Abstract
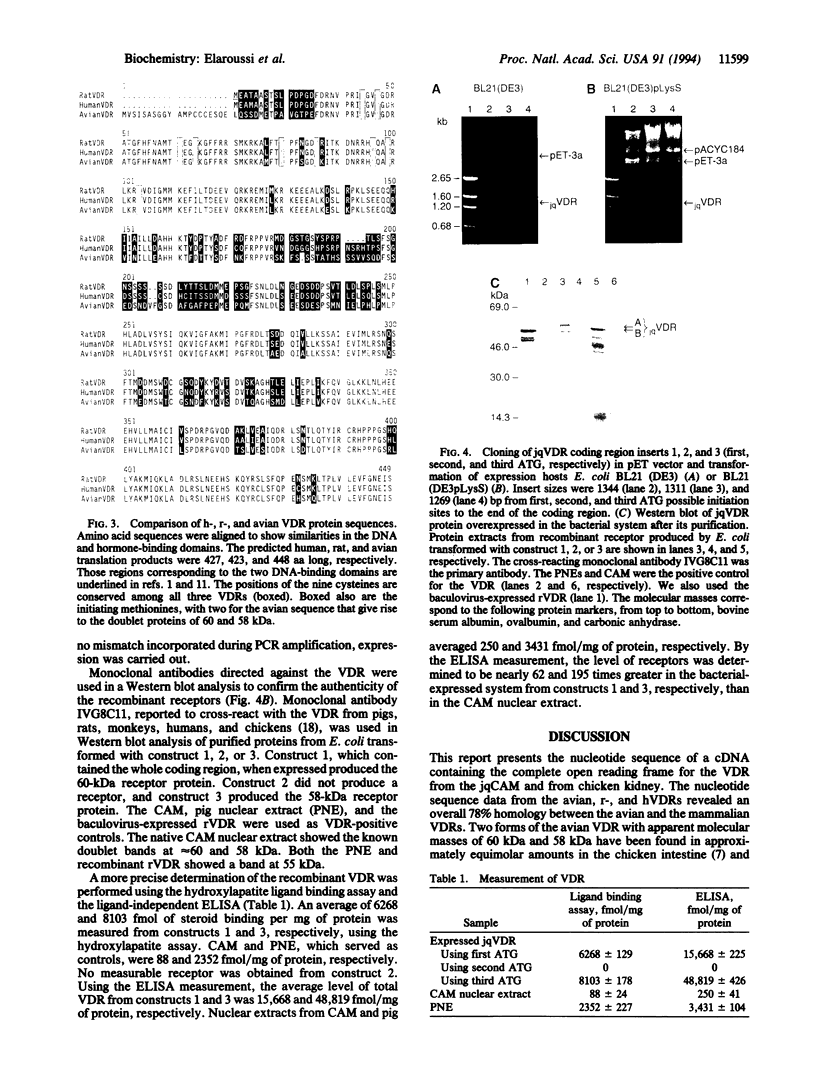
cDNA clones encoding Japanese quail chorioallantoic membrane and chicken kidney 1,25-dihydroxyvitamin D3 receptors were isolated and the total 448-amino acid (aa) sequence was deduced. The sequences of the chicken and quail receptors are identical. The nucleotide and deduced amino acid sequences of the avian receptors are similar but not identical to the reported rat or human receptor sequences. There is a 78% similarity in the nucleotide sequences and 98.5% and 87.5% similarities in the amino acid sequences of the DNA-binding and ligand-binding domains, respectively. Two avian receptor proteins (58 and 60 kDa) arise from a single mRNA transcript by alternate initiation of translation. The avian 1,25-dihydroxyvitamin D3 receptors were produced using a bacterial expression system. Form A receptor was expressed from a cloned cDNA that contains the first translation signal (ATG) and corresponds with the 60-kDa avian receptor protein, and form B receptor was initiated from the third ATG on the same mRNA transcript to give rise to the 58-kDa protein. The cysteine-rich DNA-binding domain is almost conserved among human, rat, and avian receptors. The position of the nine cysteines was conserved in all three sequences. The avian receptor differs in the second zinc finger domain, where a methionine replaces a leucine, a serine replaces an asparagine, and a lysine replaces an arginine at aa 77, 83, and 87, respectively, of the avian sequence. The increased length of the avian receptor results from a 20-aa extension of the N-terminal region. RNA hybridization indicates there is a single mRNA species of approximately 2700 bp for both the chicken and quail receptors compared to 4400 bp for the rat transcript. Surprisingly, the translated avian sequence is larger (448 aa) than the 423-aa rat receptor protein. Therefore, our results confirm that despite the difference in molecular mass between different receptor proteins, there is a similarity in gene organization such that the DNA-binding and hormone-binding domains are positionally conserved from the C terminus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. R., McDonnell D. P., Hughes M., Crisp T. M., Mangelsdorf D. J., Haussler M. R., Pike J. W., Shine J., O'Malley B. W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988 May;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burmester J. K., Wiese R. J., Maeda N., DeLuca H. F. Structure and regulation of the rat 1,25-dihydroxyvitamin D3 receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9499–9502. doi: 10.1073/pnas.85.24.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely O. M., Maxwell B. L., Toft D. O., Schrader W. T., O'Malley B. W. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987 Dec 16;149(2):493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., DeLuca H. F. Identification of the porcine intestinal 1,25-dihydroxyvitamin D3 receptor on sodium dodecyl sulfate/polyacrylamide gels by renaturation and immunoblotting. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7825–7829. doi: 10.1073/pnas.82.23.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., Prahl J. M., Hayes C. E., DeLuca H. F. Monoclonal antibodies to the porcine intestinal receptor for 1,25-dihydroxyvitamin D3: interaction with distinct receptor domains. Biochemistry. 1986 Aug 12;25(16):4523–4534. doi: 10.1021/bi00364a011. [DOI] [PubMed] [Google Scholar]
- Darwish H., DeLuca H. F. Vitamin D-regulated gene expression. Crit Rev Eukaryot Gene Expr. 1993;3(2):89–116. [PubMed] [Google Scholar]
- Elaroussi M. A., Uhland-Smith A., Hellwig W., DeLuca H. F. The role of vitamin D in chorioallantoic membrane calcium transport. Biochim Biophys Acta. 1994 Jun 1;1192(1):1–6. doi: 10.1016/0005-2736(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Yamada S., Schnoes H. K., DeLuca H. F. Specific cytosol-binding protein for 1,25-dihydroxyvitamin D3 in rat intestine. J Biol Chem. 1977 Jul 10;252(13):4501–4505. [PubMed] [Google Scholar]
- Kumar R., Schaefer J., Wieben E. The expression of milligram amounts of functional human 1,25-dihydroxyvitamin D3 receptor in a bacterial expression system. Biochem Biophys Res Commun. 1992 Dec 30;189(3):1417–1423. doi: 10.1016/0006-291x(92)90232-a. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Pike J. W., Haussler M. R. Avian and mammalian receptors for 1,25-dihydroxyvitamin D3: in vitro translation to characterize size and hormone-dependent regulation. Proc Natl Acad Sci U S A. 1987 Jan;84(2):354–358. doi: 10.1073/pnas.84.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Mellon W. S., Fivizzani M. A., Schnoes H. K., DeLuca H. F. Direct chemical synthesis of 1 alpha,25-dihydroxy[26,27-3H]vitamin D3 with high specific activity: its use in receptor studies. Biochemistry. 1980 May 27;19(11):2515–2521. doi: 10.1021/bi00552a033. [DOI] [PubMed] [Google Scholar]
- Pike J. W., Sleator N. M., Haussler M. R. Chicken intestinal receptor for 1,25-dihydroxyvitamin D3. Immunologic characterization and homogeneous isolation of a 60,000-dalton protein. J Biol Chem. 1987 Jan 25;262(3):1305–1311. [PubMed] [Google Scholar]
- Pike J. W. Vitamin D3 receptors: structure and function in transcription. Annu Rev Nutr. 1991;11:189–216. doi: 10.1146/annurev.nu.11.070191.001201. [DOI] [PubMed] [Google Scholar]
- Sandgren M. E., Deluca H. F. An immunoradiometric assay for 1,25-dihydroxyvitamin D3 receptor. Anal Biochem. 1989 Nov 15;183(1):57–63. doi: 10.1016/0003-2697(89)90171-1. [DOI] [PubMed] [Google Scholar]
- Schrader W. T., Birnbaumer M. E., Hughes M. R., Weigel N. L., Grody W. W., O'Malley B. W. Studies on the structure and function of the chicken progesterone receptor. Recent Prog Horm Res. 1981;37:583–633. doi: 10.1016/b978-0-12-571137-1.50017-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Towers T. L., Luisi B. F., Asianov A., Freedman L. P. DNA target selectivity by the vitamin D3 receptor: mechanism of dimer binding to an asymmetric repeat element. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6310–6314. doi: 10.1073/pnas.90.13.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]