Abstract
Key Clinical Message
The oral manifestations of EBV-positive mucocutaneous ulcers have a worrisome clinical appearance but relatively benign clinical course, responding well to conservative treatment. Elderly patients who develop an unexplained, persistent ulcer of the oral mucosa should have the lesion examined for EBV.
Keywords: EBV, elderly, immunosuppression, Mucocutaneous, Ulcer
Introduction
The spectrum of age-related, EBV-positive lymphoproliferative disorders (aEBVLPD) was first described by Oyama and coworkers and distinctively occurs in elderly patients without any history of immunosuppression 1. Further analysis of aEBVLPD cases revealed a heterogeneous group of diseases with very different clinical outcomes, leading to the subclassification into: reactive hyperplasia (RH), mucocutaneous ulcer (EBVMCU)/polyclonal extranodal (polyE), polyclonal nodal (polyN), and diffuse large B-cell lymphoma (DLBCL) 2,3. In this report, we present a case of age-related EBV-positive mucocutaneous ulcer that regressed completely in the course of 4 months without treatment.
Case Report
An 81-year-old lady presented to her dentist with a 3-month history of a painful, nonhealing ulcer on the left hard palate. There was no history of trauma and the ulcer did not improve after her complete upper denture was removed. Her medical history included type 2 diabetes mellitus that was managed only with diet. She was not on any immunosuppressant medication and had no other form of immunosuppression. She was a nonsmoker and did not consume alcohol. Besides the presence of a painful oral ulcer, she was well and had no systemic complaints. Clinical examination revealed a round, well-circumscribed, deep ulceration measuring 3.5 × 3.5 cm located on the posterior left hard palate extending laterally from midline to alveolar ridge and posteriorly to the hard and soft palate junction. The ulcer had a slightly raised indurated border and a tan-white granular base (Fig.1A). There were no extraoral lesions, lymphadenopathy or hepatosplenomegaly. The differential diagnoses of the ulcer included necrotizing sialometaplasia, squamous cell carcinoma, malignant salivary gland tumor, and lymphoma. An incisional biopsy was performed and sent to the Faculty of Dentistry, University of Toronto for oral pathology consultation. The initial biopsy showed pieces of oral mucosa with large areas of predominantly necrotic tissue (Fig.2A). There were scattered areas of viable tissue consisting of small blood vessels with fibrin deposits surrounded by a polymorphous infiltrate that included atypical cells (Fig.2B). The atypical cells had a large vesicular nucleus and 1 to 2 prominent nucleoli and were positive for CD20, CD30, and LMP1 (Fig.2C). In situ hybridization for Epstein–Barr virus-encoded RNA (EBER) was done at Sunnybrook Health Sciences Center and revealed scattered positive cells throughout the specimen (1–2% of the cellular infiltrate), particularly at the interface between the necrotic areas and inflamed mucosa (Fig.2D).
Figure 1.

Clinical photographs over a 4-month period showing a large palatal ulcer that migrated posteriorly and then completely resolved.
Figure 2.
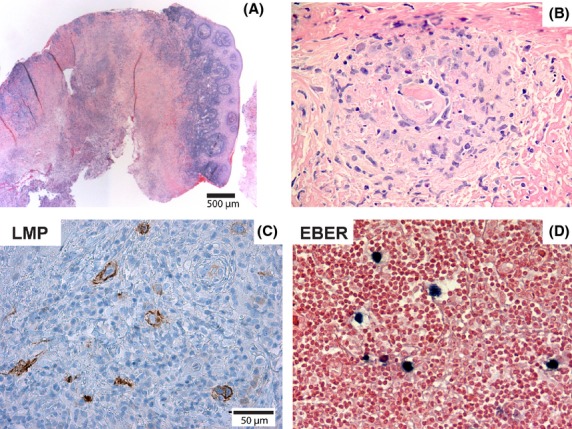
Microscopic findings in an incisional biopsy of EBV-positive oral ulcer. (A) Squamous mucosa with dense lymphoid infiltrate partly covering a large area of necrosis. (B) Small area of viable tissue within the necrotic tissue showing a polymorphous infiltrate that included atypical lymphoid cells. (C) Immunohistochemical staining demonstrated that the atypical lymphoid cells were LMP1-positive (shown here) and CD30-positive (not shown). (D) In situ hybridization for EBER showed scattered positive cells throughout the lesion.
In a follow-up visit 6 weeks after incisional biopsy, the original ulceration had extended posteriorly, encompassing parts of the soft palate. The anterior aspect of the ulcer has healed but the mucosa showed a slightly raised, irregular surface (Fig.1B). Biopsies of both the ulcer and the healed, thickened mucosa were recommended. The second biopsy of the ulcer showed a similar histological appearance as the first biopsy, again with extensive necrosis and an atypical lymphoid infiltrate (not shown). The biopsy of the healed area showed a piece of oral mucosa with a mild focal inflammatory infiltrate and no evidence of an atypical lymphoid infiltrate.
Based on the clinical and histological appearance, a diagnosis of age-related EBV-positive mucocutaneous ulcer was made. The patient implemented diet modifications and vitamin supplementation only, and no prescription medication or active treatments were used. At a follow-up visit 2 months after the second biopsy, the ulcer had healed completely (Fig.1B). At the most recent follow-up, 12 months after the second biopsy, the patient remained well and there was no recurrence of the palatal ulcer.
Discussion
EBV-positive mucocutaneous ulcer is a recently described lesion. In a comprehensive review of the clinical and histopathological features of EBVMCU, 17 cases were due to age-related immunosenescence (aEBVMCU) and nine cases were related to immunosuppressive therapy 3. The clinical presentation was that of ulceration of skin or mucosa with no evidence of systemic lymphadenopathy, hepatosplenomegaly, or bone marrow involvement. The localized nature of the ulceration was thought to reflect a minimal lapse in immunosurveillance over EBV 3. Histologically, EBVMCU regardless of cause of immunosuppression was characterized by well-circumscribed ulceration with an underlying polymorphous infiltrate of lymphoid cells that included atypical, Reed-Sternberg, and Hodgkin-like cells. Necrosis was a prominent feature in some of the cases. All cases showed CD30-positive cells. In situ hybridization for EBER showed a band-like distribution of positive cells underlying the ulcerated mucosal epithelium. Overall, the clinical course of EBVMCU was indolent and most of the age-related cases regressed spontaneously or had remitting and relapsing, localized disease. The lesions that were treated with radiotherapy or chemotherapy all underwent complete remission.
In a follow-up study, the same group reviewed 122 cases of aEBVLPD including reactive hyperplasia (RH), polyclonal extranodal (polyE), polyclonal nodal (polyN), and diffuse large B-cell lymphoma (DLBCL) 2. The majority of cases of polyclonal extranodal LPD (16 of 21 cases) were classified as EBV-positive mucocutaneous ulcer (aEBVMCU). In striking contrast with PolyN and DLBCL cases, aEBVMCU had an excellent prognosis and there were no disease-specific deaths reported in this group. The authors concluded that aEBVLPD showed a wide spectrum of clinical behavior.
There are few published reports of aEBVLPD in the oral cavity concerning patients with no history of immunosuppression 2,4,5 and there is only limited information on clinical outcome. To the best of our knowledge, there is only one report in the English published literature of aEBVLPD presenting as a palatal ulcer. In the largest series of EBVMCU 3, there was one case of a palatal lesion described as an ulcerated mass in the palate in an 80-year-old woman. The lesion underwent complete remission after radiotherapy. It is possible that there are unrecognized cases of aEBVMCU of the oral mucosa since the clinical appearance can be nonspecific and diagnosis requires the demonstration of EBV in the lesion. The absence of EBV excludes the diagnosis of aEBVMCU and requires further investigations to rule out malignant disease.
The treatment for reported cases of EBVMCU has varied from observation to radiotherapy and/or chemotherapy and the published literature does not provide conclusive guidelines for treatment 3. Dojcinov et al. 3 showed that 57% of EBVMCU patients achieve spontaneous remission while 36% received aggressive therapy with all patients achieving remission. The present case was treated empirically by supportive measures. Radiotherapy and chemotherapy were avoided because the lesion remained localized and did not compromise the ability to eat or speak. Also, the ulceration did not follow a progressive destructive course typical of lymphomas but rather started healing after the biopsy. Since the patient was not taking immunosuppressive medications, there was no opportunity to relieve immunosuppression. Increased awareness of aEBVMCU is important for correct diagnosis and management of these lesions that can mimic malignant disease requiring aggressive treatment. As reported by Attard et al. and Hart et al., further systemic investigations including imaging, bone marrow biopsy, and serology may be needed to rule out a systemic lymphoproliferative disorder 6,7.
At 14 months after the initial presentation to the dentist, the patient has remained free of lesion. She is being monitored for recurrence of the palatal ulcer or development of other areas of EBV associated lymphoproliferative disease.
Conclusion
Elderly patients who develop an unexplained, persistent ulcer of the oral mucosa should have the lesion examined for EBV. A medical history should be taken to determine if the patient is on immunosuppressive medications 7. Conservative treatment should be used if the disease remains localized. Additional reports of aEBVMCU will help to understand the factors that control the extent of EBV associated lymphoproliferative disease.
Conflict of Interest
None declared.
References
- Oyama T, Ichimura K, Suzuki R, Suzumiya J, Ohshima K, Yatabe Y, et al. Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am. J. Surg. Pathol. 2003;27:16–26. doi: 10.1097/00000478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Dojcinov SD, Venkataraman G, Pittaluga S, Wlodarska I, Schrager JA, Raffeld M, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S. Jaffe ES. EBV positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am. J. Surg. Pathol. 2010;34:405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Fukunaga S, Inoue H, Miyazaki Y, Kojima M, Ide F, et al. A case of age-related Epstein-Barr virus (EBV)-associated B cell lymphoproliferative disorder, so-called polymorphous subtype, of the mandible, with a review of the literature. Head Neck Pathol. 2013;7:178–187. doi: 10.1007/s12105-012-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague L, Cohen D. Bhattacharyya I. EBV and CD30 Positive Mucocutaneous Ulcers of the Oral Cavity, an Unusual Entity: a Series of 3 Cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;114:e52. [Google Scholar]
- Hart M, Thakral B, Yohe S, et al. EBV-positive mucocutaneous ulcer in organ transplant recipients: a localized indolent posttransplant lymphoproliferative disorder. Am. J. Surg. Pathol. 2014;38:1522–1529. doi: 10.1097/PAS.0000000000000282. [DOI] [PubMed] [Google Scholar]
- Attard AA, Praveen P, Dunn PJ. James GJ. Epstein-Barr virus-positive mucocutaneous ulcer of the oral cavity: the importance of having a detailed clinical history to reach a correct diagnosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;114:e37–e39. doi: 10.1016/j.oooo.2012.04.003. [DOI] [PubMed] [Google Scholar]


