Key Clinical Message
Iliopsoas abscesses have been reported in adult diabetic patients, but only one case has been so far reported in the pediatric diabetic literature. We report three cases of iliopsoas abscesses in three adolescents with type 1 diabetes mellitus, suggesting that an increased awareness of this condition is required for its early recognition and prompt treatment.
Keywords: Adolescents, diabetes mellitus, iliopsoas abscess, infection, psoas abscess
Introduction
Patients with diabetes mellitus (DM) are generally more susceptible to infections 1–4, but the exact underlying mechanism for the increased infection risk remains uncertain 5,6. The defects in the immune system seem to be most strongly associated with poorly controlled diabetes 2,7,8, suggesting that glycemic control may influence immune function. In most cases, patients with DM have the same infections that affect the general population 3; however, occasionally, diabetic patients will present with infections caused by rare organisms at unusual sites 9.
Iliopsoas abscess is a rare potentially life-threatening form of extraperitoneal infection, that involves the iliopsoas compartment, which contains the psoas and iliacus muscle 10. Iliopsoas abscess can be classified as either primary or secondary. Primary abscess results from hematogenous spread of an infectious process from an occult source in the body 10; local trauma with resultant intramuscular hematoma formation seems to predispose to primary iliopsoas abscess formation 11,12. The secondary abscess develops by spreading from contiguous anatomical structures, such as gastrointestinal and genitourinary tract, musculoskeletal system, or vascular tissue 11.
Before the discovery of modern antituberculosis treatment, iliopsoas abscess was a well-recognized complication of spine tuberculosis 10. Currently, medical conditions, such as defects in the immune system, DM, intravenous drug use, and alcoholism are predisposing risk factors 13.
Both primary and secondary iliopsoas abscesses have been reported in diabetic adults 14,15. To the best of our knowledge, only one case has been previously reported in an adolescent with T1DM 16. In that case, a 12-year-old boy presented with diabetic ketoacidosis (DKA) and low back pain, and was subsequently diagnosed with both a left psoas abscess and an extensive thoracolumbar spinal epidural abscess.
Here, we describe the clinical features, diagnostic findings, management and outcomes of three T1DM adolescents with primary and one case of secondary iliopsoas abscess.
Clinical Reports
Patient 1
A 14-year-old girl presented to the Pediatric Unit at the General Hospital of Trento with a 4-day history of right-sided back pain, fever, weight loss, and difficulty breathing. Her medical history was characterized by a 6-year history of poorly controlled T1DM and her most recent glycated hemoglobin (HbA1c) value was 11.1% (98 mmol/mol).
On examination, she was febrile (39°C) and dehydrated; she had Kussmaul breathing and complained tenderness in the right flank. Initial investigations showed a blood glucose of 380 mg/dL, white cell count 26.6 × 109/L with a neutrophilia of 24.2 × 109/L, C-reactive protein (CRP) of 199 mg/L, a pH of 7.35, and bicarbonate of 12 mEq/L. Urinalysis revealed 3+ glucose and 3+ ketones, and was negative for white cells. An abdominal ultrasound (US) scan showed an extensive loculated right-side perinephric abscess, measuring 73 mm in length with a 45 mm anteroposterior diameter. Abdominal computed tomography (CT) showed that the abscess extended from the perinephric space to the psoas muscle, pushing the right kidney anterolaterally (Fig.1).
Figure 1.
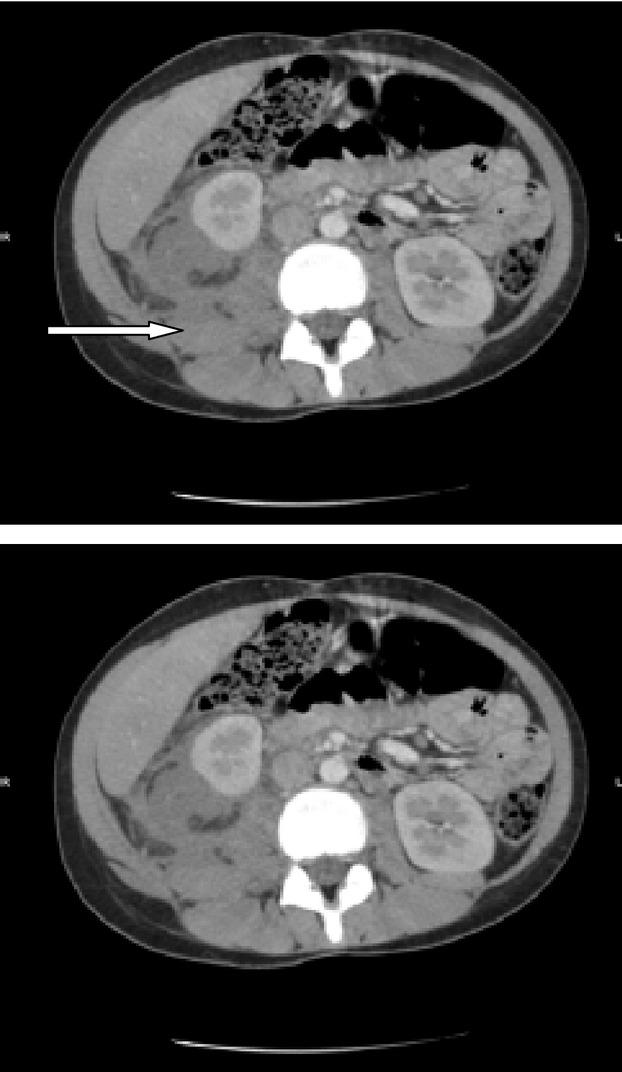
Patient 1, CT, axial view. The loculated right-sided psoas abscess extending from psoas muscle and pushing the right kidney anterolaterally.
The patient was hydrated with intravenous fluids with added potassium and an insulin infusion according to the local protocol. She was put on intravenous antibiotics ceftriaxone, gentamicin, and metronidazole to treat the infection. A percutaneous catheter was inserted under ultrasound guidance to drain the abscess. Three days later, because of the absence of improvement, the patient underwent an open abscess evacuation under general anesthesia. Staphylococcus aureus was grown from the pus, and antibiotics were changed to intravenous teicoplanin and oral ciprofloxacin. Her condition and metabolic derangements improved over the next 48 h and subcutaneous insulin was reintroduced. The duration of hospitalization was 15 days and the patient was discharged from the hospital with oral ciprofloxacin and no further complications.
Two years later, the patient re-presented with a primary right iliopsoas abscess, which was readily recognized and treated with intravenous teicoplanin and oral ciprofloxacin. Surgery was not necessary.
Patient 2
A 17-year-old girl was admitted to the Pediatric Unit at the General Hospital of Trento with a 3-day history of left-side back pain, high fever, malaise, and concomitant hyperglycemia. She had been treated at home with oral ciprofloxacin for almost 48 h.
She had a history of well-controlled T1DM diagnosed at the age of 13 years with no history of DKA. Most recent HbA1c was 7.1% (54 mmol/mol).
On examination, she had a fever (38°C) and marked tenderness on palpation of the left flank. Initial laboratory findings revealed blood glucose of 347 mg/dL, CRP of 14.9 mg/L, and mild leukocytosis 10 × 109/L. Urinalysis revealed glycosuria and ketonuria (3+). Abdominal US scan was normal and empirical antibiotic treatment with intravenous ceftriaxone was commenced. Twenty-four hours later, the left-sided back pain had progressed and abdominopelvic CT was performed. The exam revealed a voluminous nonhomogenous mass in the left psoas (Fig.2). No bony or renal tract abnormalities were noted. Treatment was changed to intravenous teicoplanin and oral ciprofloxacin, and the patient rapidly improved. She was discharged from the hospital after 15 days of intravenous therapy with instructions to continue oral ciprofloxacin therapy for 8 days. Cultures subsequently grew S. aureus. Fifteen days later, an abdominal magnetic resonance imaging (MRI) revealed a complete resolution of the abscess.
Figure 2.
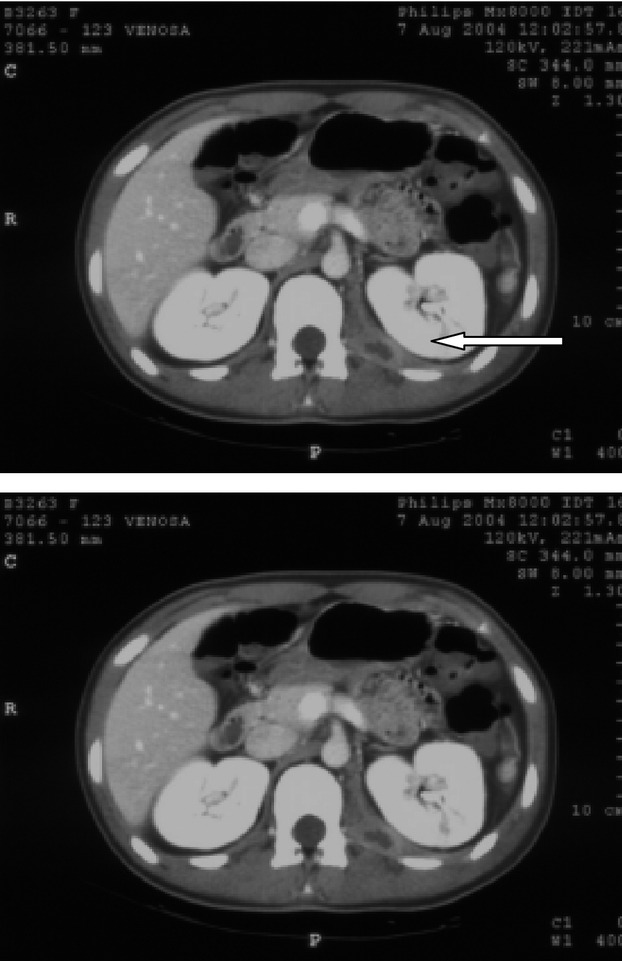
Patient 2, CT, axial view. A psoas abscess is located in the left psoas muscle.
Patient 3
A 14-year-old boy initially presented to the Emergency Department (ED) of the La Spezia General Hospital with a chief compliant of a right low back pain after a trauma during a football match. The X-ray of the pelvis revealed no fractures and the physical exam was unremarkable, so he was discharged with a prescription of nonsteroidal anti-inflammatory drugs (NSAIDs).
The patient had a medical history that was significant for poorly controlled T1DM diagnosed at age 2 years, characterized by poor compliance to self-management and inconsistent follow-up.
Fifteen days later, he returned to the ED with fever, worsening back pain, and signs of major gastric hemorrhage. He was promptly treated with multiple blood transfusions and an urgent esophagogastroduodenoscopy was performed. The exam revealed a gastric ulcer as the cause of bleeding, that was attributed to the prolonged use of NSAIDs. A noncontrast CT scan of the abdomen was also performed and documented an area of attenuation in the right psoas muscle, that seemed consistent with a voluminous psoas hematoma. He was referred to a pediatric surgeon. During hospitalization, the patient developed clinical and laboratory signs of sepsis. A CT scan revealed a voluminous right psoas abscess extending to the ipsilateral gluteus muscle. The patient was treated with intravenous antibiotic (teicoplanin, ceftriaxone, piperacillin/tazobactam) and clinical conditions gradually improved. Nineteen days later an abdominopelvic CT revealed a significant improvement of the infection. Surgery was not considered necessary. Staphylococcus aureus was isolated from blood cultures. The patient was discharged on oral antibiotic therapy (amoxicillin/clavulanate and rifampicin) with the diagnosis of psoas abscess due to hematogenous infection from S. aureus on a psoas hematoma.
Discussion
We describe three cases of primary iliopsoas abscesses and one case of secondary iliopsoas abscess occurred in three adolescents affected by T1DM. In our case series, three of the four iliopsoas abscesses were diagnosed as primary. A previous study revealed that primary iliopsoas abscesses in children accounts up to 92% of cases 12.
The classic presentation triad of iliopsoas abscess is fever, flank pain, and limitation of the hip movement 10, but in our cases fever and flank or back pain were the only presenting symptoms. That is consistent with the observation that the classic triad is present only in a small percentage of patients 17,18, favoring the frequent delay in diagnosis usually seen in patients with psoas abscess.
The iliopsoas abscess was demonstrated with abdominal US in patient 1, and with abdominopelvic CT in patients 2 and 3. In the literature, US is recommended first as a screening procedure to detect psoas abscess in patients with prolonged fever or sepsis of unknown origin 11,17, but US is highly operator dependent and retroperitoneal space can be difficult to visualize because can be obscured by bowel gas reducing drastically its sensitivity to only about 60% 10. CT is considered the “gold standard” for definitive diagnosis 17,19. Nevertheless, some authors believe that MRI is superior to CT, because of better discrimination of soft tissue and the ability to visualize the abscess wall and the surrounding structures 10. Moreover, recently, the value of Gallium-67 scanning in the diagnosis of psoas abscess in adult patients initially diagnosed as having fever of unknown origin has been reported 20.
Patient 1 and patient 3 had a history of chronically poorly controlled diabetes, confirming what has already been observed in diabetic adults, where poor glycemic control has been associated with a predisposition to unusual and more serious infections 9,14,21.
Staphylococcus aureus was the causal organism in all cases, confirming the observation that individuals with diabetes, in particular those who are insulin-dependent, have an increased risk of infection with this organism 22. Staphylococcus aureus is also the most frequent causative organism of iliopsoas abscess in diabetic adults 12.
All our patients were treated successfully with a full course of intravenous antibiotic treatment. Adjustments of the therapy were based on report of abscess fluid culture, but a better knowledge of the common causative organisms could guide the initial choice of antibiotics.
In the literature, it is reported that the mortality rate in primary psoas abscess is 2.4% and in secondary abscesses is 18.9% 10,11. The major cause of death is delayed diagnosis or inadequate therapy 11; therefore it is extremely important to recognize and treat these unusual infections promptly. The signs and symptoms of psoas abscess easily mimic other disorders, thus the differential diagnosis is extensive. Septic hip joint, arthritis of the hip, osteomyelitis of the pelvis, trauma, tumor or osteomyelitis of the spine, renal or perirenal abscess, abdominal abscess solid, or hollow viscera injury should also be considered 18.
Because of its relative infrequency and often subtle or nonspecific presentation, psoas abscess often is not considered in diabetic adolescents presenting with fever, flank, or back pain, but our cases suggest that an increased awareness of psoas abscess is required for early recognition and prompt treatment of this condition.
Acknowledgments
The authors are grateful to Nivedita Agarwal MD (Radiology Unit, S. Maria del Carmine Hospital of Rovereto, Italy) for her kind assistance in selecting figures of the manuscript.
Conflict of Interest
None declared.
References
- Jackson L. Evaluating diabetes mellitus as a risk factor for community-acquired infections. Clin. Infect. Dis. 2005;41:289–290. doi: 10.1086/431594. [DOI] [PubMed] [Google Scholar]
- Rayfield EJ, Adult MJ, Keusch GT, Brothers MJ, Nechemias C. Smith H. Infection and diabetes: the case for glucose control. Am. J. Med. 1982;72:439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- Muller I, Gorter KJ, Hak F, Goudzwaaed WI. Schellevis FG. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- Shah BR. Hux JE. Quantifying the risk of infectious disease for people with diabetes. Diabetes Care. 2003;26:510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- Knapp S. Diabetes and infection: is there a link?–A mini-review. Gerontology. 2013;59:99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- Thomsen RW. Mor A. Diabetes and risk of community-acquired respiratory tract infections, urinary tract infections, and bacteremia. Open Infect. Dis. J. 2012;6(Suppl. 1):27–39. [Google Scholar]
- Foss-Freitas MC, Foss NT, Donadi EA. Foss MC. Effect of the glycemic control on intracellular cytokine production from peripheral blood mononuclear cells of type 1 and type 2 diabetic patients. Diabetes Res. Clin. Pract. 2008;82:329–334. doi: 10.1016/j.diabres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Gallacher SJ, Thomson G, Fraser WD, Fisher BM, Gemmell CG. Mac-Cuish AC. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet. Med. 1995;12:916–920. doi: 10.1111/j.1464-5491.1995.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Rajbhandari SM. Wilson RM. Unusual infections in diabetes. Diabetes Res. Clin. Pract. 1998;39:123–128. doi: 10.1016/s0168-8227(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Mallick IH, Thoufeeq MH. Rajendran TP. Iliopsoas abscesses. Postgrad. Med. J. 2004;80:459–462. doi: 10.1136/pgmj.2003.017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenwald I, Abrahamson J. Cohen O. Psoas abscess: case report and review of the literature. J. Urol. 1992;147:1624–1626. doi: 10.1016/s0022-5347(17)37650-4. [DOI] [PubMed] [Google Scholar]
- Bresee JS. Edwards MS. Psoas abscess in children. Pediatr. Infect. Dis. J. 1990;9:201–206. doi: 10.1097/00006454-199003000-00011. [DOI] [PubMed] [Google Scholar]
- Tomich EB. Della-Giustina D. Bilateral psoas abscess in the emergency department. West J. Emerg. Med. 2009;10:288–291. [PMC free article] [PubMed] [Google Scholar]
- Lansdown AJ, Downing A, Roberts AW. Martin D. Psoas abscess formation in suboptimally controlled diabetes mellitus. Case Rep. Med. 2011;2011:249325. doi: 10.1155/2011/249325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnik Oblak M, Oblak C. Stankovic S. Psoas and spinal epidural abscess in a diabetic patient– case report. Diabetes Res. Clin. Pract. 2005;68:274–277. doi: 10.1016/j.diabres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Ladhani S, Phillips SD. Allgrove J. Low back pain at presentation in a newly diagnosed diabetic. Arch. Dis. Child. 2002;87:543–545. doi: 10.1136/adc.87.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern CH, Hu SC, Kao WF, Tsai J, Yen D. Lee CH. Psoas abscess: making an early diagnosis in the ED. Am. J. Emerg. Med. 1997;15:83–88. doi: 10.1016/s0735-6757(97)90057-7. [DOI] [PubMed] [Google Scholar]
- Goldberg BO, Hedges JR. Stewart DW. Psoas abscess. J. Emerg. Med. 1984;1:533–537. doi: 10.1016/0736-4679(84)90007-6. [DOI] [PubMed] [Google Scholar]
- Zissin R, Gayer G, Kots E, Werner M, Shapiro-Feinberg M. Hertz M. Iliopsoas abscess: a report of 24 patients diagnosed by CT. Abdom. Imaging. 2001;26:533–539. doi: 10.1007/s002610000201. [DOI] [PubMed] [Google Scholar]
- Kao PF, Tsui KH, Leu HS, Tsai MF. Tzen KY. Diagnosis and treatment of pyogenic psoas abscess in diabetic patients: usefulness of computed tomography and gallium-67 scanning. Urology. 2001;57:246–251. doi: 10.1016/s0090-4295(00)00923-7. [DOI] [PubMed] [Google Scholar]
- Leibovici L, Yehezkelli Y, Porter A, Regev A, Krauze I. Harrell D. Influence of diabetes mellitus and glycemic control on characteristics and outcome of common infections. Diabet. Med. 1996;13:457–463. doi: 10.1002/(SICI)1096-9136(199605)13:5<457::AID-DIA83>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Breen JD. Karchmer AW. Staphylococcus aureus infections in diabetic patients. Infect. Dis. Clin. North Am. 1995;9:11–24. [PubMed] [Google Scholar]


