Abstract
Objectives
Staphylococcus lugdunensis can cause community- and healthcare-associated infections. This study investigated the molecular characteristics of S. lugdunensis isolates collected at our hospital and compared the characteristics of the infectious and commensal isolates.
Methods
We collected the S. lugdunensis isolates between 2003 and 2013. The antimicrobial resistance test, SCCmec typing, accessory gene regulator (agr) typing, pulsed-field gel electrophoresis (PFGE), and δ-like hemolysin activity were performed.
Results
In total, 118 S. lugdunensis isolates were collected, of which 67 (56.8%) were classified into the infection group and 51 (43.2%) into the commensal group. The oxacillin resistance rate was 36.4%. The most common SCCmec types were SCCmec types V (51.4%) and II (32.6%). In total, 34 pulsotypes were identified. The PFGE typing revealed five clones (pulsotypes A, J, M, N, and P) at our hospital. Pulsotypes A and N caused the spread of high oxacillin resistance. In total, 10.2% (12 of 118) of the isolates lacked δ-like hemolysin activity. Compared with the infection group, the commensal group showed a higher percentage of multiple drug resistance and carried a higher percentage of SCCmec type II (11 of 22, 50% and 3 of 21, 14.3%) and a lower percentage of SCCmec type V (8 of 22, 36.4% and 14 of 21, 66.7%). The commensal group (27 PFGE types) showed higher genetic diversity than did the infection group (20 PFGE types). No difference was observed in the distribution of the five main pulsotypes, agr typing, and the presence of δ-like hemolysin activity between the two groups.
Conclusions
Five main clones were identified at our hospital. The commensal group showed higher genetic diversity, had a higher percentage of multidrug resistance, and carried a higher percentage of SCCmec type II and a lower percentage of SCCmec type V than did the infection group.
Introduction
Staphylococcus lugdunensis, belonging to the group of coagulase-negative staphylococci (CoNS), was first reported by Freney et al. in 1988 [1]. Although the incidence rate of S. lugdunensis infection was low [2, 3], increasing numbers of patients with S. lugdunensis infective endocarditis have been reported in recent 20 years [4, 5]. It has also emerged as a pathogen that causes various community- and healthcare-associated infections, such as those of the bloodstream, bones and joints, skin and soft tissues, and the central nervous system [6, 7].
Like other CoNS species, S. lugdunensis is a commensal skin flora of humans [8]. It can be transmitted between hospitalized patients and hospital environments and causes invasive infections in patients with impaired skin integrity and indwelling catheters and foreign devices [9, 10]. The organism is generally assumed to colonize the human skin and then cause invasive infection. However, little is known about the molecular epidemiology of commensal isolates.
Compared with the high prevalence of oxacillin resistance among Staphylococcus aureus and other CoNS in hospital environments, that of S. lugdunensis has been reported to be low [3, 6, 9, 11, 12]. However, in our recent study, a high rate of oxacillin resistance was observed in patients with invasive S. lugdunensis infection, with a 20.8% resistance rate [7]. In addition, erythromycin (25%) and clindamycin (18.8%) resistance was observed. The most common SCCmec type was type V, and all these isolates were accounted for healthcare-associated infections [7]. S. lugdunensis isolates carrying SCCmec type V were also reported in central Taiwan [13].
In addition to SCCmec typing, the accessory gene regulator (agr) locus also serves as a crucial molecular marker of staphylococci. According to polymorphisms, the agr gene can be divided into four types in S. aureus [14], and two types in S. lugdunensis [15]. Many virulence factors of staphylococci are regulated by the agr locus. Extensive research has been conducted on the agr locus of S. aureus, and different agr types have been associated with different virulence profiles and human diseases [16]; however, the association between agr typing and S. lugdunensis infection is unclear. Like S. aureus and S. epidermidis, S. lugdunensis can produce S. lugdunensis synergistic hemolysin, which belongs to the family of phenol-soluble modulin (PSM) peptides [17]. This hemolysin is controlled by the agr locus. This δ-like hemolysin produced serves as a surrogate maker of the agr function in staphylococci [18].
Although the molecular characteristics of pathogenic isolates have been studied [19, 20], little is known about the characteristics of commensal populations. Understanding the molecular characteristics of infectious and commensal isolates can facilitate identifying the cause of the high resistance rate, control the spread of resistant strains, and establish a background database for further study on pathogenic isolates. The aim of this study was to investigate the molecular characteristics of the S. lugdunensis isolates collected at our hospital over 10 years and to compare the characteristics of the isolates causing infection and those considered contaminants or commensals by using various methods, including pulsed-field gel electrophoresis (PFGE) typing, SCCmec typing, agr typing, and hemolytic activity analysis.
Materials and Methods
Clinical setting
This study was conducted at Chang Gung Memorial Hospital (CGMH) in Taoyuan, Taiwan. The hospital is a 3700-bed university-affiliated hospital and tertiary referral medical center in northern Taiwan. This study was approved by the Chang Gung Medical Foundation Institutional Research Board (approval number: 103-3231B). All S. lugdunensis isolates were obtained from blood or sterile body fluid cultures in our clinical microbiology laboratory between May 2003 and 2013. Clinical data, including the patient age, sex, types of samples, and infectious foci, were retrospectively collected using chart review and analyzed anonymously.
Case definition
Clinically significant bacteremia was defined as occurring in patients when two consecutive positive blood cultures were obtained for S. lugdunensis. Patients with a single positive blood culture were considered to have clinically significant bacteremia if they experienced one or more of the following: prolonged fever, hypotension, leukocytosis or neutropenia with a left-shifted differential, or disseminated intravascular coagulopathy combined with risk factors for infections caused by skin flora, including long-term intravascular catheterization, peritoneal dialysis or hemodialysis, or extensive postsurgical infections with CoNS [21, 22]; otherwise, positive results were attributed to contamination of the blood culture, and the isolate was classified as commensal.
Healthcare-associated infections were defined as occurring in patients with the following: (1) S. lugdunensis infection identified after 48 hours of admission to the hospital; (2) a history of hospitalization, surgery, dialysis, or residence in a long-term care facility within 1 year of the positive culture date; or (3) a permanent indwelling catheter or percutaneous medical device present at the time of culture. Cases that had none of the above features were classified as community associated [23].
Microbiological methods
All S. lugdunensis isolates were first identified using Gram staining and biochemical methods (catalase positive, coagulase negative, pyrrolidonyl arylamidase positive, and ornithine decarboxylase positive). Furthermore, all isolates were verified using the polymerase chain reaction (PCR) method described by Noguchi et al. [24] and a Bruker Biotyper (database 2.0) matrix-assisted laser desorption ionization/time of flight mass spectrometry system [25].
Antimicrobial susceptibility testing
The minimal inhibitory concentration (MIC) of oxacillin was determined using agar dilution methods. The oxacillin resistance was defined as an MIC of 4 mg/mL or greater, according to the Clinical and Laboratory Standards Institute guidelines [26]. Susceptibility testing of penicillin, clindamycin, erythromycin, and trimethoprim–sulfamethoxazole was performed using the disk diffusion method and interpreted according to the Clinical and Laboratory Standards Institute guidelines [26].
SCCmec typing
SCCmec typing of the S. lugdunensis isolates was performed using a multiplex PCR for identifying the ccr gene complex, the mec gene complex, and specific structures in the adjacent regions as described previously when the oxacillin MIC was ≥4 μg/mL [27].
agr typing
Two PCR primers (SL_agr-F and SL_agr-R) were designed to detect the agr genes of the S. lugdunensis isolates. We then determined the agr type through PCR amplification of the conserved sequences from the agrC gene, with two forward primers and one reverse primer (SL_agr-1-F, SL_agr-2-F/SL_agr-R). All primers were designed using a computer-assisted analysis of the genomic DNA sequences from GenBank accession numbers AF173933 and AF346728 and the whole genome sequence data of our two S. lugdunensis isolates. All primer sequences are listed in Table 1.The agr specificity types were identified on the basis of the expected product sizes (586 bp for agr type I and 771 bp for agr type II) (Fig 1).
Table 1. Primers used in polymerase chain reaction amplification and sequencing of the agr locus in this study.
| Primer | 5′-3′ Sequence | Gene | Purpose | Size of amplicon (bp) |
|---|---|---|---|---|
| SL_agr-F/SL_agr-R | ATAATGATACCAAGGAGCGTG/ CGAACCTTTAGCTTATCTGTACC | agrB/agrC | screening | 1626 |
| SL_agr-1-F/SL_agr-R | CTGTCATCCTTAGTGTAATTGCTG/ CGAACCTTTAGCTTATCTGTACC | agrC | agr I typing | 586 |
| SL_agr-2-F/SL_agr-R | GCCGGCATAATAGTCCCTTCTG/CGAACCTTTAGCTTATCTGTACC | agrC | agr II typing | 771 |
Fig 1. Multiplex PCR for agr gene typing of Staphylococcus lugdunensis.

(Lanes M: marker; 19: S. lugdunensis No. 19; 20: S. lugdunensis No. 20; 21: S. lugdunensis No. 21; 22: S. lugdunensis No. 22).
Pulsed-field gel electrophoresis
Genomic DNAs of the S. lugdunensis isolates were digested with SmaI and separated using PFGE as described previously [28]. Lambda ladder DNA was used as a molecular weight marker for PFGE. The PFGE patterns were analyzed, and a dendrogram was constructed using BioNumerics software 6.0 (Applied Maths; Texas, USA). Percent similarities were identified on a dendrogram derived using the unweighted pair group method by using arithmetic averages and based on Dice coefficients. Band position tolerance and optimization were set at 1.25 and 0.5%, respectively [28]. A similarity coefficient of 80% was selected to define closely related strains (clones).
Hemolytic activity
The δ-like hemolysin activity was analyzed by performing a cross-streaking test, perpendicular to RN4220, which produces only β-hemolysin on a Columbia blood agar plate. We used ATCC 25923 S. aureus as the agr-positive control and the S. aureus strain Mu 50 as the agr-negative control.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences software Version 18.0 (SPSS Inc.; Chicago, IL, USA). The frequency between the two groups was analyzed by performing a z-test.
Results
In total, 118 S. lugdunensis isolates were collected from 118 patients at CGMH. The mean age of patients was 56.0 ± 28.0 years, and 61 patients (51.7%) were male. All isolates tested were confirmed using matrix-assisted laser desorption ionization/time of flight mass spectrometry and PCR methods. Most of the isolates were collected from the blood culture (n = 105, 89%) (Table 2). In total, 67 isolates (56.8%) caused infections, and 51 isolates (43.2%) were considered contaminants and classified as commensals. Among the isolates involved in infection, 59 (50.0%) caused healthcare-associated infections and 8 (6.8%) caused community-associated infections. The leading causes of infection were primary (n = 29, 24.6%) and catheter-related bacteremia (n = 6, 8.5%). The foci of infection are shown in Table 3.
Table 2. 118 Staphylococcus lugdunensis isolates obtained from various samples.
| Sample | Number | Percentage (%) |
|---|---|---|
| Blood | 105 | 89.0 |
| Ascites | 6 | 5.1 |
| Body fluid | 2 | 1.7 |
| Synovial fluid | 2 | 1.7 |
| Amniotic fluid | 1 | 0.8 |
| Cerebrospinal fluid | 1 | 0.8 |
| Pleural effusion | 1 | 0.8 |
Table 3. Distribution of infection group and commensal group of 118 Staphylococcus lugdunensis isolates.
| Number | Percentage (%) | |
|---|---|---|
| Infection group | 67 | 56.8 |
| Primary bacteremia | 29 | 24.6 |
| Catheter-related | 10 | 8.5 |
| Arteriovenous graft/fistula | 6 | 5.1 |
| Bone and joints | 6 | 5.1 |
| Infective endocarditis | 4 | 3.4 |
| Intra-abdomen | 4 | 3.4 |
| Skin and soft tissue | 3 | 2.5 |
| CAPD peritonitis | 2 | 1.7 |
| Central nervous system | 1 | 0.8 |
| Genital system | 1 | 0.8 |
| Lung | 1 | 0.8 |
| Commensal group | 51 | 43.2 |
Antimicrobial susceptibility
The results regarding antibiotic resistance are shown in Table 4. Analysis of the 118 S. lugdunensis isolates revealed that 90 (76.3%) were resistant to penicillin, 43 (36.4%) to oxacillin, 40 (33.9%) to erythromycin, 33 (28.0%) to clindamycin, and only 2 (1.7%) to trimethoprim–sulfamethoxazole. All oxacillin-resistant S. lugdunensis isolates emerged after the year 2009 and were distributed as follows: 4 of 9 isolates (44%) in 2009, 12 of 30 (40%) in 2010, 11 of 29 (37.9%) in 2011, 11 of 22 (50%) in 2012, and 5 of 9 (35.7%) in 2013. Among the 43 oxacillin-resistant isolates, 18 (41.9%) were also resistant to clindamycin and erythromycin and one (0.8%) was also resistant to erythromycin and trimethoprim–sulfamethoxazole. Compared with the infectious isolates, the commensal isolates showed a higher percentage of multiple drug resistance (p < 0.05). No difference was observed in the penicillin and oxacillin resistance rates.
Table 4. Antibiotic resistance, distribution of SCCmec types and agr types, and δ-hemolytic activity among infectious and commensal Staphylococcus lugdunensis isolates.
| Total, n = 118, Number (%) | Infection, n = 67, Number (%) | Commensal, n = 51, Number (%) | p value | |
|---|---|---|---|---|
| Antibiotic resistance | ||||
| Penicillin-R | 90 (76.3) | 48 (71.6) | 42 (82.4) | 0.1773 |
| Oxacillin-R | 43 (36.4) | 21 (31.3) | 22 (43.1) | 0.187 |
| SCCmec type II 1 | 14/43 (32.6) | 3/21 (14.3) | 11/22 (50.0) | 0.012 |
| SCCmec type IV | 2/43 (4.7) | 1/21 (4.8) | 1/22 (4.5) | 0.976 |
| SCCmec type V | 22/43 (51.2) | 14/21 (66.7) | 8/22 (36.4) | 0.047 |
| SCCmec type Vt | 3/43 (7.0) | 2/21 (9.5) | 1/22 (4.5) | 0.522 |
| SCCmec type NT | 2/43 (4.7) | 1/21 (4.8) | 1/22 (4.5) | |
| Clindamycin-R | 33 (28.0) | 13 (19.4) | 20 (39.2) | 0.017 |
| Erythromycin-R | 40 (33.9) | 17 (25.4) | 23 (45.1) | 0.025 |
| TMP–SMX-R | 2 (1.7) | 1 (1.5) | 1 (2.0) | 0.841 |
| agr type | ||||
| I | 53 (44.9) | 27 (40.3) | 26 (51.0) | 0.246 |
| II | 65 (55.1) | 40 (59.7) | 25 (49.0) | 0.246 |
| δ-hemolysin activity | ||||
| nonhemolytic | 12(10.2) | 6 (9.0) | 6 (11.8) | 0.617 |
| Main pulsotypes | ||||
| A | 25 (21.2) | 17 (25.4) | 8 (15.7) | 0.201 |
| J | 14 (11.9) | 10 (14.9) | 4 (7.8) | 0.238 |
| M | 8 (6.8) | 5 (7.5) | 3 (5.9) | 0.728 |
| N | 12 (10.2) | 5 (7.5) | 7 (13.7) | 0.262 |
| P | 7 (5.9) | 4 (6.0) | 3 (5.9) | 0.984 |
NT = nontypeable; R = resistant; TMP–SMX = trimethoprim–sulfamethoxazole
1 The total number of SCCmec types was the number of isolates with oxacillin resistance.
SCCmec typing
The SCCmec typing results are summarized in Table 4. Among the 43 oxacillin-resistant isolates, SCCmec could be classified in 41 isolates, and 2 isolates were untypeable. The most common SCCmec type was type V (22 of 43, 51.2%), followed by type II (14 of 43, 32.6%). A higher frequency of SCCmec type V (66.7%, 14 of 21) than that of SCCmec type II (14.2%, 3 of 21) was observed in patients with infections (n = 21) caused by oxacillin-resistant S. lugdunensis. All infectious oxacillin-resistant isolates were isolated from patients with healthcare-associated infections. Among the 22 commensal isolates, SCCmec type II (11of 22, 50%) was the most frequently detected, followed by SCCmec type V (8 of 22, 36.4%). The difference in the distribution of SCCmec types was statistically significant between the infection and commensal groups (p < 0.05) (Table 4). All S. lugdunensis isolates with SCCmec type II were resistant to oxacillin, erythromycin, and clindamycin. Most SCCmec type V isolates were resistant to oxacillin and sensitive to clindamycin and erythromycin (21 of 22, 95.2%).
agr typing
The agr gene in most isolates could be divided into two agr types according to the PCR product size. Large PCR products were observed in our two isolates (SL32 and SL44). Further analysis of the PCR products using sequencing suggested an insertion sequence at the agr locus. The sequencing results showed that SL32 possessed agr type I, and SL44 possessed type II. In total, 53 isolates (44.9%) possessed agr type I, and 65 (55.1%) possessed agr type II. The distribution of the infectious isolates was as follows: 27 isolates (40.3%) carried type I, and 40 isolates (59.7%) carried type II. The distribution of the commensal isolates was as follows: 26 isolates (51.0%) possessed type I, and 25 isolates (49.0%) possessed type II. No statistical difference was observed between the infection and commensal groups (Table 4). In addition, most isolates with SCCmec type II carried agr type I (13 of 14, 92.9%), and all isolates with SCCmec type V carried agr type II (22 of 22, 100.0%) (p < 0.05). The results showed a strong relationship between agr and SCCmec types in oxacillin-resistant isolates.
Hemolytic activity
Upon screening for δ-like hemolysin activity, we found that 12 (10.2%) of the 118 isolates lacked synergistic hemolytic activity; of these isolates, six belonged to the infection group, and six belonged to the commensal group. The percentage of nonhemolytic isolates was similar between the infection and commensal groups (Table 4). Among the nonhemolytic isolates, three isolates (3 of 53, 5.7%) possessed agr type I, and nine isolates (9 of 65, 13.8%) possessed agr type II. The percentage of nonhemolytic isolates with agr types I and II was similar (p = 0.144).
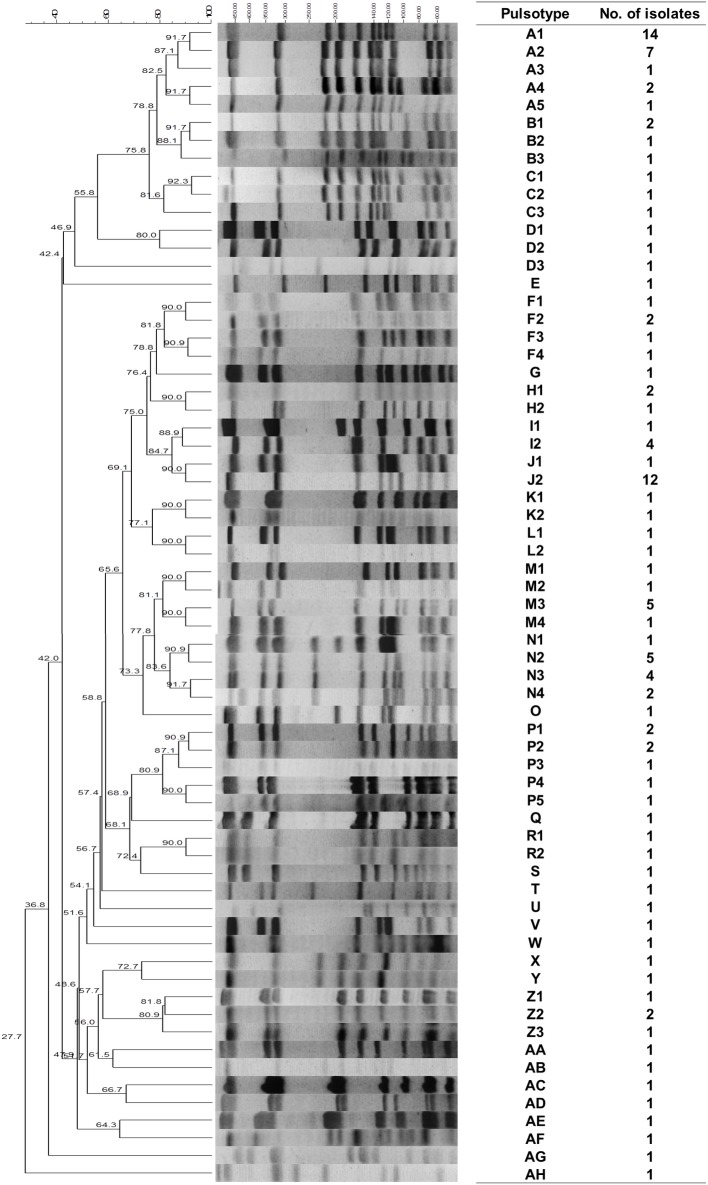
Pulsed-field gel electrophoresis typing
The dendrogram is shown in Fig 2. The 118 S. lugdunensis isolates collected at our hospital comprised a total of 34 PFGE types; however, more than half of the isolates (55.9%) belonged to five main pulsotypes (A, J, M, N, and P). Nineteen pulsotypes were represented by a single isolate each. Overall, pulsotype A (25/118, 21,2%) was the predominant pulsotype, followed by pulsotype J (14/118, 11.9%), pulsotype N (12/118, 10.2%), pulsotype M (8/118, 6.8%), and pulsotype P (7/118, 5.9%). The five pulsotypes differed in their resistant patterns. More than half of the isolates (n = 21, 84.0%) of pulsotype A were resistant only to oxacillin and carried SCCmec type V–agr type II (17/25, 68.0%), while most isolates (n = 11, 91.7%) belonging to pulsotype N showed multidrug resistance and carried SCCmec type II–agr type I (9/12, 75.0%) (Table 5). Compared with isolates of pulsotypes A and N, those belonging to pulsotypes J, M, and P were mostly oxacillin sensitive (25/29, 86.2%). Compared with the infection group (20 PFGE types), the commensal group (27 PFGE types) showed higher genetic diversity (p = 0.011). No difference was observed in the distribution of the five main pulsotypes between the infection and commensal groups (Table 4). The details of the characteristics of the five pulsotypes are shown in Table 5 and S1 Table.
Fig 2. Dendrogram of pulsed field gel electrophoresis of 118 Staphylococcus lugdunensis isolates.
Table 5. Summary of the pulsed-field gel electrophoresis results, antimicrobial resistance, and SCCmec and agr typing for Staphylococcus lugdunensis.
| PFGE type | Number (%) of isolates | Infection, n | Oxacillin resistant, n | Resistance profile 1 , n | SCCmec-agr, n |
|---|---|---|---|---|---|
| A | 25 (21.2) | HAI, 17; Commensals, 8 | 21 | O, 19; O-C-E, 2 | SCCmec V-agr II, 17; SCCmec Vt-agr II, 3; SCCmec IV-agr II, 1 |
| J | 14 (11.9) | HAI, 10; Commensals, 4 | 1 | O-C-E, 1; C-E, 4 | SCCmec II-agr I, 1 |
| M | 8 (6.8) | HAI, 5; Commensals, 3 | 2 | O-C-E, 2; C-E,1 | SCCmec II-agr I, 2 |
| N | 12 (10.2) | HAI, 4; CAI, 1; Commensals, 7 | 11 | O-C-E, 11 | SCCmec II-agr I, 9; SCCmec II-agr II, 1; SCCmec NT-agr I, 1 |
| P | 7 (5.9) | HAI, 3; CAI, 1; Commensals, 3 | 1 | O-E-SXT, 1; C-E, 2; E, 2; E-SXT, 1 | SCCmec IV-agr I, 1 |
| other | 52 (44.1) | HAI, 20; CAI, 6; Commensals, 26 | 7 | O, 5; O-C-E, 2; C-E, 8; E, 3 | SCCmec V-agr II, 5; SCCmec II-agr I, 1; SCCmec NT-agr I, 1 |
CAI = community-associated infection; HAI = healthcare-associated infection; NT = nontypeable; PFGE = pulsed-field gel electrophoresis
1C-E, clindamycin and erythromycin resistance; E, erythromycin resistance; E-SXT, erythromycin and trimethoprim–sulfamethoxazole resistance; O, oxacillin resistance; O-C-E, oxacillin, clindamycin, and erythromycin resistance; O-E-SXT, oxacillin, erythromycin, and trimethoprim–sulfamethoxazole resistance
Discussion
In this study, we analyzed general molecular characteristics and compared the molecular characteristics of the S. lugdunensis isolates at our hospital in the infection and commensal groups. The two most common SCCmec types carried by oxacillin-resistant S. lugdunensis at our hospital were types II and V. Both agr types I and II were equally distributed in the studied S. lugdunensis isolates. In addition, we identified five main clones at our hospital. Pulsotypes A and N were responsible for high oxacillin resistance. A comparison of the characteristics of the infectious S. lugdunensis and commensal isolates revealed that the commensal group had a higher percentage of multiple drug resistance than did the infection group. The commensal group also showed higher genetic diversity than did the infection group. The isolates belonging to the commensal group carried SCCmec type II more frequently and SCCmec type V less frequently compared with those belonging to the infection group. No difference was observed in the distribution of agr types and in the presence of hemolysin activity between the two groups.
SCCmec typing is a crucial epidemiological technique for analyzing oxacillin-resistant staphylococci. Studies showed that healthcare-associated methicillin-resistant S. aureus (MRSA) infections were caused by strains carrying SCCmec types I, II, and III but rarely by those carrying types IV and V [29]. At our hospital, the most common oxacillin-resistant S. lugdunensis isolates causing healthcare-associated infections carried SCCmec type V, followed by SCCmec type II. However, among the isolates considered commensals, the isolates carrying SCCmec type II were more common than those carrying SCCmec type V. Keito et al. in 2011 reporting a gene named psm-mec, which was present in the SCCmec type II cassette, regulated the virulence of S. aureus. The psm-mec encoded RNA and peptides could promote biofilm formation and reduced the dissemination ability of staphylococci [30, 31]. The SCCmec type V isolates, which did not carry psm-mec, showed higher colony spreading ability. This may partially explain our finding that the isolates carrying SCCmec type II caused fewer infection episodes than did those carrying SCCmec type V. Many studies on the epidemiology of MRSA infection have revealed that the community-associated MRSA SCCmec types have infiltrated healthcare settings [32]. More recent studies have shown the presence of MRSA strains carrying SCCmec types IV and V in healthcare-associated settings in many countries [33–35]. An increase in the number of MRSA strains carrying SCCmec type V was not only associated with skin and soft tissue infections but also with invasive infections. Our study showed that both SCCmec types II and V are the major SCCmec types in healthcare settings. Recently, S. lugdunensis carrying SCCmec type V was identified in central Taiwan [13]. Our data also suggested the emergence of SCCmec type V in healthcare settings for S. lugdunensis. However, the number of cases with community-associated infections was small in our study, and further analysis of community-associated SCCmec types in S. lugdunensis is required.
In the present study, the oxacillin resistance rate was 36.4%, which was higher than that reported in other studies [9, 12]. All oxacillin-resistant isolates were found after 2009. All infection episodes caused by oxacillin-resistant isolates were healthcare-associated infections. No community-associated infection with oxacillin-resistant S. lugdunensis was found. The findings indicate that the emergence of high oxacillin resistance within S. lugdunensis was due to high antibiotic selection pressure at the hospital. Moreover, according the PFGE data, 35 (81.3%) oxacillin-resistant isolates belonged to two clones (pulsotypes A and N). These findings suggest that the increasing resistance rate can be largely attributed to clonal spread at our hospital and not to the horizontal transfer of the SCCmec cassette between the S. lugdunensis isolates. These groups of isolates could not only colonize the skin but also caused invasive infections. While these bacterial clones did not cause outbreaks, they can still cause invasive infections, especially in patients with indwelling catheters. Although we did not screen for bacteria isolated from the hospital environment and medical devices, we believe that infection control precautions are required to eliminate the bacteria.
No difference was observed in the distribution of agr types between the infection and commensal groups. The agr system plays a major role in staphylococcal pathogenesis. According to a study conducted by Jarraud et al., different agr types are associated with different patterns of S. aureus diseases [16]. For example, agr type IV is associated with generalized exfoliative syndromes. Endocarditis strains mainly possess agr types I and II. However, in our study, we did not find any relationship between agr types and the patterns of S. lugdunensis disease. One possible reason is that our bacterial isolates were mostly from patients with bacteremia. No isolates were obtained from wound infections, and the number of isolates from other clinical samples was small. However, the agr types were highly correlated with the SCCmec types, probably because these isolates belonged to the same clones.
Most of our isolates were positive for δ-like hemolysin activity. Only 10% of the isolates were nonhemolytic. This result was consistent with that of a previous report [36]. A study revealed that the synergistic hemolytic activity of S. lugdunensis is distinct from that of S. aureus [37]. Unlike the δ-like hemolysin activity of S. aureus, which is controlled by the agr-hld system, that of S. lugdunensis is encoded by the slush locus, which belongs to the PSM family and is controlled by the agr locus. In animal models, the agr-defective mutants are attenuated for virulence [38]. However, in recent studies, agr-defective S. aureus was associated with persistent bacteremia and increased mortality [39, 40]. In our study, no difference was observed in the agr function between the infection and commensal groups. Considering the impact of agr functionality on staphylococcal pathogenesis, the role of agr in S. lugdunensis infection still requires further investigation.
Our study has several limitations: first, the isolates in our study were mostly obtained from blood culture. No isolates from wound or abscess culture were included. Second, most commensal isolates were obtained from the skin flora of patients and no isolates collected from healthy volunteers were available for further comparison. Third, the infectious isolates or commensals were classified on the basis of clinical criteria. Fourth, the data were collected from a single hospital, and the number of community-associated S. lugdunensis isolates was small.
In conclusion, the two most common SCCmec types carried by S. lugdunensis were types V and II. PFGE typing revealed the presence of five main clones (pulsotypes A, J, M, N, and P) at our hospital. Compared with the infection group, the commensal group showed a higher frequency of multiple drug resistance. By contrast, the commensal group showed higher genetic diversity, as evident in PFGE typing. The infectious isolates more frequently carried SCCmec type V, and the commensal groups showed a higher frequency of SCCmec type II. No difference was observed in agr types and in the presence of δ-hemolysin activity between the infection and commensal groups.
Supporting Information
(DOCX)
Acknowledgments
This work was supported by grants from Chang Gung Memorial Hospital (CMRPG3D1771) and the National Science Council of Republic of China (Taiwan) (NSC-101-2320-B-182A-002-MY3), Taiwan.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from Chang Gung Memorial Hospital (CMRPG3D1771) (CF, Yeh) and the National Science Council of Republic of China (Taiwan) (NSC-101-2320-B-182A-002-MY3) (JJ Lu), Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1. Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont PA, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168–172. [Google Scholar]
- 2. Choi SH, Chung JW, Lee EJ, Kim TH, Lee MS, Kang JM, et al. Incidence, characteristics, and outcomes of Staphylococcus lugdunensis bacteremia. J Clin Microbiol. 2010;48(9):3346–3349. 10.1128/JCM.00609-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shin JH, Jung HJ, Lee HR, Kim JH, Kim HR, Lee JN. Prevalence, identification, and antimicrobial susceptibility of Staphylococcus lugdunensis from various clinical specimens in Korea. Jpn J Infect Dis. 2007;60(5):312 [PubMed] [Google Scholar]
- 4. Vandenesch F, Etienne J, Reverdy ME, Eykyn SJ. Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin Infect Dis. 1993;17(5):871–876. [DOI] [PubMed] [Google Scholar]
- 5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis . Clin Microbiol Rev. 2008;21(1):111–133. 10.1128/CMR.00036-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hellbacher C, Tornqvist E, Soderquist B. Staphylococcus lugdunensis: clinical spectrum, antibiotic susceptibility, and phenotypic and genotypic patterns of 39 isolates. Clin Microbiol Infect. 2006;12(1):43–49. [DOI] [PubMed] [Google Scholar]
- 7. Lin J-F, Cheng C-W, Kuo A-J, Liu T-P, Yang C-C, Huang C-T, et al. Clinical experience and microbiologic characteristics of invasive Staphylococcus lugdunensis infection in a tertiary center in northern Taiwan. J Microbiol Immunol Infect. 2014. February 13 pii: S1684-1182(14)00002-4. 10.1016/j.jmii.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 8. Herchline T, Ayers LW. Occurrence of Staphylococcus lugdunensis in consecutive clinical cultures and relationship of isolation to infection. J Clin Microbiol. 1991;29(3):419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zinkernagel A, Zinkernagel M, Elzi M, Genoni M, Gubler J, Zbinden R, et al. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection. 2008;36(4):314–321. 10.1007/s15010-008-7287-9 [DOI] [PubMed] [Google Scholar]
- 10. Widerström M, Monsen T, Karlsson C, Wiström J. Molecular epidemiology of meticillin-resistant coagulase-negative staphylococci in a Swedish county hospital: evidence of intra-and interhospital clonal spread. J Hosp Infect. 2006;64(2):177–183. [DOI] [PubMed] [Google Scholar]
- 11. Kleiner E, Monk AB, Archer GL, Forbes BA. Clinical significance of Staphylococcus lugdunensis isolated from routine cultures. Clin Infect Dis. 2010;51(7):801–803. 10.1086/656280 [DOI] [PubMed] [Google Scholar]
- 12. Wu A-B, Wang M-C, Tseng C-C, Lin W-H, Teng C-H, Huang A-H, et al. Clinical and microbiological characteristics of community-acquired Staphylococcus lugdunensis infections in Southern Taiwan. J Clin Microbiol. 2011;49(8):3015–3018. 10.1128/JCM.01138-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yen TY, Sung YJ, Lin HC, Peng CT, Tien N, Hwang KP, et al. Emergence of oxacillin-resistant Staphylococcus lugdunensis carrying staphylococcal cassette chromosome mec type V in central Taiwan. J Microbiol Immunol Infect. 2014. December 11 pii: S1684-1182(14)00249-7. 10.1016/j.jmii.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 14. Jarraud S, Lyon GJ, Figueiredo AM, Lina G, Vandenesch F, Etienne J, et al. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J Bacteriol. 2000;182(22):6517–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dufour P, Jarraud S, Vandenesch F, Greenland T, Novick RP, Bes M, et al. High genetic variability of the agr locus in Staphylococcus species. J Bacteriol. 2002;184(4):1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002;70(2):631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rautenberg M, Joo H-S, Otto M, Peschel A. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. FASEB J. 2011;25(4):1254–1263. 10.1096/fj.10-175208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donvito B, Etienne J, Greenland T, Mouren C, Delorme V, Vandenesch F. Distribution of the synergistic haemolysin genes hld and slush with respect to agr in human staphylococci. FEMS Microbiol Lett. 1997;151(2):139–144. [DOI] [PubMed] [Google Scholar]
- 19. Tseng S-P, Lin Y-T, Tsai J-C, Hung W-C, Chen H-J, Chen P-F, et al. Genotypes and phenotypes of Staphylococcus lugdunensis isolates recovered from bacteremia. J Microbiol Immunol Infect. 2013. December 31 pii: S1684-1182(13)00229-6. 10.1016/j.jmii.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 20. Liu C, Shen D, Guo J, Wang K, Wang H, Yan Z, et al. Clinical and microbiological characterization of Staphylococcus lugdunensis isolates obtained from clinical specimens in a hospital in China. BMC Microbiol. 2012;12(1): 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Souvenir D, Anderson DE, Palpant S, Mroch H, Askin S, Anderson J, et al. Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microbiol. 1998;36(7): 1923–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fadel HJ, Patel R, Vetter EA, Baddour LM. Clinical significance of a single Staphylococcus lugdunensis-positive blood culture. J Clin Microbiol. 2011;49(4):1697–1699. 10.1128/JCM.02058-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morrison MA, Hageman JC, Klevens RM. Case definition for community-associated methicillin-resistant Staphylococcus aureus . J Hosp Infect. 2006;62(2):241. [DOI] [PubMed] [Google Scholar]
- 24. Noguchi N, Goto K, Ro T, Narui K, Ko M, Nasu Y, et al. Using the tannase gene to rapidly and simply identify Staphylococcus lugdunensis . Diagn Microbiol Infect Dis. 2010;66(1):120–123. 10.1016/j.diagmicrobio.2009.03.028 [DOI] [PubMed] [Google Scholar]
- 25. Szabados F, Woloszyn J, Richter C, Kaase M, Gatermann S. Identification of molecularly defined Staphylococcus aureus strains using matrix-assisted laser desorption/ionization time of flight mass spectrometry and the Biotyper 2.0 database. J Med Microbiol. 2010;59(7):787–790. [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory StandardsInstitute. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement (CLSI Document M100-S22. 2012). Wayne, PA: Clinical and Laboratory Standards Institute; 2012
- 27. Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. 2007;51(1):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41(11):5113–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr Opin Microbiol. 2012;15(5):588–595. 10.1016/j.mib.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 30. Kaito C, Saito Y, Nagano G, Ikuo M, Omae Y, Hanada Y, et al. Transcription and translation products of the cytolysin gene psm-mec on the mobile genetic element SCCmec regulate Staphylococcus aureus virulence. PLoS Path. 2011;7(2):e1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaito C, Omae Y, Matsumoto Y, Nagata M, Yamaguchi H, Aoto T, et al. A novel gene, fudoh, in the SCCmec region suppresses the colony spreading ability and virulence of Staphylococcus aureus . PLoS One. 2008;3(12):e3921 10.1371/journal.pone.0003921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzalez BE, Rueda AM, Shelburne SA III, Musher DM, Hamill RJ, Hultén KG. Community‐associated strains of methicillin‐resistant Staphylococccus aureus as the cause of healthcare‐associated infection. Infect Control Hosp Epidemiol. 2006;27(10):1051–1056. [DOI] [PubMed] [Google Scholar]
- 33. Brennan GI, Shore AC, Corcoran S, Tecklenborg S, Coleman DC, O'Connell B. Emergence of hospital-and community-associated panton-valentine leukocidin-positive methicillin-resistant Staphylococcus aureus genotype ST772-MRSA-V in Ireland and detailed investigation of an ST772-MRSA-V cluster in a neonatal intensive care unit. J Clin Microbiol. 2012;50(3):841–847. 10.1128/JCM.06354-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care—associated blood stream infections. Clin Infect Dis. 2006;42(5):647–656. [DOI] [PubMed] [Google Scholar]
- 35. David M, Kearns A, Gossain S, Ganner M, Holmes A. Community-associated meticillin-resistant Staphylococcus aureus: nosocomial transmission in a neonatal unit. J Hosp Infect. 2006;64(3):244–250. [DOI] [PubMed] [Google Scholar]
- 36. Hebert G. Hemolysins and other characteristics that help differentiate and biotype Staphylococcus lugdunensis and Staphylococcus schleiferi . J Clin Microbiol. 1990;28(11): 2425–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Donvito B, Etienne J, Denoroy L, Greenland T, Benito Y, Vandenesch F. Synergistic hemolytic activity of Staphylococcus lugdunensis is mediated by three peptides encoded by a non-agr genetic locus. Infect Immun. 1997;65(1):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwan WR, Langhorne MH, Ritchie HD, Stover CK. Loss of hemolysin expression in Staphylococcus aureus agr mutants correlates with selective survival during mixed infections in murine abscesses and wounds. FEMS Immunol Med Microbiol. 2003;38(1):23–28. [DOI] [PubMed] [Google Scholar]
- 39. Fowler VG, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004;190(6):1140–1149. [DOI] [PubMed] [Google Scholar]
- 40. Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, et al. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother. 2011;55(3):1082–1087. 10.1128/AAC.00918-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.