Abstract
Background
Transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (HCC) is an important option as the majority of patients present with advanced disease. Data regarding treatment outcomes in patients who have undergone transjugular intrahepatic portosystemic shunts (TIPS) are limited. The present study seeks to evaluate the safety and efficacy of TACE in HCC patients with a TIPS.
Methods
A retrospective review identifying patients with HCC and concomitant TIPS who were treated with TACE was performed.
Results
From 1999 to 2014, 16 patients with HCC underwent a total of 27 TACE procedures; eight patients required multiple treatments. The median patient age at the time of the initial TACE was 60.5 years [interquartile range (IQR):52.5–67.5] with the majority being male (n = 12, 75%) and Childs–Pugh Class B (n = 12, 75%). At 6 weeks after TACE, 56.3% of patients achieved an objective response rate (complete and partial response) by mRECIST criteria. Clavien Grade 3 or higher complications occurred in 11.1% of TACE procedures. There were no peri-procedural deaths. The median progression-free (PFS) and overall survival (OS) were 9 and 22 months, respectively, when censored for liver transplantation (median follow-up: 11.5 months).
Conclusion
TACE is an effective treatment strategy for HCC in TIPS patients; albeit may be associated with higher complication rates.
Introduction
Transarterial chemoembolization (TACE) has emerged as an effective treatment strategy for patients with hepatocellular carcinoma (HCC).1,2 Unlike surgery or transplantation, which are dependent on patients having a resectable disease or the availability of suitable organs, studies have demonstrated TACE to be effective for patients with advanced disease.3 However, TACE is not without its limitations, as current treatment guidelines recommend patients have compensated liver disease along with overall good performance status when considering this approach.4,5 The presence of cirrhosis and its associated complications from portal hypertension, which include variceal bleeding and ascites, often complicates decision-making and traditionally may preclude a patient from receiving TACE.
Placement of transjugular intrahepatic portosystemic shunts (TIPS) has become a widely used technique in managing the sequelae of portal hypertension. However, as TIPS reduces hepatic perfusion by diverting portal venous flow and may potentiate arterioportal shunting, TACE has not been commonly performed in this subgroup owing to concern for potential worsening of hepatic dysfunction, a transient increase in portal hypertension and the risk of hepatic infarct.6,7 Unfortunately with the rising incidence of HCC among western countries, there has been a corresponding increase in the number of HCC cases with TIPS in need of loco-regional therapy.8 In recent years, several studies have investigated the feasibility of TACE in patients with a functional TIPS.6,9–14 While all the studies were limited by a small sample size, the rates of TACE-related complications are variable (5–70%). Owing to the paucity of data surrounding this subject and the need to identify a viable treatment option for this patient subgroup, the present study seeks to evaluate the safety and efficacy of TACE for HCC patients after TIPS placement.
Patients and methods
Approval was first obtained from the Institutional Review Board at the Medical College of Wisconsin. Using a prospectively maintained database, patients diagnosed with HCC by alpha-fetoprotein (AFP) levels, needle biopsy or diagnostic radiographic criteria along with concomitant TIPS were identified. Inclusion criteria required that all patients underwent targeted chemoembolization from 1999 to 2014. Patients who received TACE prior to TIPS placement, or subsequently underwent other liver-directed therapies, transplantation or pursued surgical resection prior to follow-up imaging post TACE were excluded. Standard clinicopathological data were abstracted, which included the aetiology of the underlying liver disease, an indication for TIPS, liver function tests (total bilirubin, AST/ALT, albumin), creatinine, platelet count, international normalized ratio (INR), AFP and parameters of advanced liver disease (presence of encephalopathy, ascites or asterixis). Model for End-Stage Liver Disease (MELD) scores and Child–Pugh classification were determined.
Standard institutional follow-up post-TIPS placement included an ultrasound (US) at 2 weeks, 3 months, 6 months, 1 year, and then annually. Portal pressure gradients were checked only at the time of TIPs placement, or if a revision was required. All patients had a documented reduction in the portal pressure gradient of less than 12 mm Hg, after each TACE intervention. TIPS venography was not routinely performed unless a patient had abnormal US findings or developed clinical symptoms consistent with shunt dysfunction. Prior to each TACE procedure, all patients underwent dedicated cross-sectional imaging [contrast enhanced computed tomography (CT) or magnetic resonance imaging (MRI)] to evaluate TIPS patency, tumour characteristics and its relationship to surrounding vasculature.
TACE procedure
All patients were pretreated with steroids, benedryl, anti-emetics and prophylactic antibiotics. Under conscious sedation, access to the common femoral artery was obtained using a standard Seldinger technique followed by placement of a 5-French (F) vascular sheath. First, a superior mesenteric and celiac arteriogram was performed to assess patency of the portal vein/TIPS, directionality of portal venous flow, as well as to evaluate for the potential of the aberrant hepatic arterial vasculature. A 3-F microcatheter was then advanced into the second or third-order branches of the right or left hepatic artery. Selective chemoembolizaiton was performed using a standard protocol consisting of 100 mg of cisplatin (if available) (Baxter, Glendale, CA, USA), 50 mg of doxorubicin (Pharmacia & Upjohn, Peapack, NJ, USA) and 10 mg of mitomycin-C (Super Gen, Dublin, CA, USA) combined with Ethiodol (Guerbet LLC, Bloomington, IN, USA) in a 1:1 ratio without polyvinyl alcohol (PVA) particles. Owing to a drug shortage, six patients did not receive cisplatin as part of their TACE regimen. Further, a history of thrombocytopenia in one patient resulted in doxorubicin being withheld. In addition, as a result of the sub-selective approach, complete delivery of the chemotherapy-Ethiodol emulsion was restricted. An average of 60% (6 cc) of the total chemotherapy dose was utilized per TACE. Successful embolization was defined as vascular stasis in the tumour arterial branches. After delivery of the drug emulsion, two patients had significant residual tumour arterial flow, which required injection of PVA particles (150–250 micron) to achieve complete stasis. Post procedure, patients were observed overnight in the hospital and discharged home the subsequent morning.
Follow-up
Post TACE, patients were seen in follow-up at 2 and 4–6 weeks. Liver function tests and tumour markers were obtained. All patients underwent contrast-enhanced CT or MRI at a median of 41 days [interquartile range (IQR): 37–60] after each TACE procedure. The treatment response of targeted lesions was characterized by the modified Response Evaluation Criteria in Solid Tumors (mRECIST).15 The objective response rate (OR) post TACE was defined as tumours that displayed either a partial or complete response based on mRECIST definitions.
Post-procedure hepatotoxicity was evaluated based on the development of one of the hepatobiliary severe adverse events (SAEs) defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03.16 Hepatotoxicity was recorded if at least one of the following abnormal laboratory values or clinical states was observed within a 6-week period after the procedure: NCI CTCAE grade 3 or 4 for serum levels of total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), the presence of severe symptoms of ascites, or clinical hepatic failure. Overall morbidity was assessed according to the Clavien–Dindo classification system.17 Grade III or higher complications were included in the study. Progression-free (PFS) and overall survival (OS) were calculated from the date of first TACE treatment to the date of radiographic evidence of disease progression (PFS), or the date of death/last follow-up (OS). Patients that underwent liver transplantation were noted as a censored event.
Statistical analysis
Discrete variables were reported as totals and frequencies, whereas continuous data were described as median values ± IQR. Survival (months) was assessed using Kaplan–Meier methods. Statistical analyses were performed with Stata/MP 10.0 for Windows (StataCorp, College Station, TX, USA).
Results
From 1999 to 2014, 16 patients with HCC and TIPS underwent a total of 27 TACE procedures. Eight of the 16 patients required at least two treatments (50%) while an additional three patients (18.8%) underwent a third TACE session. All patients had confirmation of TIPS patency by contrast-enhanced imaging prior undergoing TACE. The majority of patients were male (n = 12, 75%) and had a median age at the time of first TACE of 60.5 years (IQR: 52.5–67.5). Prior to initial TACE, the median MELD score was 12.5 (7.5–13), with the vast majority being either Childs Class A or B (n = 14, 87.5%). While current treatment guidelines often advise against routine TACE in Childs Class C patients, two individuals in the present series underwent therapy. In both patients, a super selective TACE approach involving only the third order or greater hepatic artery branches was utilized in order to minimize exposure of the normal hepatic parenchyma to the cytotoxic agents. Patient and tumour characteristics are described in Table1.
Table 1.
Patient characteristics
| Variable | TIPS group (n = 16) |
|---|---|
| Median age at first TACE (IQR) | 60.5 (52.5–67.5) |
| Male gender, n (%) | 12 (75) |
| Cause of cirrhosis, n (%) | |
| Viral Hepatitis | 7 (43.8) |
| Alcohol | 5 (31.2) |
| NASH | 2 (12.5) |
| Other | 2 (12.5) |
| TIPS indication, n (%) | |
| Ascites | 9 (56.2) |
| Bleeding | 7 (43.8) |
| Median MELD Score following TIPS Placement (IQR) | 12 (10.5–13) |
| Time between TIPS and first TACE, (months) | |
| Median (IQR) | 41 (7.5–53.5) |
| Child–Pugh Class at first TACE, n (%) | |
| A | 2 (12.5) |
| B | 12 (75) |
| C | 2 (12.5) |
| Median MELD Score at first TACE (IQR) | 12.5 (7.5–13) |
| Median dominant tumour size, cm (IQR) | 2.8 (1.9–4.5) |
| Number of tumours | |
| Single | 9 (56.2) |
| Multiple | 7 (43.8) |
| BCLC Stage, n (%) | |
| A | 3 (18.8) |
| B | 4 (25) |
| C | 7 (43.7) |
| D | 2 (12.5) |
| Number of TACE procedures, median (range) | 1 (1–3) |
TACE, transarterial chemoembolization; TIPS, transjugular intrahepatic portosystemic shunt; NASH, non-alcoholic steatohepatitis; MELD, Model for End-Stage Liver Disease; BCLC, Barcelona Clinic Liver Cancer.
Procedure-related morbidity
Within 6 weeks of each TACE session, Clavien grade 3 or higher complications occurred three times (11.1%, Table2). Of the 16 patients that underwent TACE, four patients accounted for all reported SAE complications (25%). The most common hepatobiliary serious adverse event (SAE) was the development of ascites post TACE (n = 3, 11.1%). Hepatic failure, which was defined as the evolution of encephalophathy or asterixis, occurred in two patients (7.4%). A greater frequency of per-procedure complications were observed during subsequent TACE sessions (n = 5, 45.4%) as compared with the initial treatment (n = 2, 12.5%). There were no peri-procedural (within 30 days) deaths after TACE. Of the two patients that were Childs Class C, one patient experienced reversible SAEs after two of their three TACE sessions. The first SAE was the development of encephalopathy that was controlled by lactulose. The second SAE required paracentesis to resolve the abdominal ascites. Lastly, for the two patients that required PVA embolization, neither experienced a treatment-related complication.
Table 2.
TACE-related morbidity among HCC patients with a TIPS
| First TACE (n = 16) | Subsequent TACE (n = 11) | All TACE (n = 27) | |
|---|---|---|---|
| Clavien Grade ≥III complication, n (%) | 0 | 3 (27.3) | 3 (11.1) |
| Hepatobiliary severe adverse events | |||
| Total bilirubin, n (%) | |||
| Grade 3/4: Total bilirubin > 3 × ULN | 0 | 2 (18.2) | 2 (7.4) |
| AST, n (%) | |||
| Grade 3/4: AST > 5 × ULN | 0 | 0 | 0 |
| ALT, n (%) | |||
| Grade 3/4: ALT > 5 × ULN | 0 | 0 | 0 |
| Ascites, n (%) | |||
| Grade 3: severe symptoms requiring invasive intervention | 0 | 3 (27.3) | 3 (11.1) |
| Hepatic failure, n (%) | |||
| Grade 3: asterixis, mild encephalopathy | 2 (12.5) | 0 | 2 (7.4) |
| Mortality, n (%) | |||
| Within 30 days of procedure | 0 | 0 | 0 |
TACE, transarterial chemoembolization; HCC, Hepatocellular Carcinoma; TIPS, transjugular intrahepatic portosystemic shunt.
Survival after TACE
After TACE, by mRECIST criteria, two patients (12.5%) demonstrated a complete response, seven patients (43.8%) experienced a partial response, six (37.5%) had stable disease, and one had disease progression (6.2%). The objective response (complete + partial response) and disease control (complete + partial response + stable disease) rate were 56.3% and 93.8%, respectively. The median follow-up after TACE was 11.5 months (IQR: 9.5–15.75). Of the 16 patients, three went on to liver transplantation, whereas two patients underwent additional therapies after completion of TACE: one patient required radiofrequency ablation of a segment 6 lesion; the second patient underwent stereotactic body radiation therapy followed by systemic therapy after demonstrating disease progression post TACE.
The median PFS and OS after censoring for liver transplantation was 9 and 22 months, respectively (Fig.1). Additionally, at 1 year, the OS survival rate was 73.9%. When stratifying patients by BCLC stage, all three BCLC stage A patients proceeded to transplantation. The remaining 13 patients were either BCLC stage B (n = 4), C (n = 7) or D (n = 2) and experienced a median OS of 22 months.
Figure 1.
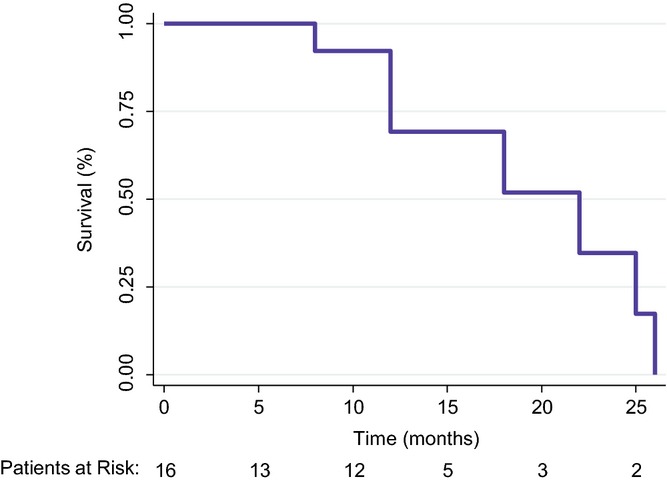
Overall survival of hepatocellular carcinoma (HCC) patients with a intrahepatic portosystemic shunt (TIPS) after Transarterial chemoembolization (TACE) (n = 16), censored for liver transplantation (n = 3)
Discussion
Patients with HCC and a concomitant TIPS pose unique challenges to clinicians when attempting to identify effective treatment options. While the presence of a TIPS traditionally underscores a patients advanced liver disease, the altered hepatic perfusion that results after TIPS placement also questions whether TACE can remain a safe and effective strategy. In the present study, TACE was successfully performed in a series of HCC patients with TIPS with low morbidity and a similar survival benefit to historical controls. These results provide further evidence to support an expanded role for TACE that now includes patients with a TIPS.
The primary concern surrounding TACE in HCC patients with a TIPS is the potential for treatment-related liver failure. Owing to the decrease in portal venous flow, TACE possesses the theoretical risk of causing further hepatic dysfunction through the disruption of the arterial vasculature, which the normal hepatic parenchyma is dependent upon. To date, there have been five series that have investigated the safety of TACE in HCC patients that possess a TIPS (Table3).6,11–14 However, a common theme shared among published series, and further demonstrated in the present study, was the assessment of TIPS patency prior to performing TACE. While no patient in the current series had evidence of a non-functional TIPS before TACE, in the absence of clinical symptoms (gastrointestinal bleeding, ascites), routine interrogation by venography to assess TIPS patency, or determination of portal pressure gradients is unnecessary. The criteria to perform TACE in TIPS patients should not be predicated on the presence of a well-functioning TIPS. Any residual portal venous flow redirected towards the liver parenchyma as a result of a stenosis in the TIPS, has the potential to decrease the associated risk of hepatic dysfunction/infarction. Instead, consideration into which TIPS patients would be suitable candidates for TACE should include those with a good performance status, absence of clinical symptoms to suggest worsening liver dysfunction, along with laboratory evidence demonstrating stable hepatic function. In the present study, patients treated demonstrated a stable MELD score after TIPS placement to the date of the first TACE.
Table 3.
Comparison in treatment related morbidity and survival outcomes among select series that utilized TACE for HCC patients with a concomitant TIPS
| Author (Year) | No. of patients | % of Childs-pugh Class A/B patients | Treatment regimen | Type of embolic material | % Morbidity | Median survival (months)b | Overall survival at 1 year (%) |
|---|---|---|---|---|---|---|---|
| Current Study | 16 | 88 | Cisplatin, Doxorubicin, Mitomycin-C | PVAa | 25 | 18 | 73.9 |
| Tesdal et al. (2006)6 | 6 | 83 | Epirubicin +/-PEI | None | 50 | NR | NR |
| Kang et al. (2012) | 20 | 90 | Cisplatin | Gelatin Sponge | 5b | 23 | 85 |
| Kohi et al. (2013)11 | 10 | 80 | Cisplatin, Doxorubicin, Mitomycin-C | Gelatin Sponge† | 70 | NR | 100 |
| Gaba et al. (2013)13 | 6 | 100 | Cisplatin, Doxorubicin, Mitomycin-C | None | 11 | NR | 71 |
| Wang et al. (2014)12 | 17 | NR | Doxorubicin, 5-fluorouracil | Gelfoama | 31.6 | NR | 88 |
TACE, transarterial chemoembolization; TIPS, transjugular intrahepatic portosystemic shunt; PEI, percutaneous ethanol injection; NR, not reported.
Not all patients received embolic material.
The complication rate not reported according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) definitions.
Unfortunately, there has been a wide variation in the reported morbidity rates among published series. A plausible explanation for the equivocal outcomes could be attributed to the lack of a uniform TACE treatment protocol. Several studies including the present have opted for a selective approach when using embolic material (e.g. PVA, foam, sponge).12 The vast majority of patients among these studies did not ultimately receive particulate embolization. Rather, the attenuation of vascular flow was achieved solely by the lipiodol emulsion. The rationale behind omitting embolic particles is to reduce the extent of ischemic necrosis of normal hepatic parenchyma that could result after TACE. In the present study, two of 16 patients received PVA. While neither patient experienced a complication, the overall morbidity rate of the collective cohort was low (25%) and comparable to the series by Wang (31.6%).12 Similarly, the study by Gaba et al.13 abandoned the use of embolic material in their series and demonstrated an 11% morbidity rate. In contrast, the study by Kohi et al.11 reported the highest frequency of complications (70%). A major procedural difference in this study, however, was the administering of the gelatin sponge after TACE. Perhaps, avoidance of embolic material in total may decrease the likelihood of treatment-related toxicity in TIPS patients. Nonetheless, the results of the present study along with published series would suggest that TACE can be safely performed for HCC patients with TIPS.
A second concern among HCC patients with a TIPS is whether overall TACE treatment efficacy is compromised. TIPS placement has been shown to increase arterioportal venous shunting, which may alter the retention rate of the lipiodol–chemotherapy emulsion within the tumour, thereby limiting the treatment success.7 For non-TIPS HCC patients, prior studies that utilized a TACE regimen consisting of cisplatin, doxorubicin and mitomycin-C, they have demonstrated median OS survival rates ranging from 15 to 18 months after TACE.18,19 Therefore, it would be reasonable to suggest that any patient with unresectable HCC treated with TACE should achieve a survival rate similar to published series. While limited by the small sample size and no comparison arm, treated patients that did not go on to transplantation in the present study, experienced a median overall survival of 22 months. As for the three patients that underwent transplantation, TACE was delivered as a bridging therapy while suitable organs became available. The average time on the liver transplant wait list experienced by the three patients was 11.7 months. Despite the presence of the TIPS, TACE effectively controlled tumour growth as reflected by all the patients remaining within Milan Criteria while awaiting transplantation. Therefore, these results reflect that the presence of a TIPS does not necessarily compromise efficacy, is safe and should be considered as an additional treatment adjunct for this difficult patient population.
In conclusion, the present study provides further evidence supporting locoregional therapies as a safe and effective therapy for HCC patients with a TIPS. While several other series have reported their outcomes surrounding this patient subgroup, treatment-related morbidity has been variable, which can be partially attributed to the lack of standardized protocols. Although the omission of embolic particles may limit the extent of ischaemic necrosis that occurs with TACE, it did not appear to affect treatment efficacy. In fact, TACE without embolic material has the potential to be a safer approach for patients with TIPS as it spares normal hepatic parenchyma from further damage. Therefore, the presence of a TIPS should not preclude HCC patients from receiving a therapy that can achieve a durable survival benefit.
Funding sources
None.
Conflicts of interest
None.
References
- Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- Miura JT, Gamblin TC. Transarterial chemoembolization for primary liver malignancies and colorectal liver metastasis. Surg Oncol Clin N Am. 2015;24:149–166. doi: 10.1016/j.soc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Diseases AAftSoL. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- Tesdal IK, Wikström M, Flechtenmacher C, Filser T, Dueber C. Percutaneous treatment of hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol. 2006;29:778–784. doi: 10.1007/s00270-005-0063-7. [DOI] [PubMed] [Google Scholar]
- Itkin M, Trerotola SO, Stavropoulos SW, Patel A, Mondschein JI, Soulen MC, et al. Portal flow and arterioportal shunting after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol. 2006;17:55–62. doi: 10.1097/01.RVI.0000191362.75969.F6. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Uchida H, Maeda M, Matsuo N, Kichikawa K, Ohishi H, et al. Combined transjugular intrahepatic portosystemic shunt and segmental Lipiodol hepatic artery embolization for the treatment of esophagogastric varices and hepatocellular carcinoma in patients with cirrhosis: preliminary report. Cardiovasc Intervent Radiol. 1995;18:9–15. doi: 10.1007/BF02807348. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Kohi MP, Naeger DM, Tong RT, Kolli KP, Taylor AG, et al. Efficacy of TACE in TIPS patients: comparison of treatment response to chemoembolization for hepatocellular carcinoma in patients with and without a transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol. 2013;36:1336–1343. doi: 10.1007/s00270-013-0698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohi MP, Fidelman N, Naeger DM, LaBerge JM, Gordon RL, Kerlan RK. Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: do two rights make a wrong? J Vasc Interv Radiol. 2013;24:68–73. doi: 10.1016/j.jvir.2012.08.032. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang H, Zhao H, Wang X, Tsauo J, Luo X, et al. Repeated transcatheter arterial chemoembolization is safe for hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Diagn Interv Radiol. 2014;20:487–491. doi: 10.5152/dir.2014.13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaba RC, Rim CM, Parvinian A. Re: Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: do two rights make a wrong? J Vasc Interv Radiol. 2013;24:1075–1076. doi: 10.1016/j.jvir.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Kang JW, Kim JH, Ko GY, Gwon DI, Yoon HK, Sung KB. Transarterial chemoembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt. Acta Radiol. 2012;53:545–550. doi: 10.1258/ar.2012.110476. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. 2010. Common Terminology Criteria for Adverse Events, version 4.03, June 14, [December 3, 2014]. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DB, Chapman WC, Cook RD, Kerr JR, Gould JE, Pilgram TK, et al. Chemoembolization of hepatocellular carcinoma: patient status at presentation and outcome over 15 years at a single center. AJR Am J Roentgenol. 2008;190:608–615. doi: 10.2214/AJR.07.2879. [DOI] [PubMed] [Google Scholar]
- Georgiades CS, Liapi E, Frangakis C, Park JU, Kim HW, Hong K, et al. Prognostic accuracy of 12 liver staging systems in patients with unresectable hepatocellular carcinoma treated with transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:1619–1624. doi: 10.1097/01.RVI.0000236608.91960.34. [DOI] [PubMed] [Google Scholar]


