Abstract
Background
A pancreatoduodenectomy (PD) is a highly advanced procedure associated with considerable post-operative complications and substantial costs. In this study the hospital costs associated with complications after PD were assessed.
Methods
A retrospective cohort study was conducted on 100 consecutive patients who underwent a pylorus-preserving (PP)PD between January 2012 and July 2013. Per patient, all complications occurring during admission or in the 30-day period after discharge were documented. All hospital costs related to the (PP)PD were defined as the costs of all medical interventions and resources during the hospitalisation period as recorded by the electronic supply tracking system.
Results
The median hospital costs ranged from €17 482 for a patient without complications to €55 623 for a patient with a post-operative haemorrhage. A post-operative haemorrhage was associated with a 39.6% increase in total hospital costs after adjusting for patient characteristics. Other factors significantly associated with an increase in total hospital costs were: the presence of a malignancy other than a pancreatic adenocarcinoma (29.4% cost increase), the severity grade of a complication (34.3–70.6% increase) and the presence of a post-operative infection (32.4% increase).
Conclusions
This study provides an in-depth analysis of hospital costs and identifies factors that are associated with substantial cost consequences of specific complications occurring after a PD.
Introduction
Health care costs are rising worldwide and, therefore, cost containment is one of the most important challenges in future medicine. Health care expenditures are considered to be at least in part influenced by the aging population, but the increase of performing specialised surgical procedures also contributes to high hospital costs.1–3 Post-operative complications also substantially increase the use of additional resources per patient and prolong the hospital stay, raising medical costs even further.4–8
A pancreatoduodenectomy (PD) is a typical example of a complex, highly specialised surgical procedure. Despite a reduction in the mortality of PD below 5% in high-volume centres, a PD is still accompanied with a substantial morbidity and post-operative complication rates are varying between 40 and 60%.9–12 Important surgical complications after PD include anastomotic leakage, in particular of the pancreatojejunostomy and leading to a pancreatic fistula (PF), haemorrhage and delayed gastric emptying (DGE).13–15
Reducing complications has become a desirable goal for quality improvement initiatives to optimise patient outcomes and to reduce hospital costs.16–18 Previous studies have already identified factors that can predict post-operative complications. Examples of such predictors are duodenal or ampullary lesions that generally present with a non-dilated pancreatic duct and a soft pancreas, which more frequently result in leakage of the pancreatic anastomosis, pancreatic fistula and a subsequently higher risk of a post-operative haemorrhage, but also pre-operative nausea, which is associated with a higher incidence of DGE and a prolonged hospital stay.19,20
Although patients at risk of developing complications after a PD can be identified, limited information is currently available about costs of specific complications.21 An in-depth cost evaluation of pancreatic surgery, in particular regarding procedures with and without specific complications, might gain insight into the economic burden of those complications. This could be helpful to predict hospital costs after pancreatic surgery. Information about hospital costs might also be helpful to suggest changes in the management of complications with the aim of reducing health care expenditures.
The aim of this study was, therefore, to quantify the cost consequences of complications occurring in hospitalised patients after a PD. Furthermore, we assessed which factors are associated with an increase in total hospital costs.
Patients and methods
Study design
A retrospective cohort study was conducted at a tertiary-referral university hospital in the Netherlands. This is a retrospective review of a database with real-time data capture. Data on a consecutive series of adult patients who underwent a PPPD or classic PD between January 2012 and July 2013 were prospectively included in this database. Patients with metastasis or local non-resectable disease during exploration were not included in this study. Data gathered during this 1.5-year observation period included the minimum follow-up period of 30 days after discharge.
The following clinical data were included: age, gender, comorbidities, American Society of Anesthesiologists (ASA) classification, type of PD, the need for a vascular resection, (histo)pathologic diagnosis, length of hospital stay, readmissions, reoperations and the length of intensive care unit (ICU) stay.
We used the STROBE statement to ensure the proper reporting of this observational study.22
Complications
All complications as documented in a local database of the Dutch National Surgical Complication Registry (Landelijke Heelkundige Complicatie Registratie, LHCR) were analysed. The LHCR was developed by the Dutch Society of Surgeons and is a slightly modified version of the Clavien–Dindo classification.23,24 The following definition of a complication was used in the registry and this study: ‘an unintended and undesired outcome or state occurring during or following medical care that is so harmful to the patients= health that it requires (adjustment of) treatment or leads to permanent damage=.25
Variables registered in the complication registry comprise patient characteristics, admission characteristics and complications occurring during admission or in the 30-day period after discharge leading to readmission, reoperation, or death.
All complications reported during morning handovers with the attendance of the complete surgical staff and residents are encoded in this registration, as well as complications reported in the discharge letter. The reliability of this complication database was independently audited.26
The complication registry categorises each complication into four grades of severity: Grade 1, temporary health disadvantage recovering without reoperation (grade 1 management includes radiological or endoscopic interventions; similar to Dindo grade I, II and IIIa); grade 2, recovery after reoperation (similar to Dindo grade IIIb); grade 3, (probably) permanent damage or function loss (similar to Dindo grade IV when permanent); and grade 4, death (similar to Dindo grade V). Patients were followed until their complication had recovered, or it was obvious that the complication resulted in permanent damage or death. When multiple complications were reported in one patient, the recorded level of severity was determined by the most severe complication. Minor complications, such as an electrolyte imbalance or fever, even without clinical consequences, were also registered in the LHCR database and were classified as severity grade 1.
Furthermore, the complication registry was searched by two investigators independently (T.B.S. and A.V.) to select three important and specific complications for pancreatic surgery, i.e. ‘post-operative haemorrhage’, ‘anastomotic leakage’ and ‘DGE’. If DGE occurred without postoperative haemorrhage or an anastomotic site leakage, the complication was labelled as ‘isolated DGE’. If a patient had a combination of post-operative haemorrhage and anastomotic leakage, the patient was analysed in both of these complication groups. If the type of complication was unclear based on the complication registry, the investigators checked the information from the discharge letter and the (electronic) medical record. Discrepancies between investigators were resolved by discussion. Admission to the ICU is not part of standard post-operative care after a PD and patients are only admitted to the ICU in case of severe complications.
Costs
We included all hospital costs per patient, including outpatient visits and readmissions that were directly related to the provided care in relation to the PD. Costs related to the diagnostic pathway before the operation and additional costs of non-related diagnoses or procedures in the past were not taken into account.
Electronic supply tracking allowed accurate determination of hospital costs for a specific diagnosis and were provided by the financial department of the hospital. Unit costs for a specific product included the front-office costs of personnel (e.g. nurses, surgeons), material use, as well as the back-office costs of facility and overhead. In our hospital, all specialists (surgeons) are employed by the hospital with a fixed yearly salary.
Total hospital costs per patient were calculated as the product-sum of volumes and unit costs of care and were subdivided into seven cost domains: ‘general diagnostics’ (e.g. laboratory, microbiology and pathology investigations), ‘imaging’ (e.g. CT or MRI scans), ‘outpatient clinic’ (e.g. visits to outpatient clinic or emergency department), ‘clinical care’ (ward care and 1-day hospital admissions), ‘surgical’ (operating room and surgical supplies), ‘ICU’ (critical care) and ‘other costs’. The cost domain ‘other costs’ included costs for blood transfusion, percutaneous drainage and similar procedures.
Incurred hospital costs in the period before the PD were not taken into account, because of the wide variety of diagnostic pathways prior to surgery, partly performed in other hospitals. The time horizon of the study was restricted to the index admission and readmissions for the treatment of complications within 30 days of initial hospital discharge. Considering the limited time horizon, no discounting of costs took place to account for time preference. Unit costs were expressed for the base year 2014. Only in-hospital costs were included in this study. Costs for additional care, such as nursing facilities or home care, were not included in this study.
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences version 21 (SPSS Inc., Armonk, NY, USA). Descriptive statistics were expressed as means and standard deviations, or medians and inter-quartile ranges (IQR), whenever appropriate. Risk differences were calculated and presented with 95% confidence intervals (CI). Student=s t and Mann–Whitney U-tests were used to analyse differences between two groups with normally or non-normally distributed continuous variables (such as costs), respectively. The chi-square test was used to compare percentages (e.g. patient characteristics) and the Kruskal–Wallis test was used to compare more than two non-normally distributed groups (e.g. the three specific complications chosen for further analysis). Kaplan–Meier estimates of total hospital costs were obtained and compared with three patient groups (without complications, with grade 1 complications, and with grade 2 complications), using log-rank test statistics. The level of significance was defined as a P-value less than 0.05.
Univariable and stepwise multivariable linear regression was used to explore possible associations between specific patient characteristics and total costs. Log-transformation of the dependent variable ‘total costs’ was performed because of its non-linear distribution. Categorical variables (e.g. post-operative diagnosis, complication severity) were recoded into dummy variables before analysing the data. Possible predictors were entered in the multivariable analysis when showing a (nearly) significant (i.e. P < 0.10) difference between patients with and without complications according to the univariable linear regression analysis. Results from the regression analyses are expressed as regression coefficients, 95% CI and P-values.
Results
Between January 2012 and July 2013, 100 consecutive adult patients underwent a PD and were included in this study. The mean age at surgery was 64.0 ± 10.0 years and most patients were males (59%). Eighty-five of the patients underwent a PPPD; the remaining patients underwent standard PD. None of the included patients received pre-operative chemo(radio)therapy.
Overall, 73% of the patients sustained one or more complications. Of the three selected complications, anastomotic leakage (PJ, HJ, GJ) was the most common (24/100). Isolated DGE and a post-operative haemorrhage were reported less frequently (in 18 and 12 patients, respectively). In eight of the latter patients, leakage and a post-operative haemorrhage occurred simultaneously. Mortality during admission and in the 30-day period after discharge was 1%. Unplanned readmission within 30 days after discharge was required in 10 patients (10%) with a grade 1 complication and in one patient (1%) with a grade 2 complication.
Characteristics of patients with and without a complication are summarised in Table1. Patients with complications after surgery more frequently showed a history of cardiac disease and hospital- and ICU stay in this group were significantly longer.
Table 1.
Characteristics of patients without or with one or more complications after a pancreatoduodenectomy
| No complication N = 27 | With ≥ 1 complication N = 73 | RD or MD (95% CI) | P-value | |
|---|---|---|---|---|
| Age at surgery in years (SD) | 64.2 (11.9) | 64.0 (9.3) | MD 0.2 (−4.3 to 4.7) | 0.105 |
| Gender (%) | ||||
| Male | 15 (55.6) | 44 (60.3) | RD −0.047 (−0.257 to 0.157) | 0.670 |
| Type of resection (%) | ||||
| Pylorus-preserving PD | 23 (85.2) | 62 (84.9) | RD 0.003 (−0.186 to 0.136) | 0.975 |
| ASA classification (%) | ||||
| I | 6 (22.2) | 16 (21.9) | 0.434 | |
| II | 20 (74.1) | 48 (65.8) | ||
| III/IV | 1 (3.7) | 9 (12.3) | ||
| Comorbidity (%) | ||||
| Cardiac disease | 0 (0.0) | 12 (16.4) | RD 0.164 (−0.266 to −0.223) | 0.025 |
| Pulmonary disease | 2 (7.4) | 5 (6.8) | RD 0.006 (−0.092 to 0.170) | 0.923 |
| Diabetes | 7 (25.9) | 14 (19.2) | RD 0.068 (−0.098 to 0.269) | 0.462 |
| Hypertension | 10 (37.0) | 19 (26.0) | RD 0.110 (−0.098 to 0.318) | 0.281 |
| Histologic diagnosis (%) | ||||
| Pancreatic adenocarcinoma | 10 (37.0) | 22 (30.1) | 0.314 | |
| Ampullary adenocarcinoma | 2 (7.4) | 6 (8.2) | ||
| Distal CBD adenocarcinoma | 5 (18.5) | 16 (21.9) | ||
| Other (pre)malignanta | 6 (22.2) | 26 (35.6) | ||
| Other benign | 4 (14.8) | 3 (4.1) | ||
| Vascular resection (%) | 3 (11.1) | 7 (9.6) | 0.822 | |
| Median length of hospital stay in days (IQR) | 8 (7–10 | 15 (10–26) | <0.001 | |
| Median length of ICU stay in days (IQR) | 0 (0–0) | 0 (0–1†) | 0.005 | |
| Severity of complications (%) | ||||
| Grade 1 | 58 (79.5) | |||
| Grade 2 | 13 (17.8) | |||
| Grade 3 | 1 (1.4) | |||
| Grade 4 | 1 (1.4) | |||
| Number of complications (%) | ||||
| 1 | 28 (38.4) | |||
| 2 | 19 (26.0) | |||
| 3 | 10 (13.7) | |||
| 4 | 6 (8.2) | |||
| ≥5 | 10 (13,7) | |||
| Type of complication (%) | ||||
| Post-operative haemorrhage | 12 (16.4) | |||
| Anastomotic leakage | 24 (32.9) | |||
| Isolated delayed gastric emptying | 18 (24.7) | |||
| Post-operative infection (local or systemic) | 33 (45.2) | |||
ASA, American Society of Anaesthesiologists; CBD, common bile duct; IQR, interquartile range; MD, mean difference; PD, pancreatoduodenectomy; RD, risk difference; SD, standard deviation; CI, confidence interval.
Italic values indicate the significance level of P <0.05
Other (pre)malignant: e.g. multiple endocrine neoplasia in the pancreatic head area or duodenal carcinoma.
Range 0–33 days.
There were no significant differences in age, gender, ASA classification, type of surgical treatment and post-operative diagnosis between the groups without or with one or more complications.
Costs related to the occurrence of a complication
The median total hospital costs per patient were €25 047 (IQR 18 430–44 600), whereas the mean total hospital costs were €37 416 (SD 29 814).
Having selected the most severe complication for each patient, 58/73 (79.5%) complications were classified as severity grade 1 (without reoperation), 13/73 (17.8%) as severity grade 2 (with reoperation), one (1/73; 1.4%) as grade 3 (permanent damage or function loss), and one as grade 4 (death). Table2 presents a comparison of hospital costs among patients without complications, those with a grade 1 or a grade 2 complication. The Kaplan–Meier curves for the three study groups are shown in Fig.1. The difference between the cost curves was statistically significant (log-rank test, P < 0.001). In a small proportion of patients, grade 1 and 2 complications caused an increase in the total hospital costs up to €150 000.
Table 2.
Analysis of costs (in Euros) for patients without and with a grade 1 or grade 2 complication after a pancreatoduodenectomy
| Median costs (IQR) | No complication N = 27 | Grade 1 complication N = 58 | Grade 2 complication N = 13 | P-value* | |||
|---|---|---|---|---|---|---|---|
| Total costs | 17 482 | 15 831–20 800 | 28 380 | 21 182–46278 | 57 060 | 40 641–90 454 | <0.001 |
| General diagnostics | 1869 | 1630–2590 | 3363 | 2299–4990 | 4731 | 3978–7195 | <0.001 |
| Imaging | 166 | 0–658 | 764 | 434–2502 | 2051 | 1033–4068 | <0.001 |
| Outpatient clinic | 1211 | 880–2144 | 1325 | 928–2289 | 1950 | 800–2661 | 0.610 |
| Clinical care | 4975 | 4422–6634 | 10 504 | 7608–15 380 | 17 690 | 14 373–36 077 | <0.001 |
| ICU | 0 | 0–0 | 0 | 0–0b | 3950 | 0–7684 | <0.001 |
| Surgical | 7772 | 7772–7772a | 7772 | 7772–7772c | 14 527 | 8674–18 530 | 0.002 |
| Other costs | 942 | 658–1.537 | 1917 | 1015–7589 | 6220 | 4640–20 131 | <0.001 |
IQR, Inter-quartile Range.
Italic values indicate the significance level of P <0.05
Range € 3683–€ 15 544;
Range € 0–€ 42 646;
Range € 3683–43 032,
Kruskal–Wallis test.
Figure 1.
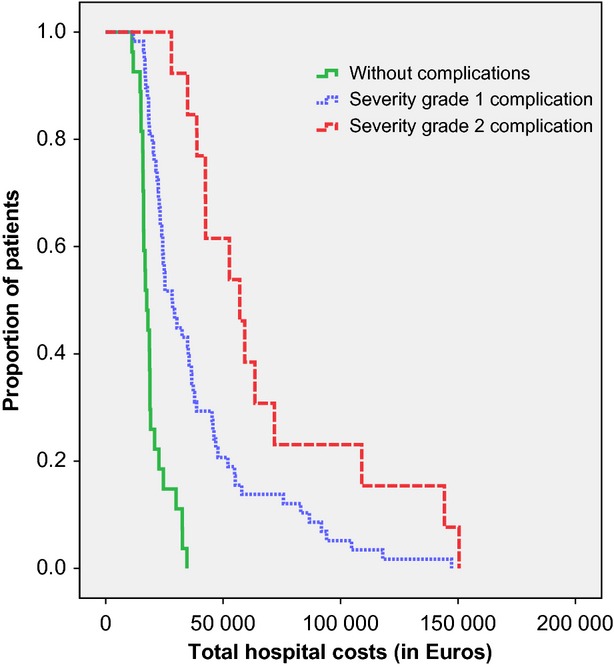
Kaplan–Meier curves of the proportion of patients without a complication or with a grade 1 or grade 2 complication and their total hospital costs
Patients with a grade 2 complication had significantly higher hospital costs (P-values ranged from <0.005 to 0.012; data not shown) than patients with a grade 1 complication in all domains except for the domains ‘general diagnostics’, ‘imaging’ and ‘outpatient clinic’. They also had a significantly longer hospital stay than those with grade 1 complications (medians 29 versus 14 days, respectively; P = 0.002). This was also true for the length of ICU stay (1 versus 0 days, respectively; P = 0.004). Patients with a grade 1 complication had a higher ASA classification (P = 0.007) and more often a history of heart disease (P = <0.001) than patients with grade 2 complications. There were no differences in other comorbidity, age, gender, type of surgical treatment and post-operative diagnosis between the two severity groups (data not shown).
Costs of common complications
Hospital costs of the three selected complications, anastomotic site leakage, isolated DGE and post-operative haemorrhage, are summarised in Table3.
Table 3.
Analysis of costs (in Euros) of the three most common complications after a pancreatoduodenectomy
| Median costs (IQR) | Post-operative haemorrhage N = 12/100 | Anastomotic leakage N = 24/100 | Isolated DGE N = 18/100 | P-value* | |||
|---|---|---|---|---|---|---|---|
| Total costs | 55 623 | 35 825–101489 | 53 760 | 24 449–90 577 | 26 825 | 22 179–39 461 | 0.016 |
| General diagnostics | 5203 | 3398–9092 | 4655 | 3023–9092 | 3907 | 2606–5555 | 0.468 |
| Imaging | 2121 | 783–3791 | 1577 | 729–3791 | 680 | 328–2882 | 0.109 |
| Outpatient clinic | 1370 | 786–1900 | 1370 | 762–2382 | 1386 | 890 –1952 | 0.885 |
| Clinical care | 14 307 | 12 310–35 700 | 17 171 | 9674–30 748 | 12 162 | 7601–15 064 | 0.117 |
| ICU | 5709 | 988–11 555 | 3950 | 0–7793 | 0 | 0–0a | 0.002 |
| Surgical | 12 885 | 7365–15 113 | 8674 | 7772–14.426 | 7772 | 7772–7772b | 0.270 |
| Other costs | 10 298 | 7589–23 098 | 6476 | 1880–10 913 | 1288 | 776–2788 | <0.001 |
Italic values indicate the significance level of P <0.05
IQR, interquartile range.
Range € 0–€ 9660.
Range € 3683–€ 24 567.
Kruskal–Wallis test.
Anastomotic leakage occurred in 24/100 patients. In eight cases, this was in combination with a late post-operative haemorrhage. Patients with an anastomotic leakage had a median length of hospital stay of 26 days (IQR 15–36 days) and 0 days ICU stay (IQR 0–4; range 0–33). There was no need for reoperation in 54.2% of the patients with an anastomotic leakage (N = 13), and accordingly these were classified as severity grade 1. Nine patients were classified as severity grade 2 (37.5%), and one patient was classified as having a grade 3 complication (because this patient needed a permanent ileostomy due to the complication). One patient died after an anastomotic leakage in combination with a late post-operative haemorrhage (grade 4). The median total hospital costs for a patient with an anastomotic leakage were €53 760, more than three times the total hospital costs of a patient without complications.
Patients with isolated DGE (occurring in 18/100 patients, all classified as grade 1) had a median length of hospital stay of 14 days (IQR 10–25) and 0 days ICU stay (IQR 0–0; range 0–4). The median total hospital costs for a patient with an isolated DGE were €26 825, which was only half of the costs for a patient with anastomotic leakage, but still more than 50% higher than the median total hospital costs of €17 482 for a patient without complications.
A post-operative haemorrhage was reported in 12/100 patie-nts. As mentioned before, in eight patients this was in combination with anastomotic leakage. In patients with a post-operative haemorrhage, the median length of hospital stay was 23 days (IQR 13–39 days) and 3 days ICU stay (IQR 0–5 days). There was no need for reoperation in five patients (5/12; 41.7%; severity grade 1) and five patients were classified as severity grade 2 (6/12; 50.0%). The median total hospital costs for a patient with a post-operative haemorrhage were €55 623.
The median hospital costs in the different domains for each of these three specific complications versus the median costs for patients without a complication are summarised in Fig.2. Clinical care and surgical costs contributed mostly to the total hospital costs of complications.
Figure 2.
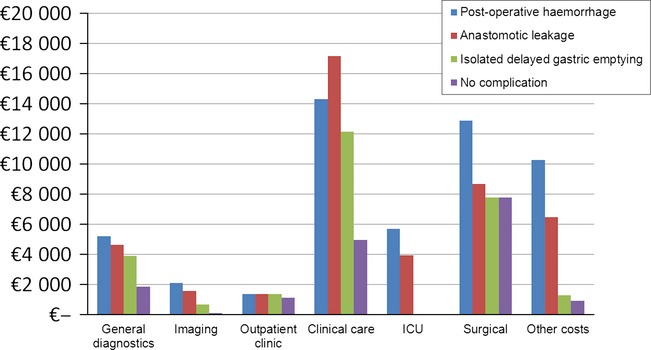
Median costs per domain by the occurrence of a specific complication after a pancreatoduodenectomy
When comparing the costs domains in patients with a post-operative haemorrhage and anastomotic leakage, none of the domains showed statistically significant differences (P-values ranged from 0.164 to 0.830). Compared with patients with an isolated DGE, the cost domains ‘total costs’, ‘ICU’ and ‘other costs’ were significantly higher in the group with a post-operative haemorrhage (P-values 0.004, 0.003, 0.001, respectively) and anastomotic leakage (P-values 0.035, 0.006, 0.003, respectively).
Regression analyses
Results of the univariable and multivariable linear regression analyses are shown in Table4. Significant predictors of total hospital costs were a histological diagnosis, complication severity, post-operative haemorrhage, anastomotic site leakage and the presence of a post-operative infection. A post-operative haemorrhage was associated with a 39.6% increase in total hospital costs. For an average patient, the total hospital costs increased with €11 485 if a post-operative haemorrhage occurred (increase from €28 973 to €40 458). The presence of a malignancy other than a pancreatic adenocarcinoma (e.g. duodenum carcinoma) was also associated with higher total hospital costs (29.4% increase). Furthermore, the occurrence of a grade 1 (34.3% increase) or a grade 2–4 (70.6% increase) complication and the presence of a post-operative infection (32.4% increase) were associated with higher hospital costs. This model explained almost 50% (R2 = 0.479) of the variance in the total hospital costs.
Table 4.
Univariable and multivariable analyses of possible factors predicting total costs
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Ratio for cost increase | 95% CI | P-value | Ratio for cost increase | 95% CI | P-value | |
| Age | 0.996 | 0.984–1.009 | 0.568 | |||
| Gender | 0.873 | 0.680–1.119 | 0.279 | |||
| Operation procedure | ||||||
| PPPD vs. standard PD | 0.882 | 0.626–1.244 | 0.471 | |||
| ASA classification | ||||||
| ASA I | RC | |||||
| ASA II | 0.894 | 0.661–1.209 | 0.462 | |||
| ASA III/IV | 0.772 | 0.608–1.556 | 0.906 | |||
| Histological diagnosis | ||||||
| Pancreatic adenocarcinoma | RC | RC | ||||
| Ampullary adenocarcinoma | 0.992 | 0.627–1.570 | 0.973 | 0.902 | 0.624–1.319 | 0.584 |
| Distal CBD adenocarcinoma | 1.419 | 1.024–1.966 | 0.036 | 1.130 | 0.622–1.493 | 0.381 |
| Other (pre)malignanta | 1.610 | 1.203–2.153 | 0.002 | 1.294 | 1.019– 1.644 | 0.035 |
| Other benign | 0.919 | 0.566–1.491 | 0.730 | 0.881 | 0.585–1.324 | 0.538 |
| Vascular resection | 0.760 | 0.506–1.140 | 0.183 | |||
| Complication severity | ||||||
| No complication | RC | RC | ||||
| Grade 1 | 1.723 | 1.355–2.190 | <0.001 | 1.343 | 1.050–1.714 | 0.019 |
| Grade 2, 3 or 4 | 2.849 | 2.061–3.940 | <0.001 | 1.706 | 1.167–2.495 | 0.006 |
| Readmission within 30 days | 1.300 | 0.881–1.917 | 0.184 | |||
| Post-operative haemorrhage | 2.163 | 1.531–3.055 | <0.001 | 1.396 | 1.002–1.950 | 0.049 |
| Anastomotic leakage | 1.892 | 1.462–2.449 | <0.001 | 1.253 | 0.957–1.637 | 0.099 |
| Delayed gastric emptying | 1.203 | 0.887–1.632 | 0.231 | |||
| Post-operative Infection (local or systemic) | 1.833 | 0.887–2.394 | <0.001 | 1.324 | 1.057–1.660 | 0.015 |
| Co morbidity | ||||||
| Heart diseaseb | 1.133 | 0.777–1.652 | 0.513 | |||
| Pulmonary diseasec | 1.057 | 0.653–1.710 | 0.820 | |||
| Diabetes | 0.932 | 0.690–1.261 | 0.647 | |||
| Hypertension | 0.879 | 0.672–1.151 | 0.346 | |||
ASA, American Society of Anesthesiologists; CBD, common bile duct; RC, reference category; PD, pancreatoduodenectomy; PPPD, pylorus-preserving pancreatoduodenectomy; CI, confidence interval.
Other (pre)malignant: e.g. multiple endocrine neoplasia in the pancreatic head area or duodenal carcinoma.
Including a history of angina pectoris, heart failure, myocardial infarction or an arrhythmia.
Including a history of asthma, chronic obstructive pulmonary disease (COPD) or pulmonary tuberculosis.
Discussion
This study showed that the total hospitals cost after a PD double if a complication occurs. In the case of a complication requiring a re-operation to recover, the total hospital costs even triple. Furthermore, the occurrence of a post-operative haemorrhage is independently associated with a 39.6% cost increase, mainly owing to the increased hospital stay. Numerous ways to reduce the length of hospital stay are currently described.27,28 Length of hospital stay can be influenced by implementing specific protocols or programmes or through cooperation with other hospitals and skilled nursing facilities.29,30 Prevention and early diagnosis of complications could also contribute to a reduction of the length of hospital stay by the implementation of the several evidence-based bundles for post-operative wound infection, pneumonia or sepsis.31
Additionally, we found that high hospital costs after a PD are associated with diagnosis, severity grade of a complication and the presence of a post-operative infection. A diagnosis other than a pancreatic adenocarcinoma, for example multiple endocrine neoplasia (MEN) in the pancreatic head area, duodenal carcinoma or suspected adenomas most of whom were likely to have had a ‘soft pancreas’ because there was no obstruction of the pancreatic duct, was a predictor of high hospital costs. In a soft pancreas, the management of complications is usually more difficult and consists of more expensive diagnostic procedures and interventions.
As a possibly feasible option in the future management of complications we recommend first optimal and early diagnostic work-up in patients with clinical post-operative problems; second, if complications are diagnosed, early intervention by non-operative procedures, becaus e these are less costly than operative interventions and showed no differences in the success rates in a previous study and will lead to a shorter hospital stay.32
Age and ASA classification did not seem to be associated with hospital costs. This is in contrast with other studies showing that these pre-operatively identifiable factors are associated with an increased risk for a complication especially with age > 70–75 years or an ASA classification of II or higher.33–37 These discrepancies could be attributed to the selection of patients for PD. Only patients in a good pre-operative performance state were accepted for surgery, and they were not excluded only based on age. This selection of patients is reflected in the fact that few patients classified as ASA III/IV (10%) were included in this study. A recent study supported our findings and also showed no association between age and morbidity after a PD.38
Because complications result in high hospital costs, it is obvious that cost savings could be achieved by reducing the incidence of complications after a PD. However, it remains difficult to act upon the presence of predicting factors for complications. In this study, predicting factors such as malnutrition and pre-operative cholangitis were not taken into account because the decision to perform surgery is based on other medical grounds and cholangitis was treated pre-operatively by antibiotics and drainage. Therefore, selection could have influenced the outcome of these predicting factors.
Previous studies showed the relationship between high hospital-volume and surgeon-volume on a lower incidence of complications and quality of care.11,39–41 It seems fair to say that cost savings can be achieved nation-wide by performing pancreatic surgery only in high-volume hospitals and by experienced surgeons.21
The overall complication rate in the present study was higher than in previous reports from our hospital as well as from other contemporary studies despite a 1% mortality.9–12 This is most likely because the patient sample from the present study contains mostly patients with a diagnosis different than pancreatic adenocarcinoma leading to more complications. Furthermore, a broader definition of a complication was used in this study, and various resources were checked to track all complications that had occurred.26 The registry also includes mild complications, such as a slight electrolyte imbalance without any clinical consequence or any form of delirium after surgery.
Some limitations to our study are worth mentioning. First, the data obtained were from consecutive patients from a single tertiary university hospital during an 18-month admission period. Therefore, the generalisability regarding costs to other hospitals or time periods is unclear. However, currently the majority of (PP)PD procedures is performed in high-volume tertiary referral hospitals. Second, we derived the costs of complications by top-down comparing hospital costs in different patient groups rather than directly attributing bottom-up which hospital resources were spent on the management of each complication. Third, we have tried to include only the hospital costs that were directly related to the PD. However, it is possible that we missed some hospital costs because they seemed not directly related (e.g. visits to the ophthalmology outpatient clinic) to the PD but might have been in reality, or vice versa. It is not likely this would have influenced our results and conclusions substantially because these additional hospital costs will be only minor. Fourth, we did not have follow-up data on potential complication-related readmissions that took place in a hospital elsewhere. However, their number would be low or even zero, because patients who underwent a PD are well informed about possible post-operative complications and the importance of returning to the index hospital.
Conclusion
By providing an in-depth analysis of hospital costs owing to complications after pancreatic surgery, the impact of complications expressed as costs are identified and clarified. With this knowledge, we can and will advocate further efforts to reduce hospital costs by shortening the length of hospital stay by implementing specific protocols or programmes or through cooperation with other hospitals and skilled nursing facilities. Inherently, efforts should be made to reduce the cost by making efforts to prevent complications and reduce the length of stay, not only to facilitate cost containment in surgical care but also to improve the quality of patient care.
Acknowledgments
We thank Mr R.N.M. Vollebregt, MSc (Department of Quality Assurance & Process Innovation of the Academic Medical Center) for his contribution to the cost data collection and Ms J.A.M.G. Tol, MD (Department of Surgery of the Academic Medical Center) for her contribution to the clinical data collection.
Funding sources
None.
Conflicts of interest
None declared.
References
- Brown ML, Fireman B. Evaluation of direct medical costs related to cancer. J Natl Cancer Inst. 1995;87:399–400. doi: 10.1093/jnci/87.6.399. [DOI] [PubMed] [Google Scholar]
- Meropol NJ, Schulman KA. Cost of cancer care: issues and implications. J Clin Oncol. 2007;25:180–186. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- van Elk R, Mot E, Franses PH. Modeling healthcare expenditures: overview of the literature and evidence from a panel time-series model. Expert Rev Pharmacoecon Outcomes Res. 2010;10:25–35. doi: 10.1586/erp.09.72. [DOI] [PubMed] [Google Scholar]
- Forster AJ, Kyeremanteng K, Hooper J, Shojania KG, van Walraven C. The impact of adverse events in the intensive care unit on hospital mortality and length of stay. BMC Health Serv Res. 2008;8:259. doi: 10.1186/1472-6963-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal R, Bates DW, Franz C, Soukup JR, Rothschild JM. Costs of adverse events in intensive care units. Crit Care Med. 2007;35:2479–2483. doi: 10.1097/01.CCM.0000284510.04248.66. [DOI] [PubMed] [Google Scholar]
- Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868–1874. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- Zoucas E, Lydrup ML. Hospital costs associated with surgical morbidity after elective colorectal procedures: a retrospective observational cohort study in 530 patients. Patient Saf Surg. 2014;8:2. doi: 10.1186/1754-9493-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med. 2006;21:177–180. doi: 10.1111/j.1525-1497.2006.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouma DJ, Nieveen van Dijkum EJ, Obertop H. The standard diagnostic work-up and surgical treatment of pancreatic head tumours. Eur J Surg Oncol. 1999;25:113–123. doi: 10.1053/ejso.1998.0612. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Howell AM, Panesar SS, Burns EM, Donaldson LJ, Darzi A. Reducing the burden of surgical harm: a systematic review of the interventions used to reduce adverse events in surgery. Ann Surg. 2014;259:630–641. doi: 10.1097/SLA.0000000000000371. [DOI] [PubMed] [Google Scholar]
- de Vries EN, Prins HA, Crolla RM, den Outer AJ, van Andel G, van Helden SH, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010;363:1928–1937. doi: 10.1056/NEJMsa0911535. [DOI] [PubMed] [Google Scholar]
- Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA. Complications and costs after high-risk surgery: where should we focus quality improvement initiatives? J Am Coll Surg. 2003;196:671–678. doi: 10.1016/S1072-7515(03)00122-4. [DOI] [PubMed] [Google Scholar]
- de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg. 2005;92:1117–1123. doi: 10.1002/bjs.5047. [DOI] [PubMed] [Google Scholar]
- Atema JJ, Eshuis WJ, Busch OR, van Gulik TM, Gouma DJ. Association of preoperative symptoms of gastric outlet obstruction with delayed gastric emptying after pancreatoduodenectomy. Surgery. 2013;154:583–588. doi: 10.1016/j.surg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Short MN, Aloia TA, Ho V. The influence of complications on the costs of complex cancer surgery. Cancer. 2014;120:1035–1041. doi: 10.1002/cncr.28527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- Marang-van de Mheen PJ, Kievit J. Automated registration of adverse events in surgical patients in the Netherlands: the current status. Ned Tijdschr Geneeskd. 2003;147:1273–1277. [PubMed] [Google Scholar]
- Goslings JC, Gouma DJ. What is a surgical complication? World J Surg. 2008;32:952. doi: 10.1007/s00268-008-9563-3. [DOI] [PubMed] [Google Scholar]
- Ubbink DT, Visser A, Gouma DJ, Goslings JC. Registration of surgical adverse outcomes: a reliability study in a university hospital. BMJ Open. 2012;2:e000891. doi: 10.1136/bmjopen-2012-000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800–809. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- Borghans I, Kool RB, Lagoe RJ, Westert GP. Fifty ways to reduce length of stay: an inventory of how hospital staff would reduce the length of stay in their hospital. Health Policy. 2012;104:222–233. doi: 10.1016/j.healthpol.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Miller TE, Thacker JK, White WD, Mantyh C, Migaly J, Jin J, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118:1052–1061. doi: 10.1213/ANE.0000000000000206. [DOI] [PubMed] [Google Scholar]
- Rotter T, Kinsman L, James E, Machotta A, Willis J, Snow P, et al. The effects of clinical pathways on professional practice, patient outcomes, length of stay, and hospital costs: Cochrane systematic review and meta-analysis. Eval Health Prof. 2012;35:3–27. doi: 10.1177/0163278711407313. [DOI] [PubMed] [Google Scholar]
- Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479–2485. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- Tol JA, Busch OR, van Delden OM, van Lienden KP, van Gulik TM, Gouma DJ. Shifting role of operative and nonoperative interventions in managing complications after pancreatoduodenectomy: what is the preferred intervention? Surgery. 2014;156:622–631. doi: 10.1016/j.surg.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Adham M, Bredt LC, Robert M, Perinel J, Lombard-Bohas C, Ponchon T, et al. Pancreatic resection in elderly patients: should it be denied? Langenbecks Arch Surg. 2014;399:449–459. doi: 10.1007/s00423-014-1183-9. [DOI] [PubMed] [Google Scholar]
- Kneuertz PJ, Pitt HA, Bilimoria KY, Smiley JP, Cohen ME, Ko CY, et al. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg. 2012;16:1727–1735. doi: 10.1007/s11605-012-1938-y. [DOI] [PubMed] [Google Scholar]
- Veltkamp SC, Kemmeren JM, van der Graaf Y, Edlinger M, van der Werken C. Prediction of serious complications in patients admitted to a surgical ward. Br J Surg. 2002;89:94–102. doi: 10.1046/j.0007-1323.2001.01963.x. [DOI] [PubMed] [Google Scholar]
- Gupta H, Gupta PK, Fang X, Miller WJ, Cemaj S, Forse RA, et al. Development and Validation of a Risk Calculator Predicting Postoperative Respiratory Failure. Chest. 2011;140:1207–1215. doi: 10.1378/chest.11-0466. [DOI] [PubMed] [Google Scholar]
- Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the Patient Safety in Surgery Study. J Am Coll Surg. 2007;204:1178–1187. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–2135. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- Ho V, Aloia T. Hospital volume, surgeon volume, and patient costs for cancer surgery. Med Care. 2008;46:718–725. doi: 10.1097/MLR.0b013e3181653d6b. [DOI] [PubMed] [Google Scholar]
- Kennedy TJ, Cassera MA, Wolf R, Swanstrom LL, Hansen PD. Surgeon volume versus morbidity and cost in patients undergoing pancreaticoduodenectomy in an academic community medical center. J Gastrointest Surg. 2010;14:1990–1996. doi: 10.1007/s11605-010-1280-1. [DOI] [PubMed] [Google Scholar]
- Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RA, et al. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485–494. doi: 10.1002/bjs.7413. [DOI] [PubMed] [Google Scholar]


