructions as modified by the WBP protocol from Treat-NMD [35]. In this protocol animals are un-restrained and conscious. Monitoring was performed in the room in which the animals were housed. The mdx mice were monitored for 2 months prior to initiation of treatment to acclimate the mice to the procedure. Mice were placed in monitoring chambers and allowed to acclimate for 15 minutes, until they were quiet and motionless, prior to recording respiratory parameters. Longer acclimation times up to 45 minutes did not change the respiratory results. Each mouse was monitored in the same chamber for each reading throughout the study to minimize variability. During acclimation and monitoring the room door was shut, and technician movement and room noise was kept to a minimum. All readings were performed between the hours of 7 and 11 AM. After the acclimation period, respiratory function was monitored for 15 minutes. Mice were assigned to groups based on tidal volume (TV) measurements made the week before taking baseline respiratory measurement. TV was chosen to normalize the groups because at the time it was one parameter that was expected to change at the time the studies were initiated [29]. TV value averaging was accomplished by randomly placing 16 mdx mice into each group and removing mice with high or low TV values until the mean TV values per group were not significantly different. This resulted in a final group size of 12. Upon initiation of dosing, mice were monitored every 2 weeks.
Echocardiography
Induction of anesthesia was performed on the 1-year-old mice in an enclosed container containing 5% isoflurane. After induction, the mice were placed on a warming pad to maintain body temperature. 1–1.5% isoflurane was supplied via a nose cone to maintain a surgical plane of anesthesia. The hair was removed from the upper abdominal and thoracic area with depilatory cream. ECG was monitored via non-invasive resting ECG electrodes. Transthoracic echocardiography was performed in the supine or left lateral position. Two-dimensional, M-mode, Doppler and tissue Doppler echocardiographic images were recorded using a Visual Sonics’ Vevo 2100 high resolution in vivo micro-imaging system. LV ejection fraction was measured from the two-dimensional long axis view. In addition systolic and diastolic dimensions and wall thickness was measured by M-mode in the parasternal short axis view at the level of the papillary muscles. Fractional shortening and ejection fraction were also calculated from the M-mode parasternal short axis view. Diastolic function was assessed by conventional pulsed-wave spectral Doppler analysis of mitral valve inflow patterns (early [E] and late [A] filling waves). Doppler tissue imaging (DTI) was used to measure the early (Ea) diastolic tissue velocities of the septal annulus and lateral annulus of the mitral valve in the apical 4-chamber view.
Data analysis and Statistics
Data were monitored and collected using FinePoint software purchased from Buxco Electronics. The data were transferred to GraphPad Prizm software for graphing and statistical analysis of the data. Comparisons between groups were done by two-way (treatment and time), repeated measures ANOVA except for baseline data, which were analyzed by one-way ANOVA using a Tukey post-hoc test. Significance was set at P < 0.05. For mdx mice, N = 12 animals per group except for the mdx 3 mg/Kg group which had only 11 animals on weeks 20 and 22. For the dko mice, the starting group sizes varied depending upon the supply of mice in the colony but the maximum number of mice available (≥ 20) was used in order to maximize the number of survivors at the end of the study. For the dko study where mortality was approximately 80% during the 8-week dosing schedule, only the respiratory data from the surviving mice are represented with the exception of dko baseline data in the S1 Table.
Abbreviations used include the following (units): Measured parameters: F, respiration rate (breaths/minute), TV, tidal volume, (ml); MV, minute volume, (ml/min); Penh, enhanced pause, (no units); PIF, peak inspiratory flow, (ml/sec); PEF, peak expiratory flow, (ml/sec); Te, expiratory time (sec); Ti, inspiratory time, (sec); Tr, relaxation time, (sec); and derived: TV/BWT, ratio of TV to body weight, (ml/Kg); Rpef, the ratio of the time from start of expiration to peak expiratory flow to Te (no units).
Histology
At the end of treatment, diaphragm muscles were removed from the mice, rinsed with ice cold PBS, formalin fixed, and embedded in paraffin. Sections (4 μm) were prepared and stained by the Histology Core at the UM. Staining procedures for the diaphragm included H&E, picosirius red, wheat germ agglutinin (WGA), and DAPI. Overlapping microscopic (10X) images (4–5 per section) were prepared for evaluation. Diaphragm muscle was cross-sectioned from regions near each end and near the middle to evaluate structure throughout the muscle. Longitudinal sections were also prepared. A minimum of 10K fibers per mdx mouse (n = 11 or 12) was analyzed. For dko mice, a minimum of 4 diaphragm muscles were analyzed per group with 5 sections per muscle. This resulted in a minimum of 1500 fibers being analyzed per group. Pathologists, blinded to sample identity evaluated the slides for centralized nuclei (CN), fiber density, fibrosis and (VC) coefficient of the minimal Feret’s diameter, a surrogate for cross sectional area [33, 34]. WGA-DAPI stained sections were analyzed using fluorescent images and Nikon Elements software (computer-assisted determination of fiber area) to evaluate the minimum Feret’s diameter from each fiber. The variance coefficient of the minimum Feret’s diameter was used as measure of improvement in muscle fiber size to avoid sectioning artifacts as recommended by the Treat-NMD neuromuscular network and others [33, 34].
Pharmacokinetic studies
C56BL/6 mice received either a single intravenous (i.v.) or an s.c. dose of P-188 NF an i.v. dose levels of 4.6 mg/kg and s.c. dose levels of 3.0 mg/kg. C56BL/6 mice received either a single intravenous or an s.c. dose of P-188 NF at intravenous dose levels of 4.6 mg/kg and s.c. dose levels of 3.0 mg/kg. Blood was collected from a group of ten animals per time-point at 0.083, 0.25, 0.5, 1, 2, 4, 8 and 12 h after dosing for the preparation of plasma from which the P-188 NF concentrations were determined by liquid chromatography-mass spectrometer methods developed at Millennium Research Laboratories, Inc. (Woburn, MA)
The plasma profiles were constructed from the mean of the plasma concentrations for each sample collection time, dose level and dose route. For the purposes of the pharmacokinetic analysis, all samples at <0 in the bioanalytical report were set to zero. All other non-numerical data were excluded from further analysis. The pharmacokinetics of the plasma profiles of the individual animals for each dose route and dose level were analyzed using a non-compartmental approach for an intravenous or extravascular dose, as appropriate, with WinNonlin ‘v’ 5.2 (Pharsight, CA). Cmax and Tmax were taken directly from the data and the AUClast calculated using the linear trapezoidal rule for the ascending portion of the plasma profile and the log trapezoidal rule for the descending portion. The half-life was determined from a linear regression of the logarithm of the P-188 NF plasma concentration versus time. The number of points used for the regression was determined from visual inspection of the data and used a minimum of 3 time points. The data were collated and tabulated, together with descriptive statistics, using WinNonlin (Certera, Princeton, NJ).
Results
Selection of dosage and route of administration
P-188 NF has been shown to have cell protective effects in several models of cell damage and in animal disease models [14, 36–39]. However, most of the in vivo work was performed using relatively high doses, 200–460 mg/Kg, and administered by i.v. or i.p. routes of administration [14, 20, 21, 40, 41]. In order for P-188 NF to become a viable therapy it must be demonstrated to work at a dosage and by a route of administration that is acceptable for daily administration to patients. Previous work in the coronary microembolism model of heart failure has shown that P-188 NF is active at an i.v. dose of 15 mg/Kg [42]. In addition, P-188 NF was shown to prevent muscle atrophy in the dysferlin-deficient SJL mouse model of limb-girdle muscular dystrophy using the s.c. route of delivery at an effective daily dose of approximately 16 mg/Kg [43]. In addition, Phrixus has data demonstrating acutely improved cardiac hemodynamics in a rat heart failure model at 4.6 mg/Kg (S1 Fig).
A pharmacokinetic (PK) study was performed with non-labeled P-188 NF to compare the PK of P-188 NF via the i.v. and s.c. routes of administration (Table 1). The doses studied were 4.6 mg/Kg i.v. (active in rat) and 3 mg/Kg s.c. It can be seen in Table 1 that the plasma exposure to P-188 NF (area under the curve (AUC) was greater in the 3 mg/Kg s.c. dose than with the 4.6 mg/Kg i.v. dose. The greater exposure at 3 mg/Kg s.c. suggests that this dose might have activity in mouse models.
Table 1. Pharmacokinetic parameters of P-188 NF dosed by the intravenous or subcutaneous routes of administration in mice.
| Parameters* | Dose level (mg/kg) | t1/2 (h) | Cmax (μg/mL) | Tmax (h) | AUClast (μg*h/mL) | AUC∞ (μg*h/mL) | F (%) |
|---|---|---|---|---|---|---|---|
| Intravenous | 4.6 | 8.2 | 23.0 1 | NC | 20.3 | 25.9 | NC |
| Subcutaneous | 3.0 | 3.9 | 7.0 | 2 | 36.9 | 42.3 | 94.1 |
* Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; AUClast, area under the curve up to the last measurement; AUC∞, area under the curve extrapolated to infinity; F, bioavailbility. NC-not calculated.
1 Calculated based on a 30g mouse with 6 ml of blood.
Respiratory function is diminished in mdx mice at 7 months of age
Baseline respiratory values (Table 2) were taken at the end of an acclimation period when the mice were 7 months of age ± 2 weeks. The mice were grouped based on baseline TV values since this value was expected to decline over time based on a literature report [29]. At 7 months of age there were several significant differences in respiratory function between wild type and mdx groups at baseline, which were similar to differences reported previously for mdx mice at 6 and 7 months of age [28, 29] including, TV, MV, PEF and Rpef, a parameter influenced by both airway obstruction and residual activity of inspiratory muscles during the initiation of expiration. Body weights of the mdx mice tended to be larger than those of the wild type mice with the 3 mg/Kg P-188 NF group being significantly larger (Table 2).
Table 2. Comparison of baseline respiratory function and body weight between groups of wild type and mdx mice at age 7 monthsg.
| Parameter | Wild type Saline | mdx Saline | mdx P-188 NF 3 mg/Kg | mdx Prednisone |
|---|---|---|---|---|
| F (breaths/min) | 426 ± 27 d | 391 ± 19 a | 414 ± 28 | 404 ± 16 |
| TV (ml) | 0.324 ± 0.024 | 0.314 ± 0.017 | 0.323 ± 0.021 | 0.321 ± 0.019 |
| TV/BW (mg/g) | 0.0093± 0.0008 | 0.0084 ± 0.0005 | 0.0083 ± 0.0005 a | 0.0088± 0.0005 |
| MV (ml/min) | 137 ± 10 d | 121 ± 10 a | 132 ± 11 | 127 ± 8 |
| MV/BW (ml/min/g) | 4.01 ± 0.32 f | 3.41 ± 0.20 c | 3.59 ± 0.24 c | 3.61 ± 0.18 b |
| Penh | 0.427 ± 0.084 | 0.424 ± 0.043 | 0.443 ± 0.057 | 0.470 ± 0.046 |
| Rpef | 0.404 ± 0.049 d | 0.325 ±0.048 b | 0.350 ± 0.060 a | 0.335 ± 0.027 b |
| PIF (ml/sec) | 10.89 ± 0.82 | 10.18 ± 0.72 | 10.70 ± 0.73 | 10.27 ± 0.63 |
| PEF (ml/sec) | 5.36 ± 0.49 d | 4.80 ± 0.40 a | 5.23 ± 0.46 | 5.10 ± 0.42 |
| Ti (sec) | 0.049 ± 0.003 | 0.052 ± 0.002 | 0.051 ± 0.003 | 0.053 ± 0.002 a |
| Te (sec) | 0.114 ± 0.014 | 0.128 ± 0.011 | 0.118 ± 0.013 | 0.123 ± 0.009 |
| BW (g) | 33.0 ± 2.5 | 35.4 ± 1.9 | 36.9 ± 2.3 c | 35.2 ± 2.3 |
a P < 0.05 vs. wild type saline.
b P = 0.001–0.01 vs. wild type saline.
c P < 0.001 vs. wild type saline.
d P < 0.05 vs. mdx saline.
e P = 0.001–0.01 vs. mdx saline.
f < 0.001 vs. mdx saline.
g all values are mean ± S.D. Analysis by 2-way ANOVA using a multiple comparison (simple effects within rows) post-hoc test.
Chronic treatment with Poloxamer 188 NF improves respiratory function in mdx mice with established respiratory dysfunction
Wild type mice were dosed once per day s.c with saline while mdx mice were dosed once per day s.c. with saline, 3 mg/kg of P-188 NF or 1 mg/kg of prednisone for 22 weeks. Respiration was monitored every 2 weeks by WBP. Fig 1, shows the overall effect of P-188 NF and prednisone treatment on TV/BW ratio, a parameter dependent upon TV, which was used to assign animals to groups. The TV/BW ratios were nearly identical at the start of the study (Table 1) and increased in both the saline and P-188 NF treated groups over the first 12 to 14 weeks of the study. The reason for this improvement is not known but could be related to the increase in fluid that both groups received from dosing. During that time (from 4–12 weeks), it appears that the saline treated group had higher TV/BW ratios, however, the saline-treated mdx mice had significantly higher TV/BW ratios (P < 0.05) only at the 12-week time point. Subsequently the 3 mg/Kg doses of P-188 NF prevented a nearly 20% decline seen in the saline-treated group that occurred from 12 to 22 weeks. It is also noteworthy that TV/BW ratio was significantly increased after 22 weeks of treatment with P-188 NF (P = 0.001 to 0.0001) or prednisone (P < 0.05), but not saline, compared to baseline. In the prednisone-treated group of mdx mice, treatment increased TV/BW early in the dosing regimen, which was maintained except at week 14 where the TV/BW was similar between the saline and prednisone-treated mdx groups. This slowing of the decline was also seen with MV/BW ratio (S11 Fig), however neither P-188 NF nor prednisone treatment improved MV/BW over baseline. Table 3 shows the overall statistically significant effects of 22 weeks of treatment on respiratory parameters. Highly statistically significant differences between the wild type saline and mdx saline groups were observed, over time, in all parameters with the exception of expiration time (Te). P-188 NF had significant effects on TV, TV/BW, MV, MV/BW, and PIF compared to saline treated mdx mice (S1–S11 Figs). Prednisone treatment, which delays the need for respiratory assistance in DMD patients [5, 6], had significant effects on TV, TV/BW, MV, MV/BW, Penh, Rpef, PIF, Ti, Te, and Tr in this model. For both P-188 NF and prednisone treatments the significance of the overall effect was established over time.
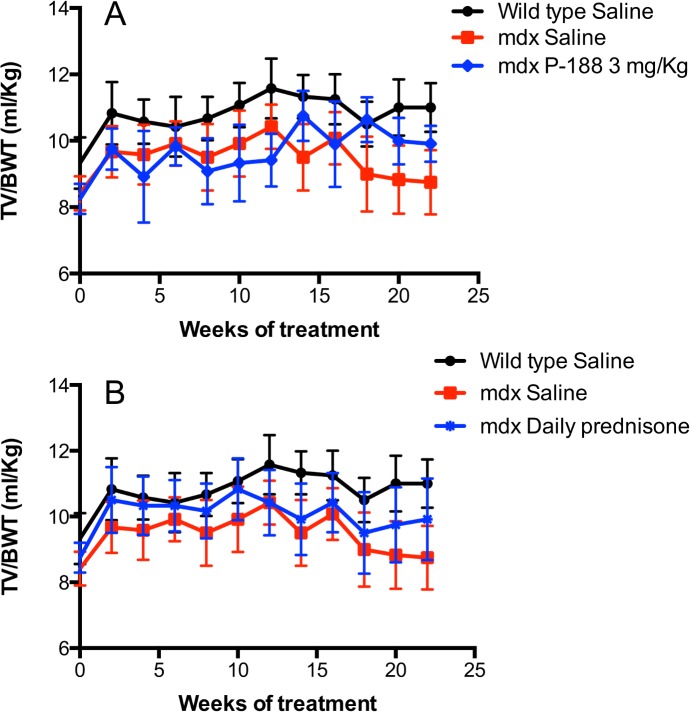
Fig 1. Effects of P-188 NF and prednisone on tidal volume/BW in the mdx mouse over time.
Mdx mice were treated QD, s.c. with P-188 NF (3 mg/Kg) or prednisone (1 mg/Kg) from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 11 for mdx 3 mg/Kg P-188 NF at 20 and 22 weeks, N = 12/group for prednisone treatment. Panel A, P < 0.0001 for the overall effect of P-188 NF 3 mg/Kg group vs. mdx saline and wild type saline. The mdx 3 mg/Kg group was also significantly greater than mdx saline at 22 weeks (P < 0.0001). Panel B, P = 0.001 to 0.0001 for mdx 1 mg/Kg prednisone group vs. mdx saline and P < 0.0001 vs. wild type saline. The mdx prednisone group was also significantly greater than mdx saline at 22 weeks (P < 0.0001).
Table 3. The main treatment effect on respiratory function in mdx mice over 22 weeks of treatment with saline or P-188 NF.
| Respiratory Function | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Two-way ANOVA 1 2 | F | TV | TV/BW | MV | MV/BW | Penh | Rpef | PIF | PEF | Ti | Te |
| Wild type saline | **** | **** | **** | **** | **** | **** | **** | **** | **** | **** | ns |
| mdx saline | nd | nd | nd | Nd | nd | nd | nd | nd | nd | nd | nd |
| mdx 3 mg/Kg P-188 NF | * | ** | ** | ** | * | ** | ** | **** | ns | ** | ns |
| mdx prednisone | ns | ** | ** | * | *** | **** | **** | **** | ns | ** | * |
1 Two-way ANOVA using a multiple comparisons (main treatment effect) post-hoc test.
2 All comparisons to mdx saline group; nd, not different; ns not significant.
**** P < 0.0001.
*** P = 0.001 to 0.0001.
** P = 0.01 to 0.001.
* P < 0.05.
P-188 NF improves cardiac hemodynamics in the mdx mouse model when administered subcutaneously.
Since P-188 NF has been previously shown to improve heart function when administered i.v. [14, 20, 40–42], it was of interest to determine if the same doses and route of administration that affected respiratory function in the mdx mouse would improve heart function in the same model. Therefore, the mice used in the respiratory study were monitored by echocardiography at the end of the 22-week treatment period. The results are shown in Table 4. Compared with the saline-treated mdx mouse group, P-188 NF treatment caused a significant increase in fractional shortening (FS), ejection fraction (EF), stroke volume (SV), and the related cardiac output (CO). In addition, treatment with P-188 NF caused a significant decreased isovolumic relaxation time. Based on a comparison with saline treatment, P-188 NF treatment at this dose and route of administration did not affect heart rate (HR) or measures of volume or wall thickness (Table 4). The results indicate that P-188 NF delivered QD s.c. at a dose of 3 mg/Kg was sufficient and appropriate to affect an improvement in heart function.
Table 4. Echocardiography results from wild type and mdx mice treated with saline or P-188 NF for 22 weeks from 7 months to 1 year of age.
| Parameters** | Wild type saline | mdx saline | mdx 3 mg/Kg | mdx prednisone |
|---|---|---|---|---|
| BW (g) | 33 ± 4 | 33 ± 3 | 34 ± 2 | 32 ± 2 |
| HR (b/m) | 406 ± 48 | 370 ± 51 | 410 ± 38 | 376 ± 50 |
| E/A | 1.8 ± 0.5 | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.7 ± 0.9 |
| IVRT (ms) | 21 ± 3 | 23 ± 3 | 18 ± 2* | 21 ± 3 |
| EF (%) | 49 ± 5 | 50 ± 4 | 58 ± 9* | 56 ± 7 |
| FS (%) | 25 ± 3 | 24 ± 3 | 31 ± 6* | 26 ± 4 |
| LVDd (μl) | 4.5 ± 0.2 | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.0 ± 0.2 |
| LVvol d (μl) | 91 ± 10 | 78 ± 13 | 73 ± 7 | 70 ± 8 |
| IVSd (mm) | 0.8 ± 0.09 | 0.8 ± 0.07 | 0.09 ± 0.07 | 0.09 ± 0.07 |
| PWd (mm) | 0.7 ± 0.1 | 0.9 ± 0.06 | 0.9 ± 0.07 | 0.8 ± 0.09 |
| SV (μl) | 37 ± 8 | 34 ± 5 | 44 ± 6* | 35 ± 8 |
| CO (ml/m) | 19 ± 4 | 15 ± 4 | 21 ± 4* | 17 ± 6 |
* P < 0.05 vs. mdx saline.
** All mice were 1 year old with 22 weeks treatment.
While the mdx mouse is an accepted model of muscular dystrophy and considered to be an appropriate model for drug testing by Treat-NMD, the model is limited by the fact that it has a mild phenotype [44]. In order to examine the effects of P-188 NF in a more severe mouse model, we obtained the heterozygous mdx/utrn+/- (het) mice, and mated the het mice to obtain mdx/utrn-/- (dko) mice which exhibit a much more severe muscular dystrophy phenotype.
P-188 NF treatment significantly slows the decline in respiratory parameters in dko mice
Dko mice are fragile and have a severely truncated life span [45–47] so in order to have enough mice survive to the end of the study groups were populated with 20 or more mice. Because of this, we performed dko studies with only a single treatment at a time and included wild type mice and dko mice treated with saline as controls with each treatment. P-188 NF treatment was run twice in order to determine reproducibility of the effects. The data were combined from these studies for analysis. Finally, while performing some pilot studies with these mice, we observed respiratory effects of P-188 NF dose of 1 mg/Kg. Based on this observation, 1 mg/Kg was chosen as the dose for the dko studies.
Three week-old dko mice had demonstrable respiratory deficits at baseline (S1 Table). The overall effects of 8 weeks of P-188 NF or prednisone treatment at 1 mg/Kg on respiration in the dko mouse model are shown in Figs 2 and 3, Table 5 and S12–S14 Figs. Compared with the saline-treated dko group, the P-188 NF treated group showed significant effects in 9 out of the 12 respiratory parameters. Prednisone treatment affected the same parameters with the exception of peak expiratory flow (PEF). Where affects were observed, they were similar for both treatment groups (Table 3). Most of the effects observed with P-188 NF can be characterized as a decrease in the rate of decline (TV, MV, TV/BW, MV/BW), or a stabilization (F, Penh, Rpef, PEF) of the respiratory parameter (Figs 2 and 3 and S12–S14). Similar effects were seen with prednisone. Since these studies were performed on mice starting at 3 weeks of age, we observed improvements in respiratory function for the first 2 weeks of each study in the dko saline and P-188 NF or prednisone treated groups that were likely due to maturation of the mice and not treatment. Either P-188 NF or prednisone treatment significantly improved respiratory parameters above what was seen at the 2 weeks of treatment time point.
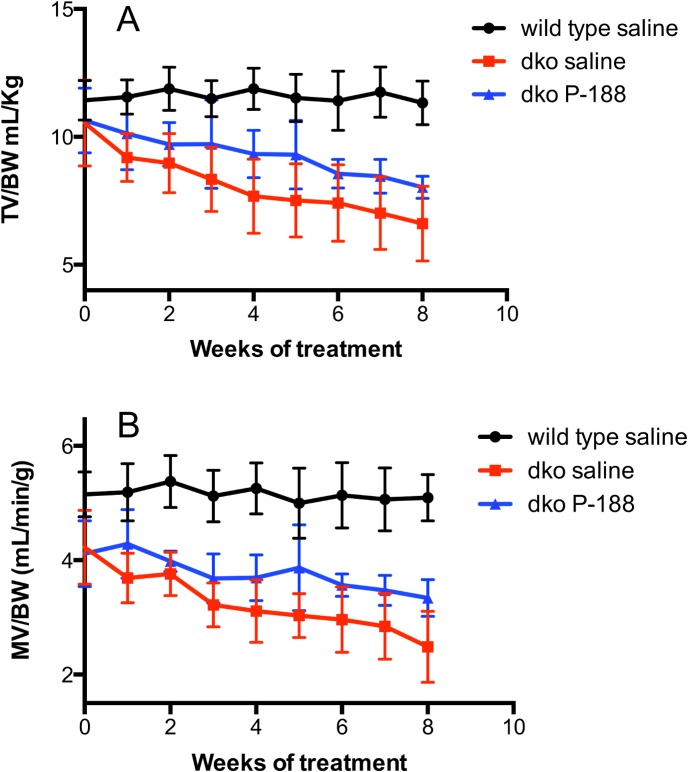
Fig 2. Effects of NF treatment on TV/BW and MV/BW in dko mice over time.
Dko mice were treated with 1 mg/Kg of P-188 NF QD, s.c, from ages 3–11 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline; the red line represents dko mice treated with saline; the blue line represents dko treated with 1 mg/Kg P-188 NF. TV/BW and MV/BW are shown in panels A, and B, respectively. Data points are means +/- S.D. N = 8/group wild type and 5/group dko saline and dko P-188 NF. For panels A and B, P < 0.0001 for dko 1 mg/Kg P-188 NF group vs. dko saline and wild type saline.
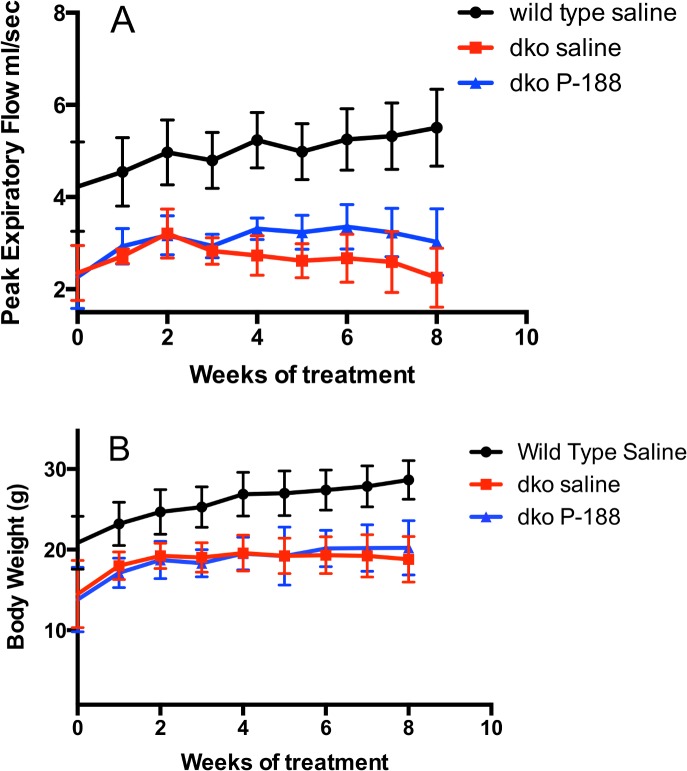
Fig 3. Effects of NF treatment on PEF and BW in dko mice over time.
Dko mice were treated with 1 mg/Kg of P-188 NF QD, s.c, from ages 3–11 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline; the red line represents dko mice treated with saline; the blue line represents dko treated with 1 mg/Kg P-188 NF. PEF and BW are shown in panels A & B, respectively. P < 0.0001 for wild type saline vs. all other groups. For panel A, P = 0.01 to 0.001 for dko 1 mg/Kg P-188 NF group vs. dko saline. For panel B, there is no significant difference between dko groups treated with saline or P-188 NF.
Table 5. Overall effect of P-188 NF and prednisone at 1 mg/Kg versus saline on respiratory parameters in the dko mouse model.
| Survivors | F | TV | TB/BW | MV | MV/BW | Penh | Rpef | PIF | PEF | Ti | Te | BW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-188 (N = 9) | * | *** | **** | **** | **** | ** | NS | ** | *** | * | NS | NS |
| Pred. (N = 6) | * | *** | *** | * | **** | ** | NS | *** | NS | * | NS | NS |
Two-way ANOVA (treatment vs. time) vs. dko saline (main treatment effect) post-hoc test.
* P< 0.05.
** P = 0.01 to 0.001.
*** P < 0.001.
**** P < 0.0001.
These results demonstrate that P-188 NF administered at 3 weeks of age in the dko mouse model can significantly delay the respiratory decline and the magnitude of the response on multiple parameters suggesting that P-188 NF has a beneficial impact on overall respiratory function.
Histology
Analysis of centralized nuclei
The absence of dystrophin in muscle cells disrupts the dystrophin glycoprotein complexes (DGC) and leads to uncontrolled influx of calcium. This triggers a set of pathological processes that lead to degradation of muscle cell proteins, cell damage and muscle wasting. The newly differentiated muscle fibers exhibit centrally located nuclei and heterogeneity of fiber size [33, 34]. The percentage of fibers with centralized nuclei (CN) from the diaphragm of each animal in the mdx study was determined (Fig 4, Panel A). Cells from the mdx saline group exhibited centralized nuclei in ~23% of the fibers compared to <1% in the wild-type-saline group. P-188 NF or prednisone treatment significantly reduced the percentage of fibers containing CN in mdx mice. The 3 mg/Kg P-188 NF and prednisone groups showed a percentage of CN of 12% and 14.4%, respectively. A decrease in the percentage of centralized nuclei was also seen in dko mice treated with either P-188 NF (22% lower) or prednisone (10% lower) (Fig 5, Panel A).
Fig 4. Histological parameters from diaphragm muscle from saline, P-188 NF, or prednisone treated mdx mice at 1 year of age.
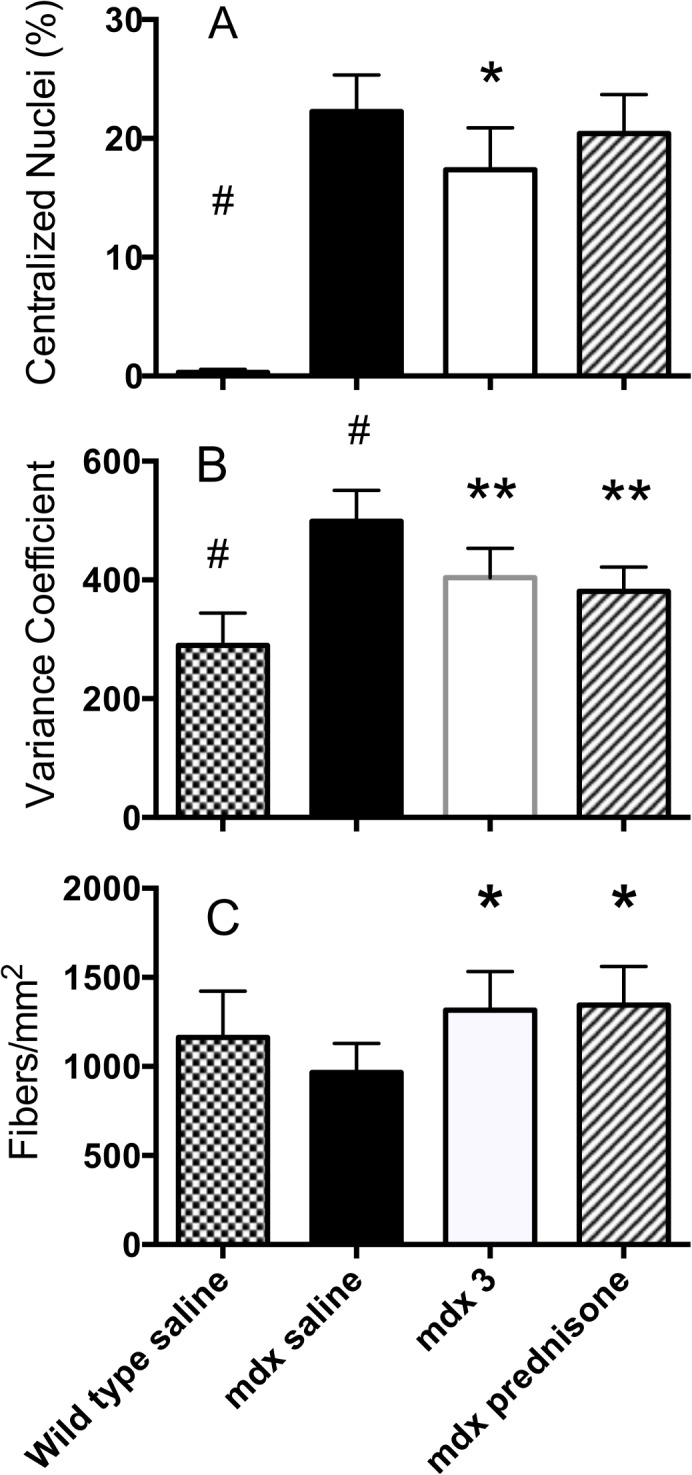
Mdx and wild type control mice were age to seven months and then randomized into groups (N = 12) based on tidal volume measurements. Mice were treated QD by s.c injection with saline, P-188 NF at 3 mg/Kg or prednisone (1 mg/Kg). At termination, diaphragm muscle was harvested, washed and embedded in paraffin. Sections from across each diaphragm were stained with wheat germ agglutinin and DAPI (Panels A & C) or lectin antibody (Panel B) and photographed. Images were analyzed using Nikon Elements software, or by direct counting. (A) Percentage of fibers with centralized nuclei; (B) Variance coefficient of the mean minimum Ferets diameter of diaphragm fibers; and (C) Fiber density. A minimum of 10,000 fibers was assessed for each parameter. * < 0.001 vs. mdx saline, # P < 0.001 vs. all other groups.
Fig 5. Histological parameters from diaphragm muscle from saline, P-188 NF, or prednisone treated dko mice at 11 weeks of age.
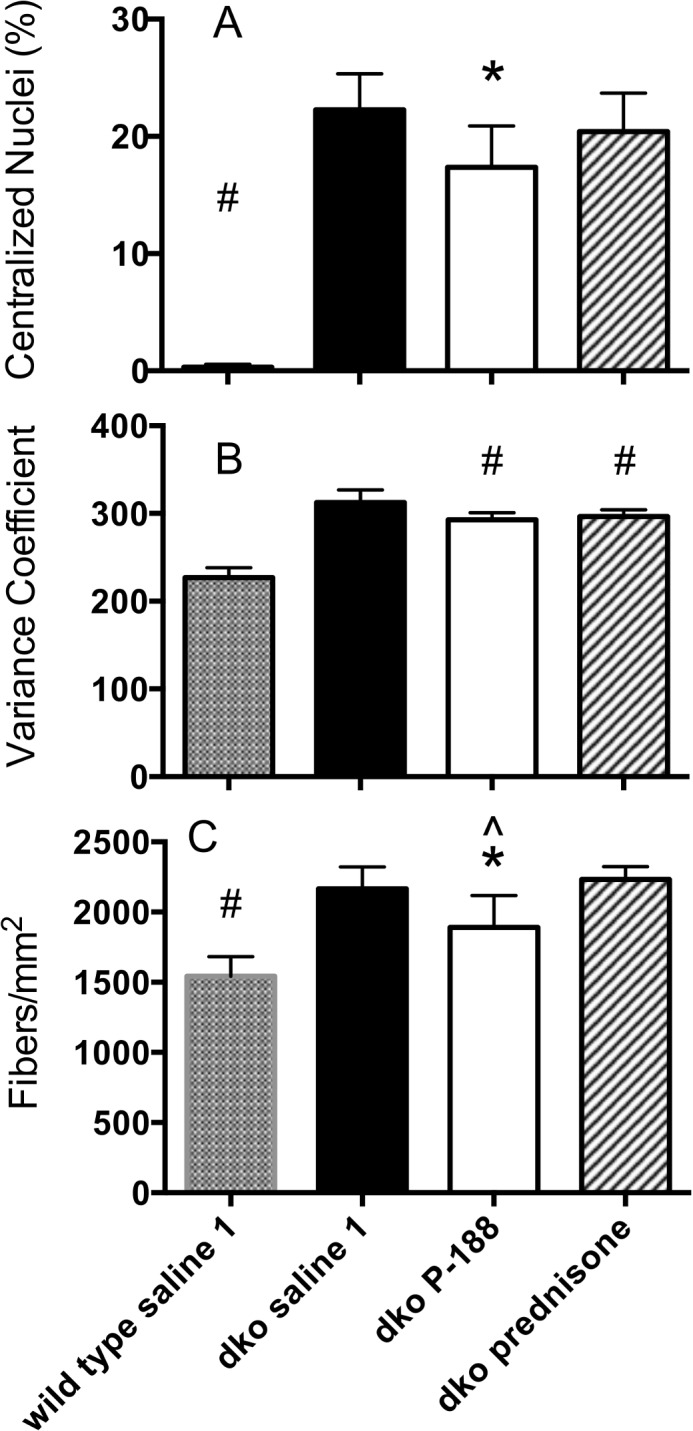
Wild type or dko mice were treated with saline, P-188 NF (1 mg/Kg) or prednisone (1 mg/Kg) QD, s.c. for 8 weeks beginning at 3 weeks of age. At termination, diaphragm muscle was harvested, washed and embedded in paraffin. Sections from across each diaphragm were stained with wheat germ agglutinin and DAPI (Panels A & C) or lectin antibody (Panel B) and photographed. Images were analyzed using Nikon Elements software, or by direct counting. (A) Percentage of fibers with centralized nuclei; (B) Variance coefficient of the mean minimum Ferets diameter of diaphragm fibers; and (C) Fiber density. * < 0.01 vs. dko saline, ** P < 0.001 vs. dko saline, # P < 0.001 vs. all other groups.
The results from these 2 studies indicate a slower rate of degeneration/regeneration in P-188 NF- and prednisone-treated mdx and dko mice compared to saline-treated mdx mice.
Analysis of fiber size and density
When skeletal muscle fibers are lost through degeneration they are replaced by a regenerative process until depletion of satellite cells. The cross-sectional area of regenerating or recently regenerated fibers is expected to be smaller and more variable than that of mature muscle fibers. To determine the variability, the variance coefficient of the Feret’s diameter of muscle fibers was determined in 4–5 cross sections from each mdx mouse diaphragm representing different sections from across the length of muscle. The results are shown in Fig 4, Panel B and Fig 5 Panel B. In the mdx mouse study, the VC calculated for diaphragms of the wild type mouse group was 280, which is in line with that reported for non-dystrophic mice (VC = 237) [33]. The mdx saline-treated group had a VC of 465, which is similar to that reported previously for 7-week-old mdx mouse diaphragm muscle (VC = 402) [33]. Treatments that prevent or slow degeneration are expected to bring the mdx VC closer to the wild type VC. It can be seen in Fig 4B that treatment with 3 mg/Kg of P-188 NF significantly shifted the mdx VC lower (380) towards that of the wild type mouse diaphragms. The same effect was seen with prednisone treatment. There was no statistical difference between the P-188 NF doses and prednisone. In addition, treatment of mdx mice with either dose of P-188 NF or prednisone increased the number of fibers per unit area of diaphragm muscle (Fig 4, Panel C).
In the dko mouse studies, the wild type mouse VC values were in line with those seen in the mdx studies. However, the VC of the minimum Feret’s diameter of 11-week old dko mice treated with saline was not as high as in saline-treated mdx mice at 1 year of age indicating less variability in fiber size (Fig 5, Panel B). This might be expected if the muscle fibers of the 11-week old dko mice regenerate efficiently. P-188 NF or prednisone treatment resulted in a small but significant decrease in VC compared to dko mice treated with saline (Fig 5, Panel B). This indicates muscle fibers in the diaphragms of mice from both treatment groups are less heterogeneous in size. Muscle fiber densities of the dko mouse groups were significantly higher than those in the wild type groups (Fig 5, Panel C) but there was no difference between the dko saline and dko P-188 NF or prednisone-treated groups. In the dko study evaluating prednisone, the fiber density in the dko mouse groups was significantly greater than those groups in the P-188 NF study. The reason for this difference is unknown. However the mice in these studies were not from the same breeding pairs, which might contribute to the difference.
H&E staining of longitudinal sections of diaphragm muscle from wild type and mdx mice revealed many features of muscle damage (S15 Fig). These included centralized nuclei, mononuclear cell accumulation, necrotic fibers, and vesicles that are presumed to be lipid vesicles. The diaphragm from the mdx mouse treated with saline at 12 months (S15C Fig) exhibits paler fibers than the other sections indicating more fibrosis, which was confirmed with Picosirius red staining (see Figs 6 and 7).
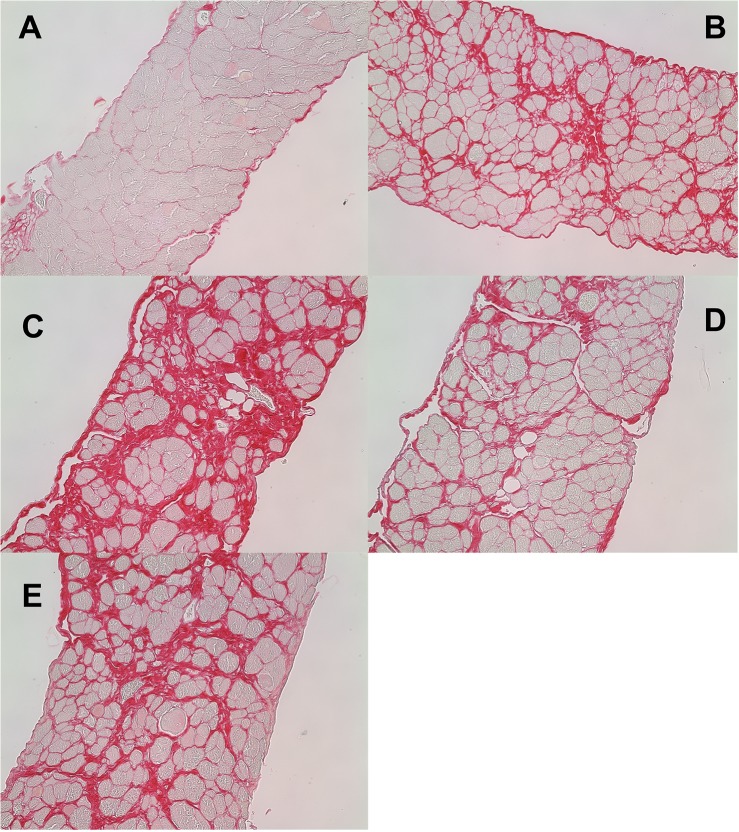
Fig 6. Fibrosis of diaphragm muscle from 1-year-old mdx and wild type control mice after 22 weeks of treatment with saline or P-188 NF.
Sections of diaphragms from the same groups of mice described in Fig 1 were stained with Picosirius Red to visualize collagen deposition. All staining was done on diaphragm muscle from 12-month old mice with the exception of Panel B. Shown in the figure are cross sections of diaphragm muscle from: Panel A, wild type control saline-treated mouse; Panel B, a 7 month old mdx mouse; Panel C, an mdx saline-treated mouse; Panel D, an mdx mouse treated with 3 mg/Kg P-188 NF; Panel E, an mdx mouse treated with 1 mg/Kg prednisone.
Fig 7. Effect of P-188 NF on collagen composition in diaphragm muscle.
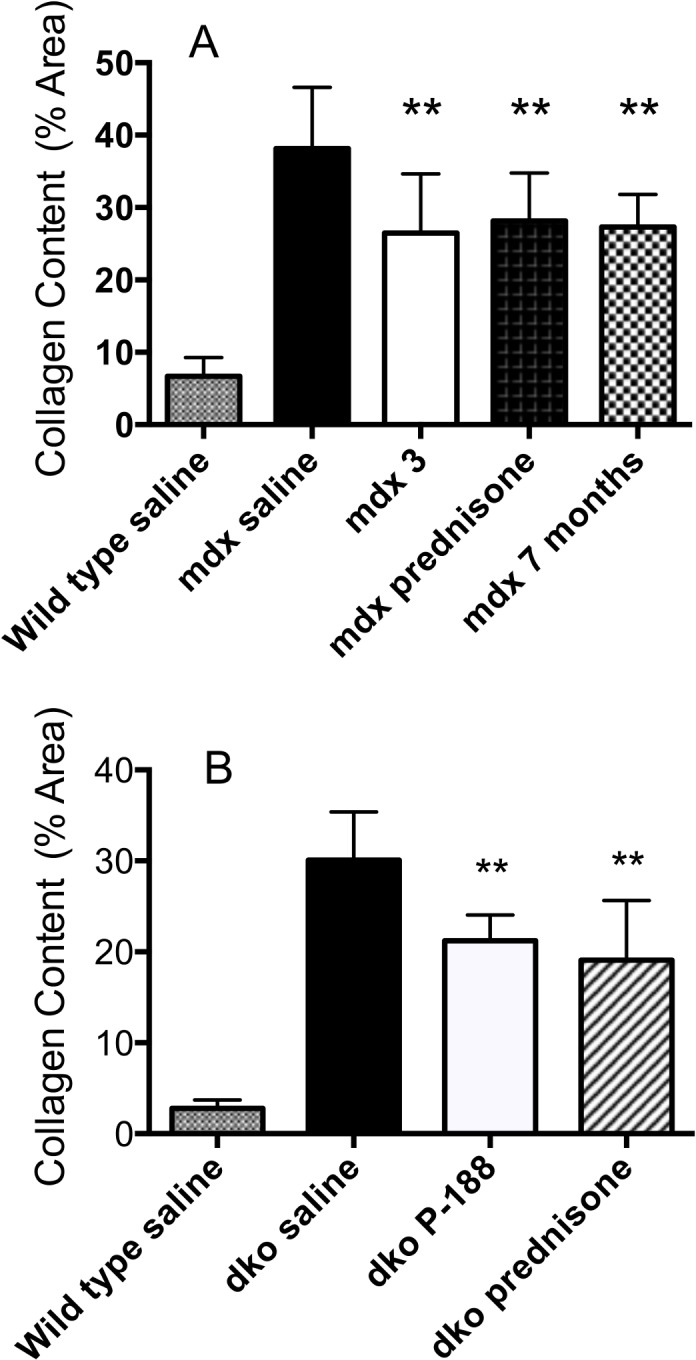
Measurements of picosirius red staining were determined from in mdx (panel A) and dko mice (panel B) at the end of the respective treatment periods. All surviving mice were use in the analysis. Staining was quantified using Nikon Elements software to determine the percentage of area stained in each section. A minimum of 6 sections/diaphragm was assessed. The wild type saline group was significantly different from all other groups, #P < 0.0001. Panel A, ** P <0.01 vs. mdx saline; Panel B ** P < 0.01 vs. dko saline.
Analysis of fibrosis
Representative cross sections of diaphragm muscle from mdx mice muscle, stained with Picosirius Red to show collagen, are shown in Fig 6. Panels A-E show wild type (saline), mdx at 7 months of age, mdx (saline) at 12 months of age, mdx P-188 NF and mdx prednisone treated groups at 12 months of age respectively. There was minor collagen deposition in the diaphragms from wild type mice at 12 months of age (Fig 6, Panel A). However, there was significant collagen deposition in the mdx mouse diaphragm at 7 months of age (Fig 6, Panel B) and remarkably more at 12 months (Fig 6, Panel C). The measureable collagen deposition in the diaphragm muscle of the mdx mice at 7 months of age was expected [28, 29]. Each of the treatment groups had much less collagen staining than the mdx saline-treated group at 12 months and look similar to that observed in 7 month old mdx mice, the age when treatment was initiated (Panels D-F). Quantitative measurement of the extent of fibrosis in mdx mice, measured as the percentage of red area/total area is shown in Fig 7, Panel A. The wild-type group showed 6.6% staining and was significantly different from all other groups (P < 0.0001). The 7-month mdx mouse group had 27.3% fibrotic area compared to 38% for the 12-month old saline treated group indicating a significant (P < 0.0001) increase in fibrosis during the study period. Treatment with either P-188 NF or prednisone resulted in a significantly lower level percentage of collagen in the cross sections of 26 and 28% for P-188 NF and prednisone, respectively, measured at 12 months of age. The 3 mg/Kg P-188 NF group had significantly less fibrosis than either the prednisone or 7 month-old mdx groups. There was no difference between the 7 month-old mdx group and the prednisone- treated group.
Diaphragm muscles from dko mice treated with P-188 NF or prednisone at 1 mg/Kg also showed a substantial decrease in fibrosis (Fig 6B), approximately a 33% reduction compared with their respective dko saline controls. These results demonstrate that P-188 NF treatment has the potential to slow or stop the deposition of collagen in the diaphragm.
Discussion
The studies reported here were undertaken to test the hypothesis that P-188 NF treatment would have functional and structural effects on contraction-induced membrane damage in diaphragm muscle. We observed significant differences in several respiratory parameters between groups of mdx mice treated for 22 weeks (Table 2) of which TV/BW and MV/BW ratio were clearly biologically meaningful (Figs 1 and S11). TV/BW was improved 12% by P-188 NF treatment compared with saline-treated mdx mice or compared to baseline. P-188 NF treatment also significantly impacted diaphragm structure in the 1-year-old mdx mice suggesting that P-188 NF treatment protected muscle fibers from degeneration. When we switched to a more severe muscular dystrophy model the biological effects of P-188 NF treatment were even more evident. There was a demonstrable slowing of the loss of respiratory function in dko mice treated for 8 weeks with P-188 NF (Table 3, and S12–S14 Figs). Maintenance of respiratory function was associated with a diaphragm muscle protective effect of P-188 NF treatment. These studies support our hypothesis that P-188 NF can protect from contraction-induced diaphragm membrane damage. It is also of note that the significant changes observed in the mdx and dko mouse studies occurred in animals with established respiratory dysfunction indicating that P-188 NF has the potential to treat, rather than just prevent, respiratory dysfunction.
Prednisone was run as a positive control in these studies. Corticosteroid therapy has been shown to delay the loss of respiratory function in DMD patients [6, 48]. The results shown here indicate that, in these 2 mouse models of muscular dystrophy, the activity of prednisone is similar to that of P-188 NF both qualitatively and quantitatively. The similarity between the effects of P-188 treatment and this standard of care therapy in mdx and dko mice is exciting since it suggests that P-188 NF might have similar positive effects in patients. With a better side effect profile than prednisone P-188 NF might be an alternative therapy for the many DMD patients, for whom the side effects of corticosteroid treatment are severe and unacceptable. In addition, P-188 NF treatment effects might be additive to those of corticosteroid treatment given the difference in their proposed mechanisms of action. Additional work will be needed to demonstrate the effects of combination P-188-prednisone treatment. While we don’t know if the functional effects in mdx mice will translate into functional improvements in DMD patients, the fact that nine of twelve respiratory parameters were affected in the same fashion by P-188 NF and prednisone treatment provides a basis for suggesting that P-188 NF might also delay loss of respiratory function in patients.
Our results showing effects of P-188 NF on respiratory function, through protection of diaphragm muscle, are in contrast to in situ results reported recently by Terry et al. [24] that show a modest drop in maximum force generated in Tibialis Anterior muscle. While the reason for the functional discrepancy is not known, we only evaluated diaphragm muscle so no direct comparison can be made. However, work by Dr. Joseph Metzger (Chair of the Phrixus Scientific Advisory Board) and colleagues have demonstrated that the route of administration of P-188 is critical to its pharmacodynamic properties with the s.c but not the i.p. route of administration conferring protection to dystrophic skeletal muscle [49]. The work presented here was performed administering P-188 s.c. while Terry et al. [24] administered P-188 i.p. The i.p. route of administration was also used in another paper that claimed that P-188 did not protect skeletal muscle [21]. Further, the Terry et al. study used Hypnorm and midazolam as anesthesia during functional evaluation. Both fentanyl (Hypnorm component) and P-188 have surfactant properties [50, 51] and have been shown to directly influence membrane structure and function [14, 52–58]. In addition, it is clear from a pharmacokinetic study that P-188 enhances the bioavailability of fentanyl from an oral transmucosal lozenge suggesting an interaction between these molecules at the membrane level [59]. The potential for interactions between these agents was not controlled for in the Terry et al. study [24]. Additional work will be required to determine if route of administration or choice of anesthesia contributed to these differences.
The histology results provide a mechanistic explanation for P-188 NF’s functional benefits. We hypothesized that P-188 NF protects diaphragm muscle fibers from membrane damage thereby decreasing the amount of regeneration taking place at any specific time. The reduced need for regenerative capacity likely preserves that capacity for a longer period. Diaphragm regeneration, as measured by centralized nuclei, has been reported to be above 40% at 20 weeks of age [60] and above 40% at 4 and 7 months of age [61] in mdx mice. The 26% level seen in the 1 year old mdx mice treated with saline (Fig 3) suggests that the active regenerative process in diaphragm muscle may be slowing compared to that in younger mice. Indeed, 16-month-old mice have been reported to have 14% centralized nuclei [62] supporting this contention. In any case, the results in Fig 3 demonstrate that diaphragm muscle has reduced regenerative capacity in 1-year-old mdx mice.
The results presented here demonstrate that daily, low dose, subcutaneous administration of P-188 NF can improve respiratory function in the mdx mouse model. Since respiratory failure is the leading cause of death in the DMD population, P-188 NF could represent an important new therapy to slow the loss of respiratory function and potentially increase life span in DMD patients.
Wild type and dko mice (3 weeks of age) were randomized into groups and evaluated for respiratory activity using un-anesthetized, un-restrained whole body plethysmography. This table shows the baseline readings for each respiratory parameter evaluated.
Supporting Information
Myocardial infarction was induced in Sprague-Dawley rats by complete ligation of the left anterior descending coronary artery and significant heart failure developed over 8-weeks of incubation as determined by echocardiography (ejection fraction < 30%) (CHF rats). Ejection fraction was measured at baseline and 2 days later the CHF rats were dosed i.v., by tail vein injection, with 1.5 mg/Kg of P-188 NF and echoed 4 hr post dose. After a 3 day washout period, the rats were dosed with 4.6 mg/kg of P-188 NF and echoed 4 hr post dose. Ejection fraction was increased at both doses of P-188. One rat in the 4.6 mg/Kg group did not respond keeping value at this dose low.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 NF at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.0001 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.05 for mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.001 for mdx 3 mg/Kg group vs. wild type saline. Panel B. P < 0.001 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.0001 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.05 for the mdx 1 mg/Kg prednisone group vs. mdx saline. P < 0.01 for the mdx P-188 NF group vs. the mdx prednisone group.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.01 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.0001 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for the wild type saline groups vs. mdx saline and mdx P-188 NF. The wild type saline and mdx prednisone groups were not significantly different. Panel A, P < 0.0001 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.0001 for the mdx 1 mg/Kg prednisone group vs. mdx saline. P < 0.05 for mdx P-188 NF vs. mdx prednisone.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.0001 for mdx 3 mg/Kg group vs. wild type saline. Panel B. P < 0.0001 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. There was no significant difference between mdx groups.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.01 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.01 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.01 for wild type saline vs. all mdx P-188 NF and P < 0.0001 vs. mdx prednisone. Panel A, Not significant for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.05 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx P-188 NF, P < 0.05 for mdx saline vs. mdx P-188 and P < 0.001 for mdx saline vs. mdx prednisone.
(TIF)
Dko mice were treated with 1 mg/Kg of P-188 NF once per day, s.c, for 8 weeks from ages 3–11 weeks. Respiration was measured weekly by WBP. The key on the right side of each panel identifies the groups and the parameter is indicated on the Y-axis. Data points are means +/- S.D. N = 8/group wild type and 5/group dko saline and dko P-188. P < 0.0001 for wild type saline vs. All mdx groups. Panel A, P < 0.05 for mdx P-188 NF vs. mdx saline. Panel B, P < 0.001 for mdx P-188 NF vs. mdx saline. Panel C, P < 0.0001 001 for mdx P-188 NF vs. mdx saline. N = 18 for wild type saline and N = 9 for mdx P-188 NF.
(TIF)
Dko mice were treated with 1 mg/Kg of P-188 NF once per day, s.c, for 8 weeks from ages 3–11 weeks. Respiration was measured weekly by WBP. The key on the right side of each panel identifies the groups and the parameter is indicated on the Y-axis. Data points are means +/- S.D. N = 8/group wild type and 9/group dko saline and dko P-188. P < 0.0001 for wild type saline vs. All mdx groups. The significance for any differences observed can be found in Table 3 in the P-188 (N = 5) row. Panel A, P < 0.01 for mdx P-188 NF vs. mdx saline. Panel B, Not significant for mdx P-188 NF vs. mdx saline. Panel C, P < 0.05 for mdx P-188 NF vs. mdx saline. N = 18 for wild type saline and N = 9 for mdx P-188 NF.
(TIF)
Dko mice were treated with 1 mg/Kg of P-188 NF once per day, s.c, for 8 weeks from ages 3–11 weeks. Respiration was measured weekly by WBP. The key on the right side of each panel identifies the groups and the parameter is indicated on the Y-axis. Data points are means +/- S.D. N = 8/group wild type and 9/group dko saline and dko P-188. P < 0.0001 for wild type saline vs. All mdx groups. The significance for any differences observed can be found in Table 3 in the P-188 (N = 5) row. Panel A, P < 0.05 for mdx P-188 NF vs. mdx saline. Panel B, Not significant for mdx P-188 NF vs. mdx saline.
(TIF)
Sections of diaphragms from the same groups of mice described in Fig 1 were stained with H&E. All staining was done on diaphragm muscle from 12-month old mice with the exception of Panel B, which shows a diaphragm from a 7 month old untreated mdx mouse. Shown in the figure are longitudinal sections of diaphragm muscle from: Panel A, wild type control saline-treated mouse; Panel B, a 7 month old mdx mouse; Panel C, an mdx saline-treated mouse; Panel D, an mdx mouse treated with 3 mg/Kg P-188 NF; Panel E, an mdx mouse treated with 1 mg/Kg prednisone.
(TIF)
(DOCX)
Acknowledgments
The authors would like to thank Dr. Joseph Metzger for helpful suggestions and critical reading of the manuscript, Dr. Leslie Browne for manuscript review, Kimber Converso-Baran for performing the echocardiography and analysis, Robert Ings, (RMI-Pharmacokinetics for PK analysis).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Small Business Innovative Research Grants R43 NS070327 from the NINDS, and R43 HL110464 from the NHLBI and a grant from the Duchenne Alliance to BEM. Phrixus Pharmaceuticals provided support in the form of salaries for authors [BEM, SK]. The funders did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the 'author contributions' section.
References
- 1. Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul. Disord. 1991; 1: 19–29. [DOI] [PubMed] [Google Scholar]
- 2. Davies KE, Pearson PL, Harper PS, Murray JM, O’Brien T, Sarfarazi M. et al. Linkage analysis of two cloned DNA sequences flanking the Duchenne muscular dystrophy locus on the short arm of the human X chromosome. Nucleic Acids Res. 1983; 11: 2303–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd Y, Buckle V, Holt S, Munro E, Hunter D, Craig I. Muscular dystrophy in girls with X; autosome translocations. J. Med. Genet. 1986; 23: 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51:919–928. [DOI] [PubMed] [Google Scholar]
- 5. Emery AE. The muscular dystrophies. Lancet. 2002; 359: 687–695. [DOI] [PubMed] [Google Scholar]
- 6. Manzur AY, Kinali M, Muntoni F. Update on the management of Duchenne muscular dystrophy. Arch Dis Child. 2008; 93:986–90. 10.1136/adc.2007.118141 [DOI] [PubMed] [Google Scholar]
- 7. Chetboul V, Escriou C, Tessier D, Richard V, Pouchelon JL, Thibault H, et al. Tissue Doppler imaging detects early asymptomatic myocardial abnormalities in a dog model of Duchenne's cardiomyopathy. Eur Heart J. 2004; 25:1934–1939. [DOI] [PubMed] [Google Scholar]
- 8. Finsterer J, Stöllberger C, Wahbi K. Cardiomyopathy in neurological disorders. Cardiovasc Pathol. 2013, pii: S1054–8807(13)00004-5. 10.1016/j.carpath.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 9. Kamogawa Y, Biro S, Maeda M, Setoguchi M, Hirakawa T, Yoshida H, et al. Dystrophin-deficient myocardium is vulnerable to pressure overload in vivo. Cardiovasc Res. 2001; 50:509–515. [DOI] [PubMed] [Google Scholar]
- 10. Melacini P, Fanin M, Angelini A, Pegoraro E, Livi U, Daniele GA, et al. Cardiac transplantation in a Duchenne muscular dystrophy carrier. Neuromuscul Disord. 1998; 8:585–590. [DOI] [PubMed] [Google Scholar]
- 11. Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990; 26:271–277. [DOI] [PubMed] [Google Scholar]
- 12. Lynch GS. Role of contraction-induced injury in the mechanisms of muscle damage in muscular dystrophy. Clin Exp Pharmacol Physiol. 2004; 31: 557–561. [DOI] [PubMed] [Google Scholar]
- 13. Menke A, Jockusch H. Extent of shock-induced membrane leakage in human and mouse myotubes depends on dystrophin. J Cell Sci. 1995; 108:727–733. [DOI] [PubMed] [Google Scholar]
- 14. Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005; 436:1025–1029. [DOI] [PubMed] [Google Scholar]
- 15. Lorin C, Vögeli I, Niggli E. Dystrophic cardiomyopathy: role of TRPV2 channels in stretch-induced cell damage. Cardiovasc Res. 2015; 106:153–162. 10.1093/cvr/cvv021 [DOI] [PubMed] [Google Scholar]
- 16. Cheng X, Zhang X, Gao Q, Ali Samie M, Azar M, Tsang WL, et al. The intracellular Ca²⁺ channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med. 2014; 20:1187–92. 10.1038/nm.3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goonasekera SA, Davis J, Kwong JQ, Accornero F, Wei-LaPierre L, Sargent MA, et al. Enhanced Ca²⁺ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy. Hum Mol Genet. 2014; 23:3706–15. 10.1093/hmg/ddu079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harisseh R, Chatelier A, Magaud C, Déliot N, Constantin B. Involvement of TRPV2 and SOCE in calcium influx disorder in DMD primary human myotubes with a specific contribution of α1-syntrophin and PLC/PKC in SOCE regulation. Am J Physiol Cell Physiol. 2013; 304:C881–94. 10.1152/ajpcell.00182.2012 [DOI] [PubMed] [Google Scholar]
- 19. Allen DG, Whitehead NP. Duchenne muscular dystrophy—what causes the increased membrane permeability in skeletal muscle? Int J Biochem Cell Biol. 2011; 43:290–4. 10.1016/j.biocel.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 20. Townsend D, Turner I, Yasuda S, Martindale J, Davis J, Shillingford M, et al. Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. J Clin Invest. 2010; 120:1140–1150. 10.1172/JCI41329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quinlan JG, Wong BL, Niemeier RT, McCullough AS, Levin L, Emanuele M. Poloxamer 188 failed to prevent exercise-induced membrane breakdown in mdx skeletal muscle fibers. Neuromuscul Disord. 2006; 16:855–864. [DOI] [PubMed] [Google Scholar]
- 22. Ryall JG, van der Poel C, Schertzer JD, Plant DR, Lynch GS. The membrane sealant poloxamer reduces membrane permeability in tibialis anterior muscle from dystrophic mdx mice. FASEB J. 2007; 21:769.28 [Google Scholar]
- 23. Ng R, Metzger JM, Claflin DR, Faulkner JA. Poloxamer 188 reduces the contraction-induced force decline in lumbrical muscles from mdx mice. Am J Physiol Cell Physiol. 2008; 295:C146–150. 10.1152/ajpcell.00017.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Terry RL, Kaneb HM, Wells DJ. Poloxamer 188 has a deleterious effect on dystrophic skeletal muscle function. PLOS one 2015. 9(3): e91221, 10.1371/journal.pone.0091221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991; 352:536–539 [DOI] [PubMed] [Google Scholar]
- 26. Liu F, Liang KW, Huang l. Systematic administration of naked DNA: Gene transfer to skeletal muscle. Mol. Intervent. 2001; 1:168–172. [PubMed] [Google Scholar]
- 27. Huang P, Cheng G, Lu H, Aronica M, Ransohoff RM, Zhou L. Impaired respiratory function in mdx and mdx/utrn +/- mice. Muscle Nerve 2011; 43: 263–267. 10.1002/mus.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nelson CA, Hunter B, Quigley LA, Girgenrath S, Weber WD, McCullough JA, et al. Inhibiting TGF-β activity improves respiratory function in mdx mice. Am. J. Pathol. 2011; 178: 2611–2621. 10.1016/j.ajpath.2011.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishizaki M, Suga T, Kimura E, Shiota T, Kawano R, Uchida Y, et al. Mdx respiratory impairment following fibrosis of the diaphragm. Neuromuscul Disord. 2008; 18:342–348. 10.1016/j.nmd.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 30. Guerron AD, Rawat R, Sali A, Spurney CF, Pistilli E, Cha HJ, et al. Functional and molecular effects of arginine butyrate and prednisone on muscle and heart in the mdx mouse model of Duchenne Muscular Dystrophy. PLoS One. 2010; 5(6): e11220 10.1371/journal.pone.0011220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou L, Rafael-Fortney JA, Huang P, Zhou X, Kaminski HJ Liu L, et al. Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J Neurol Sci. 2008; 264:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration and repair in muscular dystrophies. Annu. Rev. Physiol. 2009; 71: 37–57. 10.1146/annurev.physiol.010908.163216 [DOI] [PubMed] [Google Scholar]
- 33. Dubach-Powell J. Quantitative determination of muscle fiber diameter (minimal Feret’s diameter) and percentage of centralized nuclei. Treat NMD website, dmd_m.1.2.001, 2008 Available: http://www.treat-nmd.eu/research/preclinical/dmd-sops/. Accessed 20 September 2013. [Google Scholar]
- 34. Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord. 2004; 14:675–682. [DOI] [PubMed] [Google Scholar]
- 35.Khurana TS, Mosqueira M, Matecki S. Respiratory System Evaluation Treat NMD website, DMD_M.2.2.002, 2008; Available: http://www.treatnmd.eu/research/preclinical/dmd-sops/. Accessed July 21 2011.
- 36. Steinhardt RA. The mechanisms of cell membrane repair. A tutorial guide to key experiments. Ann. N.Y. Acad. Sci. 2005; 1066:152–165. [DOI] [PubMed] [Google Scholar]
- 37. Agarwal J, Walsh A, Lee RC. Multimodal strategies for resuscitating injured cells. Ann. N.Y. Acad. Sci. 2005; 1066:295–309. [DOI] [PubMed] [Google Scholar]
- 38. Maskarinec SA, Wu G, Lee KYC. Membrane sealing by polymers. Ann. N.Y. Acad. Sci. 2005; 1066: 310–320. [DOI] [PubMed] [Google Scholar]
- 39. Serbest G, Horwitz J, Barbee K. The effect of poloxamer-188 on neuronal cell recovery from mechanical injury. J. Neurotrauma 2005; 22: 119–132. [DOI] [PubMed] [Google Scholar]
- 40. Juneman EB, Saleh L, Lancaster JJ, Thai HM, Markham B, Goldman S. The effects of poloxamer-188 on left ventricular function in chronic heart failure after myocardial infarction. J Cardiovasc Pharmacol. 2012; 60:293–298. 10.1097/FJC.0b013e31825f6f88 [DOI] [PubMed] [Google Scholar]
- 41. Schaer GL, Spaccavento LJ, Browne KF, Krueger KA, Krichbaum D, Phelan JM, et al. Beneficial effects of RheothRx injection in patients receiving thrombolytic therapy for acute myocardial infarction. Results of a randomized, double-blind, placebo-controlled trial. Circulation. 1996; 94:298–307. [DOI] [PubMed] [Google Scholar]
- 42. Ilsar I, Wang M, Jiang A, Dye K, Markham B, Sabbah HN. Acute intervenous bolus injection of Poloxamer-188 improves left ventricular function in dogs with heart failure. J. Amer Col. Cardiol. 2010; 55(10A): A16E146. [Google Scholar]
- 43. Suzuki N, Akiyama T, Takahashi T, Komuro H, Warita H, Tateyama M, et al. Continuous administration of poloxamer 188 reduces overload-induced muscular atrophy in dysferlin-deficient SJL mice. Neurosci Res. 2012;72:181–186. 10.1016/j.neures.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 44. Weir AP, Burton EA, Harrod G, Davies KE. A- and B-utrophin have different expression patterns and are differentially up-regulated in mdx muscle. J Biol Chem. 2002. 277:45285–45290. [DOI] [PubMed] [Google Scholar]
- 45. Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997; 90:729–738. [DOI] [PubMed] [Google Scholar]
- 46. Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997; 90:717–727. [DOI] [PubMed] [Google Scholar]
- 47. Call JA, Ervasti JM, Lowe DA. TAT-μUtrophin mitigates the pathophysiology of dystrophin and utrophin double-knockout mice. J Appl Physiol. 2011; 111:200–205. 10.1152/japplphysiol.00248.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bach JR, Martinez D, Saulat B. Duchenne muscular dystrophy: the effect of glucocorticoids on ventilator use and ambulation. Am J Phys Med Rehabil. 2010; 89:620–624. 10.1097/PHM.0b013e3181e72207 [DOI] [PubMed] [Google Scholar]
- 49. Houang EM, Haman K, Filareto A, Perlingeiro RC, Bates F, Lowe DA, et al. Membrane stabilizing copolymers confer marked protection to dystrophic skeletal muscle. MDA Scientific Sessions, March 2015. (abstract). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee RC, River LP, Pan FS, Ji L, Wollmann RL. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc Natl Acad Sci U S A. 1992; 89:4524–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edmond-Seal J, Prys-Roberts C. Pharmacology of drugs used in neuroleptanalgesia. Brit, J. Anesth. 1970; 42: 207–216. [DOI] [PubMed] [Google Scholar]
- 52. Duncan DJ, Hopkins PM, Harrison SM. Negative inotropic effects of tumour necrosis factor-α are ameliorated by alfentanil in rat ventricular myocytes. Brit. J. Pharmacol. 2007; 150: 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brooks ZLS, Browne NJ, Reilly CS. The dose-dependent effects of Fentanyl on rat skeletal muscle microcirculation in vivo. Anesth. Analg. 2003; 96: 456–462. [DOI] [PubMed] [Google Scholar]
- 54. Hertsens R, Jacob W, Van Bogaert A. Effect of hypnorm chloralosane and pentobarbital on the ultra structure of the inner membrane of rat heart mitochondria. Biochim. Biophys. Acta 1984; 769: 411–418. [DOI] [PubMed] [Google Scholar]
- 55. Blair JR, Pruett JK, Introna RPS, Adams RJ, Balser JS. Cardiac electrophysiologic effects of fentanyl and sufentanil in canine cardiac purkinje fibers. Anesth. 1989; 71: 565–570. [DOI] [PubMed] [Google Scholar]
- 56. Cui GL, Sandvik AK, Munkvold B, Waldum HL. Effects of gastric agents on gastrin-stimulated and histamine-stimulated gastric acid secretion in the totally isolated vascularly perfused rat stomach. Scand. J. Gastroenterol. 2002; 37: 750–753. [DOI] [PubMed] [Google Scholar]
- 57. Lee B, Firestone MA. Electron density mapping of triblock copolymers associated with model biomembranes: insights into conformational states and effect on bilayer structure. Biomacromol. 2008; 9: 1541–1550 [DOI] [PubMed] [Google Scholar]
- 58. Padanilam JT, Bischof JC, Lee RC, Cravalho EG, Tompkins RG, Yarmush ML, et al. Effectiveness of poloxamer 188 in arresting calcien leakage from thermally damaged isolated skeletal muscle cells. Ann. N Y Acad. Sci. 1994; 720: 111–123. [DOI] [PubMed] [Google Scholar]
- 59. Fisher A, Watling M, Smith A Knight A. Pharmacokinetic comparisons of three nasal fentanyl formulations; pectin, chitosan and chitosan-poloxamer 188. Int J Clin Pharmacol Ther. 2010; 48:138–145. [DOI] [PubMed] [Google Scholar]
- 60. Willmann R, Possekel S, Dubach-Powell J, Meier T, Ruegg MA. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul Disord. 2009;1 9:241–249. [DOI] [PubMed] [Google Scholar]
- 61. Turgeman T, Hagai Y, Huebner K, Jassai DS, Genin O, Nagler A, et al. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscul Disord. 2008; 18:857–868. 10.1016/j.nmd.2008.06.386 [DOI] [PubMed] [Google Scholar]
- 62. Gayraud J, Matecki S, Hnia K, Mornet D, Prefaut C, Mercier J, et al. Ventilation during air breathing and in response to hypercapnia in 5 and 16 month-old mdx and C57 mice. J Muscle Res Cell Motil. 2007; 28:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Myocardial infarction was induced in Sprague-Dawley rats by complete ligation of the left anterior descending coronary artery and significant heart failure developed over 8-weeks of incubation as determined by echocardiography (ejection fraction < 30%) (CHF rats). Ejection fraction was measured at baseline and 2 days later the CHF rats were dosed i.v., by tail vein injection, with 1.5 mg/Kg of P-188 NF and echoed 4 hr post dose. After a 3 day washout period, the rats were dosed with 4.6 mg/kg of P-188 NF and echoed 4 hr post dose. Ejection fraction was increased at both doses of P-188. One rat in the 4.6 mg/Kg group did not respond keeping value at this dose low.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 NF at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.0001 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.05 for mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.001 for mdx 3 mg/Kg group vs. wild type saline. Panel B. P < 0.001 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.0001 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.05 for the mdx 1 mg/Kg prednisone group vs. mdx saline. P < 0.01 for the mdx P-188 NF group vs. the mdx prednisone group.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.01 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.0001 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for the wild type saline groups vs. mdx saline and mdx P-188 NF. The wild type saline and mdx prednisone groups were not significantly different. Panel A, P < 0.0001 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.0001 for the mdx 1 mg/Kg prednisone group vs. mdx saline. P < 0.05 for mdx P-188 NF vs. mdx prednisone.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.0001 for mdx 3 mg/Kg group vs. wild type saline. Panel B. P < 0.0001 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. There was no significant difference between mdx groups.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx groups. Panel A, P < 0.01 for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.01 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.01 for wild type saline vs. all mdx P-188 NF and P < 0.0001 vs. mdx prednisone. Panel A, Not significant for mdx 3 mg/Kg group vs. mdx saline. Panel B. P < 0.05 for the mdx 1 mg/Kg prednisone group vs. mdx saline.
(TIF)
Mdx mice were treated QD, s.c. with P-188 NF or prednisone from age 7 months to 12 months ± 2 weeks. The black line represents wild-type mice (C57BL/10 SnJ) treated with saline. The red line represents mdx mice treated with saline. The blue line represents mice treated with 3 mg/Kg P-188 NF (Panel A) or 1 mg/Kg prednisone (Panel B). Data points are means +/- S.D. N = 12/group for both groups, except N = 11 for mdx 3 mg/Kg P-188 at 20 and 22 weeks. P < 0.0001 for wild type saline vs. all mdx P-188 NF, P < 0.05 for mdx saline vs. mdx P-188 and P < 0.001 for mdx saline vs. mdx prednisone.
(TIF)
Dko mice were treated with 1 mg/Kg of P-188 NF once per day, s.c, for 8 weeks from ages 3–11 weeks. Respiration was measured weekly by WBP. The key on the right side of each panel identifies the groups and the parameter is indicated on the Y-axis. Data points are means +/- S.D. N = 8/group wild type and 5/group dko saline and dko P-188. P < 0.0001 for wild type saline vs. All mdx groups. Panel A, P < 0.05 for mdx P-188 NF vs. mdx saline. Panel B, P < 0.001 for mdx P-188 NF vs. mdx saline. Panel C, P < 0.0001 001 for mdx P-188 NF vs. mdx saline. N = 18 for wild type saline and N = 9 for mdx P-188 NF.
(TIF)
Dko mice were treated with 1 mg/Kg of P-188 NF once per day, s.c, for 8 weeks from ages 3–11 weeks. Respiration was measured weekly by WBP. The key on the right side of each panel identifies the groups and the parameter is indicated on the Y-axis. Data points are means +/- S.D. N = 8/group wild type and 9/group dko saline and dko P-188. P < 0.0001 for wild type saline vs. All mdx groups. The significance for any differences observed can be found in Table 3 in the P-188 (N = 5) row. Panel A, P < 0.01 for mdx P-188 NF vs. mdx saline. Panel B, Not significant for mdx P-188 NF vs. mdx saline. Panel C, P < 0.05 for mdx P-188 NF vs. mdx saline. N = 18 for wild type saline and N = 9 for mdx P-188 NF.
(TIF)
Dko mice were treated with 1 mg/Kg of P-188 NF once per day, s.c, for 8 weeks from ages 3–11 weeks. Respiration was measured weekly by WBP. The key on the right side of each panel identifies the groups and the parameter is indicated on the Y-axis. Data points are means +/- S.D. N = 8/group wild type and 9/group dko saline and dko P-188. P < 0.0001 for wild type saline vs. All mdx groups. The significance for any differences observed can be found in Table 3 in the P-188 (N = 5) row. Panel A, P < 0.05 for mdx P-188 NF vs. mdx saline. Panel B, Not significant for mdx P-188 NF vs. mdx saline.
(TIF)
Sections of diaphragms from the same groups of mice described in Fig 1 were stained with H&E. All staining was done on diaphragm muscle from 12-month old mice with the exception of Panel B, which shows a diaphragm from a 7 month old untreated mdx mouse. Shown in the figure are longitudinal sections of diaphragm muscle from: Panel A, wild type control saline-treated mouse; Panel B, a 7 month old mdx mouse; Panel C, an mdx saline-treated mouse; Panel D, an mdx mouse treated with 3 mg/Kg P-188 NF; Panel E, an mdx mouse treated with 1 mg/Kg prednisone.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.