Key Clinical Message
Treatment of patients with refractory Hodgkin lymphoma is a significant issue. We report a patient with Proteus syndrome and relapsed Hodgkin lymphoma, whose remission was finally achieved after brentuximab vedotin therapy, allowing her to receive a haploidentical stem cell transplant. The possible relationship between both disorders was discussed.
Keywords: Brentuximab vedotin, children, Hodgkin lymphoma, Proteus syndrome
Introduction
Proteus syndrome (PS) is a rare disease caused by a somatic activating mutation in AKT1, proving the hypothesis of somatic mosaicism and implicating activation of the PI3K-AKT pathway in the characteristic clinical findings of overgrowth and tumor susceptibility in this disorder 1–3. First described by Cohen and Heyden in 1979, Wiedemann et al. in 1983 named this distinct entity PS. The incidence of PS is approximately 1:100,000–1,000,000 1,2. We report a case of PS combined with refractory Hodgkin lymphoma (HL) and discuss therapeutic options for PS patients with oncological diseases.
Case Report
A 14-year-old female was admitted to the oncohematology department with enlargement of the peripheral and mediastinal lymph nodes. Asymmetry of her scalp bones and gum hyperplasia were noted at birth. The diagnosis of PS was made phenotypically on the basis of existing scalp and oral cavity deformation, antimongoloid eyes, chest kyphosis, and intact intellect. No genetic changes were found by conventional cytogenetics, and there was no possibility to perform DNA sequencing. Since the age of 11, she had been diagnosed with hyperplasia of the myometrium, polymenorrhea, and chronic posthemorrhagic anemia. The patient had administered contraceptives and hemostatic therapy.
At 14 years of age, the patient was noted to have enlargement of the neck lymph nodes and a 37.8°C fever. Chest computed tomography (CT) imaging, lymph node biopsy were performed and nodular sclerosis HL stage IIB was diagnosed (Fig.1). Treatment was administered according to EuroNet-PHL Trials, for risk group 2, and consisted of four courses of chemotherapy (2OEPA/2COPDac) plus radiation therapy of the involved fields (20 Gy) 4. Complete remission was achieved and the patient was followed up according to the protocol.
Figure 1.
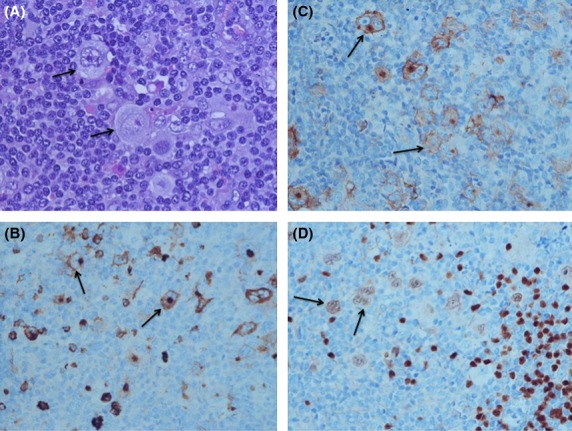
Pathological and immunohistochemical findings of HL, nodular sclerosis type: At high magnification large Reed-Sternberg cells are seen (A, Hematoxylin-Eosin ×600). The large cells express CD15 (B, ×400), CD30 (C, ×400), Pax5 (D, ×400).
After 6 months, the first early recurrence involving the supraclavicular, axillary lymph nodes, mediastinum lymph nodes, and lungs was diagnosed with biopsy confirmation of the same variant of the disease (Fig.2). The patient received four courses (2IEP, 2DHAP) of second-line treatment and peripheral blood stem cell (PBSC) harvesting (Figure3) 5,6. But at the end of chemotherapy, the mediastinal lymph nodes were observed to be growing again.
Figure 2.
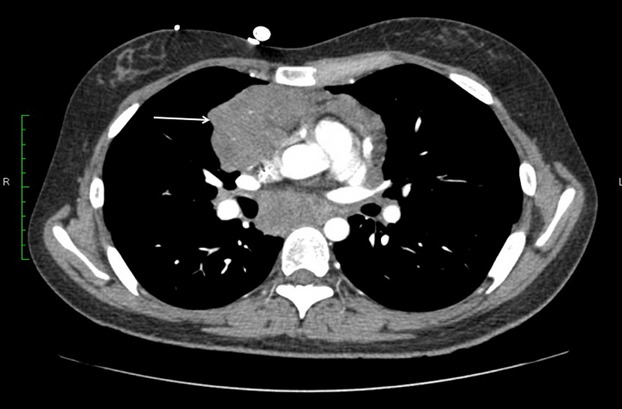
Computer tomography findings at diagnosis, showing large mediastinal mass (arrow).
Figure 3.
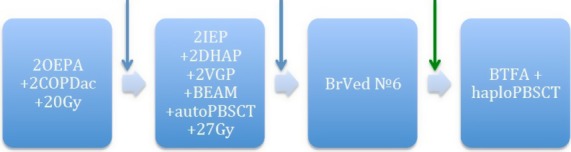
Treatment course: OEPA – vincristine 1.5 mg/m2/day №3, etoposide 125 mg/m2/day №5, prednisone 60 mg/m2/day №15, doxorubicine 40 mg/m2/day №2; COPDac – vincristine 1.5 mg/m2/day №2, prednisone 40 mg/m2/day №15, cyclophosphamide 500 mg/m2/day №2, dacarbasine 250 mg/m2/day №3; IEP – ifosphamide 2000 mg/m2/day №5, etoposide 125 mg/m2/day №5, prednisone 100 mg/m2/day №5, DHAP – dexamethasone 24 mg/m2/day №4, Ara-C 2000 mg/m2/twice a day №1, cisplatine 100 mg/m2/day №1; VGР – vinorelbine 25 mg/m2, gemcitabine 1000 mg/m2, prednisolone 100 mg/m2, BEAM – etoposide 200 mg/m2/day №4, melphalan125 mg/m2/day №1, Ara-C 200 mg/m2/twice a day №4, carmustine 100 mg/m2/day №1; BrVed – Brentuximab vedotine 1.8 mg/kg/day; BTFA, bendarmustine 240 mg/m2/day, treosulphane 42 mg/m2, fludarabine 150 mg/m2, antitymocyte globuline 50 mg/kg; PBSCT, peripheral blood stem cell transplantation (auto – autologous, haplo – haploidentical); 20 Gy, involved field irradiation; blue arrow – progression; green arrow – response.
The patient received two more courses of VGP (vinorelbine 25 mg/m2, gemcitabine 1000 mg/m2, prednisolone 100 mg/m2) with a positive response, and autologous stem cell transplantation was subsequently performed. The conditioning regimen consisted of BEAM. A radiation dose of 27 Gy was then carried out on the involved fields 6,7.
At the final examination 4 months after radiation therapy, chest CT revealed new lesions in the mediastinum and both lungs. Positron emission tomography (PET) scans showed prominent metabolic activity in these lesions. We proceeded with salvage therapy of brentuximab vedotin (1.8 mg/kg every 3 weeks). The only side effect was general deterioration and fever after first administration. No acute reactions or neuropathy were noted during further brentuximab vedotin treatment.
A CT scan after three courses of therapy revealed a remarkable response. After six courses of therapy the CT data showed good partial remission – dramatic shrinkage of mediastinal masses and lung lesions, with a small amount of contrast enhancement and calcifications.
As we did not find either related or unrelated donors the patient received a haploidentical transplant from her father with αβ T-cell receptor depletion. The conditioning regimen consisted of bendamustine, treosulfan, fludarabine, and antithymocyte globulin. Transplant engraftment was noted at day 19 post transplant 8,9.
In the early and late post-transplant period, the patient suffered from different complications including late acute graft-versus-host disease (GVHD) of the skin, chronic GVHD of the gut, myometrium hyperplasia with bleeding, bacterial and respiratory syncytial virus pneumonia, pericarditis, chronic heart insufficiency, chronic glomerulonephritis, and avascular necrosis of the joints. The patient received multimodal treatment.
Now, more than 1 year after transplant, according to the last examination (CT, PET), complete remission is confirmed, the girl is doing well, studying in the University.
Discussion
PS is a rare sporadic disease that causes mosaic overgrowth of tissues of all germ layers. The most frequently affected tissues are bone, skin, adipose, and central nervous system. One of the major clinical findings is distorting, progressive overgrowth that typically starts between ages 6–18 months, a key finding in PS is distortion of the skeletal architecture. Other clinical features include cerebriform connective tissue nevi, linear verrucous epidermal nevus, adipose dysregulation, vascular malformations, organomegaly, bilateral ovarian cystadenoma, parotid monomorphic adenoma, deep vein thrombosis, and bullous pulmonary degeneration 1,2.
Diagnosis is established by clinical features but can be confirmed by molecular methods. In more than 90% of PS cases, the somatic mosaic mutation in gene AKT1 (c.49G>A [p. Glu17Lys]) located at 14q32.33, is detected 1–3.
The major concern for managing PS is surgical correction of skeletal deformities and bullous pulmonary disease. The prognosis of PS depends mainly on localization of lesions. The majority of manifestations are not life-threatening and do not reduce lifespan with adequate treatment. The most critical situations are deep vein thrombosis, pulmonary embolism, and bullous pulmonary disease 1.
PS can be associated with some oncological diseases. During the second decade of life, the most specific tumors are cystadenoma and parotid monomorphic adenoma. Other tumors reported are meningioma, testicular tumor, astrocytoma, optic nerve tumor, and breast intraductal papilloma. Patients may have multiple tumors simultaneously 1–3.
HL is the malignant tumor of lymphoid tissue with specific granulomatous histologic structure. HL is one of the most frequent of all childhood malignant neoplasms with morbidity of 0.7–0.9 per 100,000 children. The prognosis of HL is favorable and according to international trials data the 5-year event-free survival is approximately 88%, and the overall survival is 97% 10,11.
Classical HL accounts for approximately 95% of cases, and is subdivided into nodular sclerosis, mixed cellularity, lymphocyte-rich classical, and lymphocyte depletion variants. Tumor cells morphologically present as Reed–Sternberg cells with the immunohistochemical profile CD30+, CD15+, and PAX+. HL is a radiosensitive and chemosensitive tumor; however, in some cases the disease is refractory to first-line therapy. To improve the results of refractory and relapsed patients while reducing late side effects, recent approaches have been based on new agents such as monoclonal antibodies, HDAC-inhibitors, mTOR inhibitors, and others 12,13. One of the most promising agents is brentuximab vedotin, a CD30-directed antibody-drug conjugate. After interaction of anti-CD30 and CD30-antigen on the tumor cell membrane, this complex is internalized and a soluble linker is enzymatically degraded, releasing the antitubulin drug monometylauristatine A, and leading to apoptosis. The predominant side effect of brentuximab vedotin is neuropathy 14,15. To decrease cytostatic loading brentuximab vedotin seems an excellent therapeutic option, for heavy pretreated relapsed patients, who need PBSCT 16–18.
It is still not well understood which of the prognostic factors plays the main role: age, initial tumor volume, erythrocyte sedimentation rate, B-symptoms, or unknown molecular contributors. In our case, PS is speculated to play a role in such disease behavior, and only haploidentical PBSCT had curative effect. Certainly, the PI3K-AKT pathway plays the role in Proteus Syndrome, and we know that Hodgkin lymphoma is characterized by high mTOR activity in 93% of the cases, and Bcl-xL and NF-kappaB expression correlated with this mTOR activity 19. High mTOR activity is observed in the case of both favorable and unfavorable clinical response. The presence of mTOR activity probably indicates that the inclusion of mTOR inhibition in the therapy of Hodgkin-lymphomas may be feasible and beneficial, especially when standard protocols are ineffective, and it may also allow dose reduction to decrease late treatment toxicity. Most likely, the combination of mTOR inhibitors with other agents will offer the highest efficiency for achieving the best clinical response.
The development of new agents for the treatment of refractory HL gives new hope to heavily pretreated patients. Further trials are needed to understand possible links pathogenesis and to establish the best treatment choice for children with HL and underlying genetic diseases.
Acknowledgments
This study was supported in part by Charity Foundation “Gift of life”.
Conflict of Interest
Authors declare no competing financial interests in relation to the work described.
References
- Angurana SK, Angurana RS, Panigrahi I. Marwaha RK. Proteus syndrome: clinical profile of six patients and review of literature. Indian J. Hum. Genet. 2013;19:202–206. doi: 10.4103/0971-6866.116117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM., Jr Proteus syndrome review: molecular, clinical, and pathologic features. Clin. Genet. 2014;85:111–119. doi: 10.1111/cge.12266. [DOI] [PubMed] [Google Scholar]
- Lindhurst MJ, Wang JA, Bloomhardt HM, Witkowski AM, Singh LN, Bick DP, et al. AKT1 gene mutation levels are correlated with the type of dermatologic lesions in patients with Proteus syndrome. J. Invest. Dermatol. 2014;134:543–546. doi: 10.1038/jid.2013.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauz-Körholz C, Hasenclever D, Dörffel W, Ruschke K, Pelz T, Voigt A, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J. Clin. Oncol. 2010;28:3680–3686. doi: 10.1200/JCO.2009.26.9381. [DOI] [PubMed] [Google Scholar]
- Schellong G, Dörffel W, Claviez A, Körholz D, Mann G, Scheel-Walter HG, et al. Salvage therapy of progressive and recurrent Hodgkin’s disease: results from a multicenter study of the pediatric DAL/GPOH-HD study group. J. Clin. Oncol. 2005;23:6181–6189. doi: 10.1200/JCO.2005.07.930. [DOI] [PubMed] [Google Scholar]
- Isidori A, Piccaluga PP, Loscocco F, Guiducci B, Barulli S, Ricciardi T, et al. High-dose therapy followed by stem cell transplantation in Hodgkin’s lymphoma: past and future. Expert. Rev. Hematol. 2013;6:451–464. doi: 10.1586/17474086.2013.814451. [DOI] [PubMed] [Google Scholar]
- Cole PD, Schwartz CL, Drachtman RA, de Alarcon PA. Chen L. Trippett TM Phase II study of weekly gemcitabine and vinorelbine for children with recurrent or refractoryHodgkin’s disease: a children’s oncology group report. J. Clin. Oncol. 2009;27:1456–1461. doi: 10.1200/JCO.2008.20.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghesquières H, Stamatoullas A, Casasnovas O, Morschhauser F, Gyan E, Gabarre J, et al. Clinical experience of bendamustine in relapsed or refractory Hodgkin lymphoma: a retrospective analysis of the French compassionate use program in 28 patients. Leuk. Lymphoma. 2013;54:2399–2404. doi: 10.3109/10428194.2013.776165. [DOI] [PubMed] [Google Scholar]
- Moskowitz AJ, Perales PA, Jr, Hamlin MA, Gerecitano J, Horwitz SM, Matasar MJ, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J. Clin. Oncol. 2013;31:456–460. doi: 10.1200/JCO.2012.45.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L. I Schafer A. Hodgkin’s Lymphoma. In:Goldman’s Cecil Medicine, 192, 1228-1233, Copyright © 2012, 2008, 2004, 2000, 1996, 1991, 1988, 1982, 1979, 1975, 1971, 1963, 1959, 1955 by Saunders, an imprint of Elsevier Inc.
- Bope ET. Kellerman RD. Hematology. In: Conn’s Current Therapy 2014, Section 12, 820-824. Copyright © 2014, 2013, 2012, 2011, 2010, 2009, 2008 by Saunders, an imprint of Elsevier Inc.
- Batlevi CL. Younes A. Novel therapy for Hodgkin lymphoma. Hematol. Am. Soc. Hematol. Educ. Program. 2013;2013:394–399. doi: 10.1182/asheducation-2013.1.394. [DOI] [PubMed] [Google Scholar]
- von Tresckow B. Engert A. Refractory Hodgkin lymphoma. Curr. Opin. Oncol. 2013;25:463–469. doi: 10.1097/01.cco.0000432524.62475.60. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Viviani S, Anastasia A, Vitolo U, Luminari S, Zaja F, et al. Brentuximab vedotin in relapsed/refractory Hodgkin’s lymphoma: the Italian experience and results of its use in daily clinical practice outside clinical trials. Haematologica. 2013;98:1232–1236. doi: 10.3324/haematol.2012.083048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland AM, Li JX, Wasco LE, Aziz MT. Lowe DK. Brentuximab vedotin: a CD30-directed antibody-cytotoxic drug conjugate. Pharmacotherapy. 2013;33:93–104. doi: 10.1002/phar.1170. [DOI] [PubMed] [Google Scholar]
- Gibb A, Jones C, Bloor A, Kulkarni S, Illidge T, Linton K, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013;98:611–614. doi: 10.3324/haematol.2012.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, Kumar L. Iqbal N. Update on salvage options in relapsed/refractory hodgkin lymphoma after autotransplant. ISRN Oncol. 2014;2014:605691. doi: 10.1155/2014/605691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illidge T, Bouabdallah R, Chen R, Gopal AK, Moskowitz CH, Ramchandren R, et al. Allogeneic transplant following brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk. Lymphoma. 2015;56:703–710. doi: 10.3109/10428194.2014.930852. [DOI] [PubMed] [Google Scholar]
- Mark A, Hajdu M, Váradi Z, Sticz TB, et al. Characteristic mTOR activity in Hodgkin-lymphomas offers a potential therapeutic target in high risk disease–a combined tissue microarray, in vitro and in vivo study. BMC Cancer. 2013;13:250. doi: 10.1186/1471-2407-13-250. [DOI] [PMC free article] [PubMed] [Google Scholar]


