Key Clinical Message
We report a 53-year-old man diagnosed with Richter syndrome. He was heavily pretreated and was refractory to prior therapy. He received rituximab and ibrutinib, and achieved a significant response after 1 month of therapy. Our case illustrates the importance of investigation of rituximab and ibrutinib in Richter’s syndrome.
Keywords: Ibrutinib, Richter, Richter syndrome, Richter’s transformation, rituximab
Case Presentation
We report a 53-year-old man who was diagnosed with chronic lymphocytic leukemia (CLL) in 2012, after presenting to the emergency room with a white blood cell count of 23,200 with 78% lymphocytes and enlarged cervical nodes. A bone marrow biopsy was performed; pathologic findings were consistent with CLL without cytogenetic or FISH abnormalities. Immunoglobulin heavy chain testing was not performed. He completed six cycles of fludarabine, cyclophosphamide, and rituximab (FCR). However, a CT scan 3 months after treatment completion showed extensive cervical, axillary, mediastinal, and retroperitoneal adenopathy. He was then started on rituximab and bendamustine, but treatment was discontinued early because of rash. Figure1, panel A, shows the PET scan performed prior to starting third line therapy with single agent ofatumumab. Due to concern about possible transformation, he was then started on a dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin, (R-EPOCH) regimen, with persistent disease. Biopsy of a left axillary lymph axillary node confirmed transformation to diffuse large B cell lymphoma, nongerminal center origin. Richter score at the time of confirmed transformation was 1. He was then treated with rituximab, ifosfamide, carboplatin, and etoposide (R-ICE) and rituximab, dexamethasone, high dose cytarabine, and carboplatin (R-DHAC) and high-dose cyclophosphamide, with suboptimal response (Fig.1, Panel B). Treatment regimens are shown in Table1.
Figure 1.
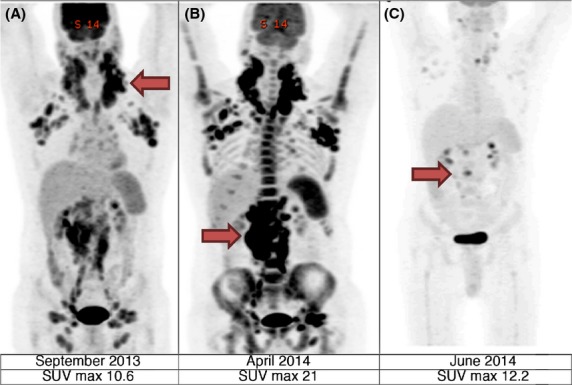
PET scan with arrows pointing to areas of maximum standardized uptake value (SUV).
Table 1.
Diagnosis and treatment timeline
| Aug-12 | Feb-13 | May-13 | Jun-13 | Sep-13 | Nov-13 | Jan-14 | Feb-14 | Apr-14 | May-14 | Jun-14 |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosed with CLL | FCR × 6 cycles | CT scan demonstrated recurrence | Rituximab and Bendamustine × 2 cycles | Ofatumumab × 5 cycles | R-EPOCH × 4 cycles | Richter confirmed. PET scan no response | R-ICE × 1 cycle; R-DHAC × 3 | PET scan without response | Ibrutinib 420 mg daily and Rituximab weekly × 5 | PET response – eligible for transplant |
CLL, chronic lymphocytic leukemia; FCR, fludarabine, cyclophosphamide, and rituximab.
The patient then began taking a combination of rituximab (375 mg/m2 weekly) and ibrutinib (420 mg daily). Within one month of starting therapy, no lymph nodes were clinically palpable and PET scan showed significant response in the majority of involved lymph nodes (Fig.1, panel C). He never developed ibrutinib-associated lymphocytosis. The response continued for 3 months. During evaluation for allogeneic transplant, he developed progressive disease and elected to forego further therapy and later died.
Discussion
Richter syndrome describes the transformation of CLL into an aggressive lymphoma. It is considered to have a worse prognosis than either CLL or de novo diffuse large B cell lymphoma. About 1% of CLL cases annually transform to a more aggressive lymphoma. While most transformations are to diffuse large B cell lymphoma, transformations to other disorders, such as Hodgkin lymphoma, have been reported 1. Risk factors for transformation include advanced Rai stage at diagnosis and lymph nodes >3 cm on physical examination 2. Since Richter syndrome may not involve all lymph nodes simultaneously, it is important to obtain a PET scan to guide biopsy of suspected areas prior to initiation of therapy 2. A standard uptake value (SUV) max of ≥5 is sensitive and specific for diagnosing Richter syndrome; however an SUV max of ≥10 is independently associated with a shorter overall survival 3. The prognosis for patients with Richter syndrome is poor, and hematopoietic transplant is recommended in patients who do respond to therapy. Our patient had a Richter score of 1 at diagnosis which correlates with a median survival of 1.12 years 4. In addition, he had no response to multiple regimens, and the combination of ibrutinib and rituximab was initially given with palliative intent.
Ibrutinib is an oral covalent inhibitor of Bruton’s tyrosine kinase. This kinase is located downstream from the B cell receptor and is necessary for the activation of pathways linked to CLL cell survival 5. Several recently published studies reported better progression-free and overall survival and response rates in patients with CLL without evidence of transformation 6,7. An unpublished phase 1b/II trial was conducted to study the combination of ofatumumab and ibrutinib reported a partial response in 2 of 3 study participants with Richter syndrome 8. Further, ibrutinib has shown activity combined with rituximab and bendamustine in diffuse large B cell lymphoma 9. We speculate ibrutinib may inhibit downstream signals important for malignant B cell proliferation; however the effect was not durable possibly due to the development of acquired resistance to ibrutinib 10. Importantly, our patient did not have lymphocytosis during therapy and previous data has suggested that prolonged lymphocytosis may decrease risk of relapse 10. To our knowledge, no previous reports have described using the combination of ibrutinib and rituximab in a heavily pretreated patient with Richter syndrome. In this patient, lymphadenopathy rapidly diminished after 4 weeks of a combined regimen of rituximab and ibrutinib.
Conclusion
This case highlights the importance of further investigation of combined ibrutinib and rituximab for patients with Richter syndrome.
Conflict of Interest
None declared.
References
- Tsimberidou AM, O’Brien S, Kantarjian HM, Koller C, Hagemeister FB, Fayad L, et al. Hodgkin transformation of chronic lymphocytic leukemia: the M. D. Anderson Cancer Center experience. Cancer. 2006;107:1294–1302. doi: 10.1002/cncr.22121. [DOI] [PubMed] [Google Scholar]
- Parikh SA, Kay NE. Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123:1647–1657. doi: 10.1182/blood-2013-11-516229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi L, Keating MJ, Marom EM, Truong MT, Schlette EJ, Sargent RL, et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood. 2014;123:2783–2790. doi: 10.1182/blood-2013-11-536169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimberidou AM, O’Brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, et al. Clinical outcomes and prognostic factors in patients with Richter’s syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J. Clin. Oncol. 2006;24:2343–2351. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]
- Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–3295. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglowski SM, Jones JA, Flynn J, Andritsos LA, Maddocks K, Blum K, et al. A phase Ib/II study evaluating activity and tolerability of BTK inhibitor PCI-32765 and Ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and related diseases. J. Clin. Oncol. 2012;30 (suppl; abstr 6508) [Google Scholar]
- Maddocks K, Christian B, Jaglowski S, Flynn J, Jones JA, Porcu P, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;125:242–248. doi: 10.1182/blood-2014-08-597914. [DOI] [PubMed] [Google Scholar]
- Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]


