Key Clinical Message
Extensive full-thickness skin wounds are quite common in domestic animals. In these report, following the failure of reconstructive surgery, adipose tissue-derived mesenchymal stem cells, and platelet-rich plasma were successfully used in a dog to improve speed and quality of skin tissue healing, avoiding suffering, and debilitating effects.
Keywords: Dog, mesenchymal stem cells, platelet-rich plasma, regenerative medicine, wound healing
Introduction
In companion animals, the healing of large skin defects resulted from traumatic accidents can be challenging to treat since they need a complex management consisting of accurate debridement of damaged and necrotic tissues, local and/or systemic infection control, protection of the underlying tissues and induction of cutaneous tissue regeneration 1,2. To this aim, surgical tissue reconstruction (i.e., skin graft) and medical treatments are often associated to reach complete anatomical and functional recovery of the damaged area, reducing debilitating side effects, and pain.
The present work describes a successful therapeutic approach based on the synergic application of Platelet-Rich Plasma (PRP) and autologous Mesenchymal Stem Cells (MSCs) in an adult Labrador male dog. The patient was involved in a traumatic accident that caused a very large tissue defect (approximately 30 × 35 cm, width × length) of the superior area of the left thigh, that could not be resolved by surgery.
Case History
A 1-year -old Labrador male dog, weight 33 kg, was referred about 8–12 h after being struck by a train. The dog was conscious, well oriented in space and cooperating, but presented an extensive loss of skin tissues of the superior area of the left thigh (approximately 30 × 35 cm, width × length) (Fig.1). The wound extended through all layers of the skin and into underlying tissues. The muscles, although widely exposed, were not damaged. The lesion was classified as a class 2 avulsion injury, 6–12 h old, with large loss of integumentary structures 1. Furthermore, the dog presented fractures of the coccygeal vertebra with partial avulsion of the tail, bilateral lesions of the Achilles tendons, a fracture of the left calcaneal bone, and several cutaneous wounds. The animal did not show neurological deficits, but exhibited orthopedic deficits associated to the bilateral tendon injuries. In particular, the first orthopedic evaluation showed the presence of lameness (grade 3) of the hind limbs. Edema of both Achilles tendons was present at the insertion to os calcaneus, where a 2 cm linear skin wound was present bilaterally. The wounds were probably caused by a sharp object.
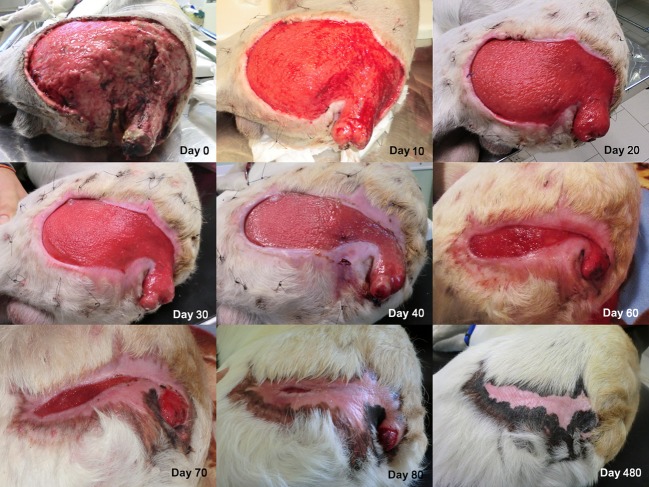
Figure 1.
Images of the wound from day 0 to day 480. A faster tissue growth was observed where wound margins strongly adhered to the underlying tissue. At day 90 the wound margins were closed.
Assessment of Therapeutic Options and Treatment
The first day a lavage of the joints with physiological saline was performed, the left calcaneal bone fragment was removed, and the skin wounds were closed. Both lesions of the Achilles tendons were 1 cm in length and 0.5 cm in depth, parallel to the tendon fibers. We decided not to close the tendon lesions, to avoid fibrotic scar formation, but instead to proceed with a regenerative therapy (see below). No bandage, casting or external fixation was performed. With regard to the main lesion of the left hind limb, after the evaluation of the elasticity of the safe surrounding skin, we decided to perform a two-step surgery. We first opted to close the wound with the surrounding skin, to reduce as much as possible the tissue exposition, thus creating a suitable environment for second intention healing of the residual exposed part of the wound. A second surgery to perform a rotational flap or, alternatively, a skin graft to completely close the wound was scheduled 20–30 days later 3,4. Moreover, it was decided to proceed with the amputation of the tail. The fifth day after surgery, diffused necrosis of the edge of the skin, and the presence of purulent material were observed, thus we decided to remove the necrotic and contaminated tissue. Although the patient was a large size breed, he weighed only 33 kg and there was not enough skin available to perform a free skin graft. Consequently, we decided to proceed with a regenerative therapy. In particular, we opted for a biologic and cellular therapy, to promote second intention healing with open wound management, based on the application of platelet-rich plasma (PRP) and adipose tissue-derived mesenchymal stem cell (MSCs). We reasoned that the association between MSCs and PRP could provide an appropriate set of biological mechanisms to speed up tissue growth, improve the quality of tissue regeneration, and control infection.
Materials and methods used for the preparation of MSCs and PRP are described in Appendix. PRP was applied either by dripping or spraying over the wound surface (every 48–72 h), as well as by injecting the platelet concentrate along the attached wound edge (every 4–6 days). MSCs were applied by spraying the cells over the wound surface (five applications at day 11, 17, 23, 31, 41). Figure 3 reports the timing of PRP and MSCs applications. For each cell application, an aliquot of the cells was sprayed on a culture dish and growth in DMEM to confirm their vitality (Fig.2). The lesions of the Achilles tendons were treated by injecting under ultrasonographic guidance a single dose of 2 × 106 MSCs resuspended in 1 mL of PRP inside the lesions 5,6. Since the cutaneous defect was extremely extended, a wound managing strategy and planning was decided based on the “TIME system” 7. The medication of the wound was performed daily, starting with the removal of old bandage, followed by the irrigation with lactated Ringer’s solution, debridement of the necrotic tissue, and application of PRP and/or cell therapy. The hydration of the wound was ensured by means of Connettivina (Fidia Farmaceutici, Padova, Italy) or Hypermix bandage (RI.MOS., Modena, Italy). When PRP or cells were not employed, Hypermix oil and Hypermix dressing were applied, to protect the tissue. No antibiotic or disinfectant was applied locally. To prevent the movement of the bandage, a variation of “tie-over dressing/bandage technique” was applied 8.
Figure 2.

Sprayed MSCs culture. To assess MSCs viability and replication after spraying, an aliquot of cells was cultured in DMEM for 72 h. PRP induced a partial gelation of the culture medium, therefore cells distributed and grew on different focal planes.
Systemic antibiotic therapy was administered as follow: Cefazolin (Cefazolina TEVA, Milano, Italy) 30 mg/kg intravenous every 8 h from day 1 to 15; Cefadroxil monohydrate (Cefa Cure tabs, Intervet Italia, Milano, Italy) p/o 20 mg/kg from day 16 to 30; Marbofloxacin (Marbocyl Vetoquinol, Forlì, Italy) 2 mg/kg from day 31 to 60 and amoxycillin and clavulanic acid (Synulox Pfizer, Roma, Italy) p/o from day 61 to 80. The huge area of the wound and rare episodes of fever in the first weeks suggested the use of the antibiotic therapy. Anyway, no signs of bacterial infection were observed after the onset of regenerative therapy. Analgesic therapy was administered with Tramadol (Altadol Formevet, Milano, Italy) 2 mg/kg, every 8 h for the first 15 days. Anti-inflammatory therapy was given with Carprofen (Rimadyl Pfizer, Roma, Italy) 2 mg/kg p/o, for 30 days, while gastric protection was provided with Omeprazolum (Omeprazolo Laboratori Alter, Milano, Italy) 10 mg p/o.
Before the daily care, for the first 20 days, the animal was sedated with butorphanol (Butorphanol Nargesic ACME, Reggio Emila, Italy) 0.2 mg/kg and dexmedetomidine (Dexdomior Orion Pharma, Milano, Italy) 5 microgr/kg; afterwards, since the dog did not exhibit pain sensitivity, the sedation was suspended.
Outcome and Follow-up
A granulation tissue was visible after 4 days following the first PRP application. For a better perception of the speed of new tissue growth, as well as of the reduction in the exposed area, we measured every day the amplitude of the wound, in the same position. Interestingly, the curve depicted in Fig.3 fits a hyperbolic curve as described by Cukjati et al. 9. The ratio tissue growth/time reduced gradually during the period, with a fast growth during the first period (about 2.5 cm/day in the first month) and then a gradual fall during the remnant days. A complete closure of the wound occurred 3 months after the start of the regenerative therapy (Fig.3). The wound never presented signs of infection or necrosis during and following the regenerative therapy. The lameness of the hind limbs and the edema of the Achilles’s tendon disappeared 20 days after intra-lesional application of MSCs and PRP. Pigmentation of the skin appeared after about 2 months.
Figure 3.
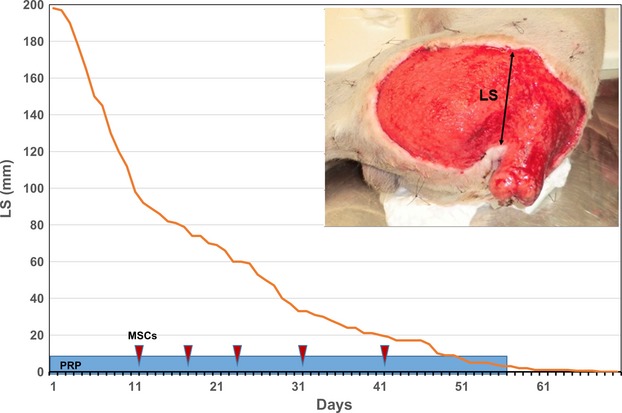
Wound extent assessment. Advancement of the wound margins, identified by the LS (Lesion Size) line, as a function of time. The graph fits with a hyperbolic curve with a faster healing rate during the first weeks. Light blue rectangle indicates the duration of PRP treatment; red arrows indicate the MSC treatments.
Discussion
In recent years, MSCs and platelet-derived products have gained a strong attention in veterinary medicine since they are supposed to enhance tissue regeneration and wound healing 10. Although a definitive knowledge about the molecular and cellular mechanisms through which PRP and MCS exert their effects is still lacking, several clinical and experimental reports confirm their efficacy for the treatments of patients both in veterinary and human medicine 11–14. Recently, a positive effect of the application of MSCs in healing of experimentally induced skin wounds has been reported in the dog 15. MSCs are likely to play a central role in normal wound healing. MSCs respond to wound healing cytokines and migrate to region of inflammation creating a favorable immune environment 16. MSCs secrete a plethora of effector molecules and produce microvesicles containing signaling proteins, mRNAs, miRNAs 17,18. By means of these mechanisms, they reprogram resident immune cells, control inflammation, activate tissue progenitors cells, promote angiogenesis. MSCs also regulate leukocytes (T and B cells, natural killer cells) function and activate resident macrophages to produce anti-inflammatory cytokines. Furthermore, MSCs attenuate fibrotic tissue deposition by producing anti-fibrotic molecules. In particular, COX2 and PGE2 upregulation are probably key elements of immunomodulatory mechanisms of the MSCs as well as of their antiscarring effects 16.
Large cutaneous lesions, where poor blood supply, tissue necrosis, excessive scarring, inflammation, and bacterial contamination are possible complications, would take significant advantages from the application of regenerative therapies based on the synergic action of PRP and MCS 16,19–22.
Here, we report the case of a particularly large cutaneous lesion in a dog hit by a train. The area involved in the lesion was too extended to allow a successful reconstructive surgery. After the failure of the first surgery aimed to close the wound with the surrounding skin, the physical conditions of the patient were serious, although not critical. The wound was highly contaminated and the area of necrosis extended. Furthermore, although the patient was a Labrador, he weighed only 33 kg and there was not enough skin to perform a free skin graft. Consequently, we opted for a regenerative therapy based on PRP and MSCs application. Since the main lesion was particularly extended to reach a homogeneous distribution of the therapeutics, we sprayed the whole injured area with both PRP and MCS. Parallel in vitro cell cultures showed that sprayed MSCs could survive and growth inside a PRP-derived gel (Fig.2), thus supporting this route of therapy administration. Connettivina and Hypermix dressing were used to improve tissue hydration when PRP and MSCs were not applied. The application of PRP and autologous adipose tissue-derived MSCs probably contributed to the positive outcome, inducing a prompt formation of granulation tissue, a strong adhesion of the wound edges to the underlying tissue and a rapid tissue growth, starting from wound margin. Furthermore, no sign of excessive scarring, tissue contraction, and fragile re-epithelialization, sometimes associated to second intention healing, was observed.
In addition to the amelioration of the tissue growth, PRP and MSCs probably contributed to a faster wound healing providing an antibacterial and anti-inflammatory environment 23,24. Platelet concentrates exhibit antimicrobial properties against different microorganisms, inhibiting bacterial growth 25,26. A therapeutic effect against bacterial infections has been proposed also for MSCs. Antimicrobial properties are probably do to their capacity to reduce inflammatory response and to increase macrophages and monocytes phagocytosis 27,28. Furthermore, MSCs secrete GM-CSF, a cytokine that exert significant antimicrobial activity 27. As a matter of fact, following the regenerative therapy, no sign of bacterial contamination was evidenced, even though the exposed area was vast and extended to the perianal region (Fig.1).
These results suggest that MSCs and PRP are indeed an effective therapeutic option to manage soft tissue wounds where a large amount of tissue is destroyed, especially when surgery alone cannot guarantee satisfactory results. Regenerative therapy can be considered by surgeon both to improve the quality of tissue regeneration and to speed up the wound healing process. Finally, this report outlines that in clinical veterinary medicine, effective collaboration of the owner, and a highly collaborative approach between clinical and biomedical staffs, are essential to lead to a successful healing of complicated wounds and ensure the return of the patients to an excellent quality of life.
Acknowledgments
The authors are grateful to Giuseppe Bertaccini for his valuable technical support.
Appendix Mesenchymal stem cells and platelet-rich plasma preparation
Mesenchymal stem cells preparation: For the preparation of MSCs, three grams of healthy subcutaneous adipose tissue were collected along the edge of the wound, minced into small pieces under sterile conditions, and washed several times with phosphate buffer saline solution (PBS) containing penicillin (1000 IU/mL) and streptomycin (100 mg/mL) (Sigma-Aldrich Corp., ST. Louis, MO). The sample was then digested in 15 mL of a 0.75 mg/mL solution of type I collagenase (Sigma-Aldrich Corp.) dissolved in Dulbecco’s modified Eagle’s low glucose medium (DMEM) (Lonza, Inc. Rockland, ME) under mild shaking for 1 h at 37°C. After digestion, the remaining tissue fragments were removed, while the cell suspension was filtered through a 50 μm nylon mesh and then centrifuged at 250 × g for 10 min. The supernatant was removed and the cell pellet resuspended in DMEM containing 10% v/v fetal bovine serum (FBS) (Lonza, Inc.), 1000 IU/mL penicillin and 100 mg/mL streptomycin. Finally, the cells were seeded in 25 cm2 tissue culture flasks at the concentration of 5.0 × 104 cells/cm2, and incubated at 37°C in 5% CO2. Subcultures were performed when cells reached about 80% confluence. At each passage, MSCs were seeded at 5.0 x103 cells/cm2. Cells were used at passage three and four. Before application, cell viability was validated by trypan blue exclusion test. Cell viability was always >90%. To assess MSCs viability and replication after spraying, an aliquot of the cells used for the therapy was sprayed on a cell culture dish and was cultured in DMEM containing 10% v/v fetal bovine serum (FBS) and antibiotics for 72 h.
The expression of cell surface markers CD13, CD34, CD44, CD45, CD90, CD105 has been assessed by RT-PCR (data not shown). Cell cultures were positive to CD44, CD90, and CD105, and negative to CD45 and CD13 expression. Cells were also positive to CD34 expression, as often reported for adipose tissue-derived MSCs 29. Furthermore, cells were positive to adipogenic, osteogenic, and chondrogenic differentiation (Appendix Fig.1).
Platelet-rich plasma preparation: Platelet-rich plasma (PRP) was obtained from autologous venous blood collected with citrate-phosphate-dextrose. Fresh blood samples (about 25–30 mL) were centrifuged at 150 g for 30 min at 4°C in a 50 mL tube. The erythrocyte fraction was discarded, while the plasma, enriched with platelets, was further centrifuged at 800 g for 15 min. The platelet pellet was collected and the platelets were counted (CELL-DYN 3500R haematology analyser, Abbott, Roma, Italy) and diluted at a final concentration of 1.0 × 109 cell/mL. Blood sampling was repeated at 10–15 days intervals. Platelet-Rich Plasma was applied soon after preparation (PRP), or as Platelet-Rich Plasma lysate (PRPlys). In this case PRP was aliquoted immediately after preparation, frozen and stored at −80°C until use.
Figure 4.
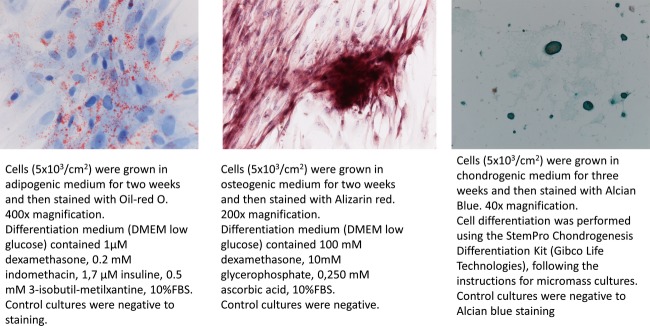
Three lineage differentiation of adipose tissue-derived MSCs. MSCs were positive to adipogenic, osteogenic and chondrogenic differentiation.
Conflict of Interest
The authors report no conflict of interest relevant to this article.
References
- Pavletic MM, editor. Atlas of small animal wound management and reconstructive surgery. 3rd edn. Hoboken, NJ: Wiley Blackwell; 2010a. Basic principles of wound management; pp. 32–43. [Google Scholar]
- Williams J. Decision making in wound closure. In: Williams JM, Moores A, editors. BSAVA manual of canine and feline wound management and reconstruction. 2nd edn. Hoboken, NJ: Wiley Blackwell; 2009. pp. 25–36. [Google Scholar]
- Mayhew P. Tension-relieving techniques and local skin flaps. In: Williams JM, Moores A, editors. BSAVA manual of canine and feline wound management and reconstruction. 2nd edn. Hoboken, NJ: Wiley Blackwell; 2009. pp. 69–99. [Google Scholar]
- Pavletic MM, editor. Atlas of small animal wound management and reconstructive surgery. 3rd edn. Hoboken, NJ: Wiley Blackwell; 2010b. Local flap; pp. 307–336. [Google Scholar]
- Sánchez M, Albillos J, Angulo F, Santisteban J. Andia I. Platelet-rich plasma in muscle and tendon healing. Oper. Tech. Orthop. 2012;22:16–24. [Google Scholar]
- Riccò S, Renzi S, Del Bue M, Conti V, Merli E, Ramoni R, et al. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int. J. Immunopathol. Pharmacol. 2013;26(1 Suppl):61–68. doi: 10.1177/03946320130260s108. [DOI] [PubMed] [Google Scholar]
- Pope J. Wound aethiology and classification. In: Williams JM, Moores A, editors. BSAVA manual of canine and feline wound management and reconstruction. 2nd edn. Hoboken, NJ: Wiley Blackwell; 2009. pp. 15–24. [Google Scholar]
- Anderson D. Management of open wounds. In: Williams JM, Moores A, editors. BSAVA manual of canine and feline wound management and reconstruction. 2nd edn. Hoboken, NJ: Wiley Blackwell; 2009. pp. 37–53. [Google Scholar]
- Cukjati D, Rebersek S. Miklavcic D. A reliable method of determining wound healing rate. Med. Biol. Eng. Comput. 2001;39:263–271. doi: 10.1007/BF02344811. [DOI] [PubMed] [Google Scholar]
- Harman RJ. Stem cell therapy in veterinary dermatology. Vet. Dermatol. 2013;24:90–96. doi: 10.1111/vde.12000. e23–4. [DOI] [PubMed] [Google Scholar]
- De Bakker E, Van Ryssen B, De Schauwer C. Meyer E. Canine mesenchymal stem cells: state of the art, perspectives as therapy for dogs and as a model for man. Vet. Q. 2013;33:225–233. doi: 10.1080/01652176.2013.873963. [DOI] [PubMed] [Google Scholar]
- De Schauwer C, Van de Walle GR, Van Soom A. Meyer E. Mesenchymal stem cell therapy in horses: useful beyond orthopedic injuries? Vet. Q. 2013;33:234–241. doi: 10.1080/01652176.2013.800250. [DOI] [PubMed] [Google Scholar]
- Engebretsen L, Steffen K, Alsousou J, Anitua E, Bachl N, Devilee R, et al. IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br. J. Sports Med. 2010;44:1072–1081. doi: 10.1136/bjsm.2010.079822. [DOI] [PubMed] [Google Scholar]
- Tambella AM, Attili AR, Dini F, Palumbo Piccionello A, Vullo C, Serri E, et al. Autologous platelet gel to treat chronic decubital ulcers: a randomized, blind controlled clinical trial in dogs. Vet. Surg. 2014;43:726–733. doi: 10.1111/j.1532-950X.2014.12148.x. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lee JH, Lyoo YS, Jung DI. Park HM. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet. Dermatol. 2013;24:242–253. doi: 10.1111/vde.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WM, Nesti LJ. Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res. Ther. 2012;3:20. doi: 10.1186/scrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio SR, Pegtel DM. Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem HK. Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakos K, Lyras DN, Verettas D, Tilkeridis K. Tryfonidis M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. J Foot Ankle Surg. 2009;16:137–141. doi: 10.1016/j.injury.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Sommeling CE, Heyneman A, Hoeksema H, Verbelen J, Stillaert FB. Monstrey S. The use of platelet-rich plasma in plastic surgery: a systematic review. J. Plast. Reconstr. Aesthet. Surg. 2013;66:301–311. doi: 10.1016/j.bjps.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Sorrell J. Caplan A. Topical delivery of mesenchymal stem cells and their function in wounds. Stem Cell Res. Ther. 2010;1:30. doi: 10.1186/scrt30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk SW. Theoret C. Translating stem cell therapies: the role of companion animals in regenerative medicine. Wound Repair Regen. 2013;21:382–394. doi: 10.1111/wrr.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park C. Park HM. Curative effect of autologous platelet-rich plasma on a large cutaneous lesion in a dog. Vet. Dermatol. 2009;20:123–126. doi: 10.1111/j.1365-3164.2008.00711.x. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol. Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago L, Bortolin M, Vassena C, Romanò CL, Taschieri S. Del Fabbro M. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: an in vitro study. PLoS ONE. 2014;9:e107813. doi: 10.1371/journal.pone.0107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidnezhad M, Varoga D, Wruck CJ, Podschun R, Sachweh BH, Bornemann J, et al. Platelets display potent antimicrobial activity and release human beta-defensin 2. Platelets. 2012;23:217–223. doi: 10.3109/09537104.2011.610908. [DOI] [PubMed] [Google Scholar]
- Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J. Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- Uzbas F, May ID, Parisi AM, Thompson SK, Kaya A, Perkins AD, et al. Molecular physiognomies and applications of adipose-derived stem cells. Stem Cell Rev. 2014 doi: 10.1007/s12015-014-9578-0. doi: 10.1007/s12015-014-9578-0 (accessed: 11 december 2014) [DOI] [PubMed] [Google Scholar]