Abstract
Interleukin-5 (IL5) is a T cell derived glycoprotein that stimulates eosinophil production and activation. In the mouse, but apparently not in the human, it is active on B cells. The murine and human IL5 polypeptides exhibit 70% sequence similarity and yet display distinct species-specific activity. Whilst mouse and human IL5 are equally active in human cell assays, human IL5 is 100-fold less active than murine IL5 in mouse cell assays. Two restriction sites were utilized to divide the human and mouse sequences into three fragments. Hybrid molecules consisting of all combinations of these fragments were constructed and expressed. In the human cell assays [using bone marrow or the erythroleukaemic cell line (TF-1)] all the hybrid proteins generated activity comparable to that of the human and mouse IL5. This implies that replacing different domains does not result in detrimental effects to the tertiary structure of the molecule. In the mouse cell assays [using bone marrow or the pro-B cell line (B13)] the hybrids clearly identified the importance of residues in the C terminus for biological activity. The changing of only eight residues in this region of human IL5, to those of mouse IL5, resulted in the hybrid producing biological activity comparable to mouse IL5. In addition, competition binding assays showed that this region probably interacts with the receptor.
Full text
PDF

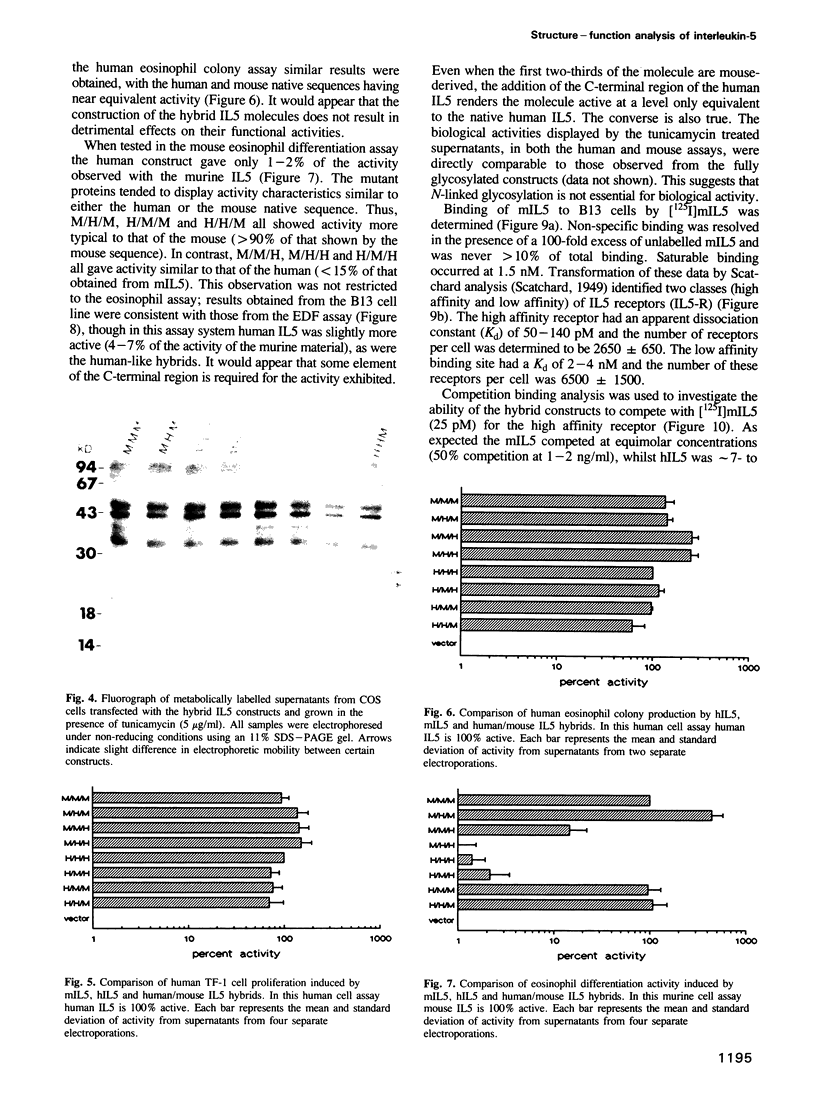
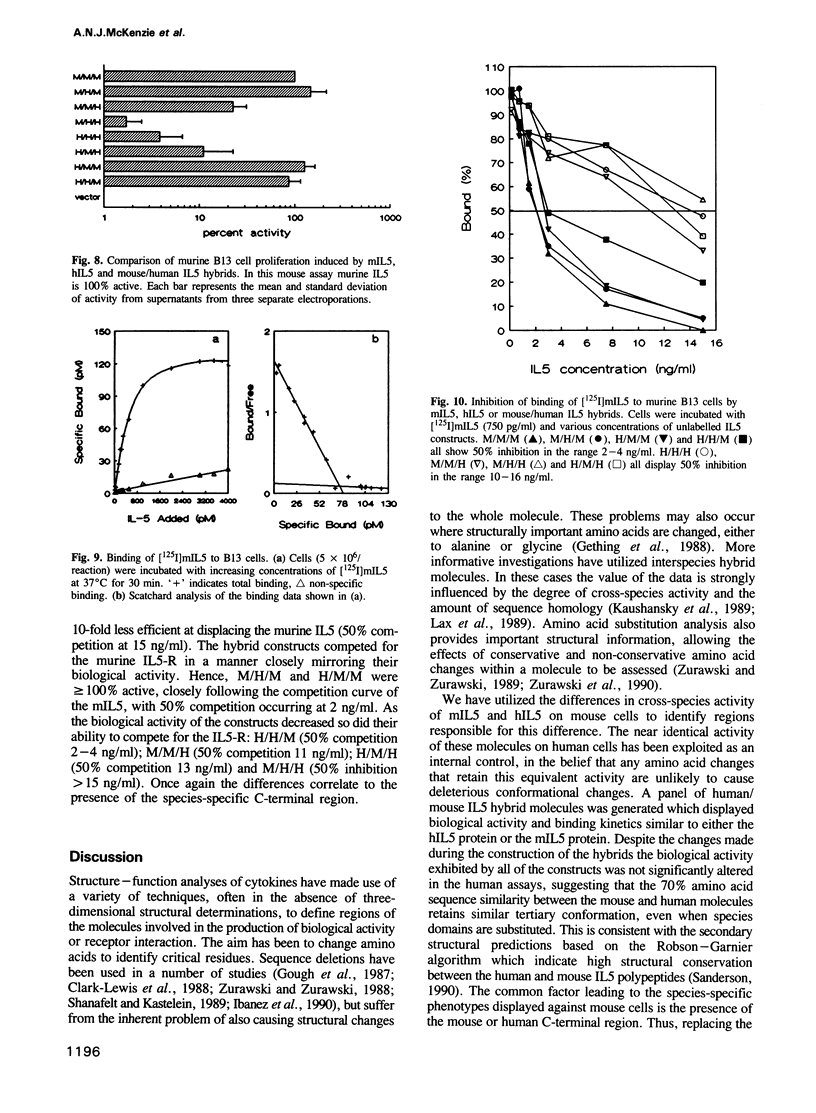



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell H. D., Sanderson C. J., Wang Y., Hort Y., Martinson M. E., Tucker W. Q., Stellwagen A., Strath M., Young I. G. Isolation, structure and expression of cDNA and genomic clones for murine eosinophil differentiation factor. Comparison with other eosinophilopoietic lymphokines and identity with interleukin-5. Eur J Biochem. 1988 Jun 1;174(2):345–352. doi: 10.1111/j.1432-1033.1988.tb14104.x. [DOI] [PubMed] [Google Scholar]
- Campbell H. D., Tucker W. Q., Hort Y., Martinson M. E., Mayo G., Clutterbuck E. J., Sanderson C. J., Young I. G. Molecular cloning, nucleotide sequence, and expression of the gene encoding human eosinophil differentiation factor (interleukin 5). Proc Natl Acad Sci U S A. 1987 Oct;84(19):6629–6633. doi: 10.1073/pnas.84.19.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Lopez A. F., To L. B., Vadas M. A., Schrader J. W., Hood L. E., Kent S. B. Structure-function studies of human granulocyte-macrophage colony-stimulating factor. Identification of residues required for activity. J Immunol. 1988 Aug 1;141(3):881–889. [PubMed] [Google Scholar]
- Clutterbuck E., Shields J. G., Gordon J., Smith S. H., Boyd A., Callard R. E., Campbell H. D., Young I. G., Sanderson C. J. Recombinant human interleukin 5 is an eosinophil differentiation factor but has no activity in standard human B cell growth factor assays. Eur J Immunol. 1987 Dec;17(12):1743–1750. doi: 10.1002/eji.1830171210. [DOI] [PubMed] [Google Scholar]
- Dent L. A., Strath M., Mellor A. L., Sanderson C. J. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990 Nov 1;172(5):1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotsika E. N., Sanderson C. J. A fluorometric assay for determining cell growth in lymphocyte proliferation and lymphokine assays. J Immunol Methods. 1987 Dec 4;105(1):55–62. doi: 10.1016/0022-1759(87)90413-3. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Adler B., Boose J. A., Gerard R. D., Madison E. L., McGookey D., Meidell R. S., Roman L. M., Sambrook J. Variants of human tissue-type plasminogen activator that lack specific structural domains of the heavy chain. EMBO J. 1988 Sep;7(9):2731–2740. doi: 10.1002/j.1460-2075.1988.tb03127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Grail D., Gearing D. P., Metcalf D. Mutagenesis of murine granulocyte/macrophage-colony-stimulating factor reveals critical residues near the N terminus. Eur J Biochem. 1987 Dec 1;169(2):353–358. doi: 10.1111/j.1432-1033.1987.tb13619.x. [DOI] [PubMed] [Google Scholar]
- Harada N., Kikuchi Y., Tominaga A., Takaki S., Takatsu K. BCGFII activity on activated B cells of a purified murine T cell-replacing factor (TRF) from a T cell hybridoma (B151K12). J Immunol. 1985 Jun;134(6):3944–3951. [PubMed] [Google Scholar]
- Heldin C. H., Ernlund A., Rorsman C., Rönnstrand L. Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J Biol Chem. 1989 May 25;264(15):8905–8912. [PubMed] [Google Scholar]
- Ibáez C. F., Hallbök F., Ebendal T., Persson H. Structure-function studies of nerve growth factor: functional importance of highly conserved amino acid residues. EMBO J. 1990 May;9(5):1477–1483. doi: 10.1002/j.1460-2075.1990.tb08265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., Shoemaker S. G., Alfaro S., Brown C. Hematopoietic activity of granulocyte/macrophage colony-stimulating factor is dependent upon two distinct regions of the molecule: functional analysis based upon the activities of interspecies hybrid growth factors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1213–1217. doi: 10.1073/pnas.86.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989 Aug;140(2):323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lax I., Bellot F., Howk R., Ullrich A., Givol D., Schlessinger J. Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J. 1989 Feb;8(2):421–427. doi: 10.1002/j.1460-2075.1989.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Gillis D., Park L. S., Clark S., Vadas M. A. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7022–7026. doi: 10.1073/pnas.86.18.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Sanderson C. J., Gamble J. R., Campbell H. D., Young I. G., Vadas M. A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988 Jan 1;167(1):219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie A. N., Ely B., Sanderson C. J. Mutated interleukin-5 monomers are biologically inactive. Mol Immunol. 1991 Jan-Feb;28(1-2):155–158. doi: 10.1016/0161-5890(91)90099-6. [DOI] [PubMed] [Google Scholar]
- Miller J., Malek T. R., Leonard W. J., Greene W. C., Shevach E. M., Germain R. N. Nucleotide sequence and expression of a mouse interleukin 2 receptor cDNA. J Immunol. 1985 Jun;134(6):4212–4217. [PubMed] [Google Scholar]
- Minamitake Y., Kodama S., Katayama T., Adachi H., Tanaka S., Tsujimoto M. Structure of recombinant human interleukin 5 produced by Chinese hamster ovary cells. J Biochem. 1990 Feb;107(2):292–297. doi: 10.1093/oxfordjournals.jbchem.a123041. [DOI] [PubMed] [Google Scholar]
- Mita S., Hosoya Y., Kubota I., Nishihara T., Honjo T., Takahashi T., Takatsu K. Rapid methods for purification of human recombinant interleukin-5 (IL-5) using the anti-murine IL-5 antibody-coupled immunoaffinity column. J Immunol Methods. 1989 Dec 20;125(1-2):233–241. doi: 10.1016/0022-1759(89)90098-7. [DOI] [PubMed] [Google Scholar]
- Mita S., Tominaga A., Hitoshi Y., Sakamoto K., Honjo T., Akagi M., Kikuchi Y., Yamaguchi N., Takatsu K. Characterization of high-affinity receptors for interleukin 5 on interleukin 5-dependent cell lines. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2311–2315. doi: 10.1073/pnas.86.7.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Binding of the differentiation-inducer, granulocyte-colony-stimulating factor, to responsive but not unresponsive leukemic cell lines. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3765–3769. doi: 10.1073/pnas.81.12.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Warren D. J., Holman M., Popham A. M., Sanderson C. J., Klaus G. G. Interleukin 4 (B-cell growth factor II/eosinophil differentiation factor) is a mitogen and differentiation factor for preactivated murine B lymphocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5228–5232. doi: 10.1073/pnas.83.14.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaetinck G., Van der Heyden J., Tavernier J., Faché I., Tuypens T., Fischkoff S., Fiers W., Devos R. Characterization of interleukin 5 receptors on eosinophilic sublines from human promyelocytic leukemia (HL-60) cells. J Exp Med. 1990 Sep 1;172(3):683–691. doi: 10.1084/jem.172.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A. G., Melchers F., Palacios R. Monoclonal antibodies reactive with the mouse interleukin 5 receptor. J Exp Med. 1989 May 1;169(5):1693–1701. doi: 10.1084/jem.169.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Campbell H. D., Young I. G. Molecular and cellular biology of eosinophil differentiation factor (interleukin-5) and its effects on human and mouse B cells. Immunol Rev. 1988 Feb;102:29–50. doi: 10.1111/j.1600-065x.1988.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., O'Garra A., Warren D. J., Klaus G. G. Eosinophil differentiation factor also has B-cell growth factor activity: proposed name interleukin 4. Proc Natl Acad Sci U S A. 1986 Jan;83(2):437–440. doi: 10.1073/pnas.83.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Warren D. J., Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985 Jul 1;162(1):60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt A. B., Kastelein R. A. Identification of critical regions in mouse granulocyte-macrophage colony-stimulating factor by scanning-deletion analysis. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4872–4876. doi: 10.1073/pnas.86.13.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Scott P., Cheever A. W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci U S A. 1990 Jan;87(1):61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Dutton R. W. Production of a B cell growth-promoting activity, (DL)BCGF, from a cloned T cell line and its assay on the BCL1 B cell tumor. J Exp Med. 1982 Dec 1;156(6):1821–1834. doi: 10.1084/jem.156.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki S., Tominaga A., Hitoshi Y., Mita S., Sonoda E., Yamaguchi N., Takatsu K. Molecular cloning and expression of the murine interleukin-5 receptor. EMBO J. 1990 Dec;9(13):4367–4374. doi: 10.1002/j.1460-2075.1990.tb07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Harada N., Mita S., Matsumoto M., Takahashi T., Kikuchi Y., Yamaguchi N. T cell-replacing factor (TRF)/interleukin 5 (IL-5): molecular and functional properties. Immunol Rev. 1988 Feb;102:107–135. doi: 10.1111/j.1600-065x.1988.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Tominaga A., Takahashi T., Kikuchi Y., Mita S., Naomi S., Harada N., Yamaguchi N., Takatsu K. Role of carbohydrate moiety of IL-5. Effect of tunicamycin on the glycosylation of IL-5 and the biologic activity of deglycosylated IL-5. J Immunol. 1990 Feb 15;144(4):1345–1352. [PubMed] [Google Scholar]
- Zurawski S. M., Imler J. L., Zurawski G. Partial agonist/antagonist mouse interleukin-2 proteins indicate that a third component of the receptor complex functions in signal transduction. EMBO J. 1990 Dec;9(12):3899–3905. doi: 10.1002/j.1460-2075.1990.tb07610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski S. M., Zurawski G. Identification of three critical regions within mouse interleukin 2 by fine structural deletion analysis. EMBO J. 1988 Apr;7(4):1061–1069. doi: 10.1002/j.1460-2075.1988.tb02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski S. M., Zurawski G. Mouse interleukin-2 structure-function studies: substitutions in the first alpha-helix can specifically inactivate p70 receptor binding and mutations in the fifth alpha-helix can specifically inactivate p55 receptor binding. EMBO J. 1989 Sep;8(9):2583–2590. doi: 10.1002/j.1460-2075.1989.tb08397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]