Abstract
Background
Centralization of a pancreatoduodenectomy (PD) leads to a lower post-operative mortality, but is unclear whether it also leads to improved radical (R0) or overall resection rates.
Methods
Between 2004 and 2009, pathology reports of 1736 PDs for pancreatic and peri-ampullary neoplasms from a nationwide pathology database were analysed. Pre-malignant lesions were excluded. High-volume hospitals were defined as performing ≥ 20 PDs annually. The relationship between R0 resections, PD-volume trends, quality of pathology reports and hospital volume was analysed.
Results
During the study period, the number of hospitals performing PDs decreased from 39 to 23. High-volume hospitals reported more R0 resections in the pancreatic head and distal bile duct tumours than low-volume hospitals (60% versus 54%, P = 0.035) although they operated on more advanced (T3/T4) tumours (72% versus 58%, P < 0.001). The number of PDs increased from 258 in 2004 to 394 in 2009 which was partly explained by increased overall resection rates of pancreatic head and distal bile duct tumours (11.2% in 2004 versus 17.5% in 2009, P < 0.001). The overall reported R0 resection rate of pancreatic head and distal bile duct tumours increased (6% in 2004 versus 11% in 2009, P < 0.001). Pathology reports of low-volume hospitals lacked more data including tumour stage (25% versus 15%, P < 0.001).
Conclusions
Centralization of PD was associated with both higher resection rates and more reported R0 resections. The impact of this finding on overall survival should be further assessed.
Introduction
Pancreatic adenocarcinoma is the fourth cause of cancer mortality worldwide with nearly 37 000 deaths annually in the United Stated and 78 000 deaths in the European Union.1 As a result of the late occurrence of symptoms and the aggressive tumour biology, less than 20% of patients are suitable candidates for curative surgery.1 The median survival after an R0 resection in these patients is 22 months whereas the survival for non-resectable patients is only 14 months.2
A number of studies have outlined the relationship between hospital volume, post-operative mortality, and survival for patients undergoing surgery for pancreatic and peri-ampullary neoplasms.3–12 It has been postulated that both short- and long-term improvements in the outcome of a pancreatic resection are mainly as a result of improvements in the quality of surgery and peri-operative care.7 In patients with pancreatic ductal adenocarcinoma (PDAC), the ratio of nodal involvement and, to a somewhat lesser extent, the negative resection margin (R0) are the most important prognostic factors for long-term survival. It has been suggested that high-volume hospitals achieve more R0 resections but data are lacking.13–15 Some have suggested that supporting specialities, such as pathology, may also be of better quality in high-volume hospitals16 but evidence is lacking.
In the Netherlands, a recent nationwide guideline led to the implementation of a minimum volume of 20 annual procedures for high-risk surgery such as a pancreateoduodenectomy (PD). A recent analysis demonstrated that in the period 2005 to 2009 centralization occurred in the Netherlands, which resulted in a decreased post-operative mortality from 9.8% to 5.1%.5 Two additional studies showed that both in the south and the west of the Netherlands, centralization was associated with an increased 2-year survival.7,8 It is unknown which factors have contributed to the increased survival after the centralization of PD. Does detection bias play a role, or is the surgical technique somehow improved, leading to an increase in radical (R0) resections?
The aim of the present work was to analyse the impact of centralization of PD in the period 2004–2009 in the Netherlands on resection rates and reported R0 resections of pancreatic and peri-ampullary neoplasms and the quality of pathology reports.
Methods
The summaries of the pathology reports of all the PDs carried out in the Netherlands between 2004 and 2009 (6 years) were obtained from the Dutch nationwide pathology database (PALGA).17 After 2009, these data were no longer collected for all hospitals by PALGA. Only patients who underwent a PD for pancreatic head or a peri-ampullary neoplasm [including pancreatic, cholangio, ampullary and duodenum adenocarcinoma, neuroendocrine tumours, malignant intraductal papillary mucinous neoplasm (IPMN) and mucinous cystadenocarcinoma] were included. PDs carried out for benign or pre-malignant disease, metastases to the pancreas and peri-ampullary region (such as renal cell carcinoma) or (auto-immune) pancreatitis, total pancreatectomies and left-sided pancreatectomies, or reports which did not mention the tumour type nor the type of surgery were excluded. Finally, data on the number of pancreas, ampulla, duodenum and distal bile duct tumours in the Netherlands for the period 2004–2009 were collected from the Netherlands Cancer Registry managed by the Comprehensive Cancer Centre Netherlands (CCCNL).18
Variables
The following variables were recorded for each entry: age, gender, year of surgery, hospital volume, radicality of the resection, tumour size, histological type, pTN status, tumour differentiation, ingrowth in surrounding organs and the number of resected lymph nodes. If the pTN status was not stated in the report, it was classified according to the AJCC criteria, 7th edition19 but only if enough information, such as ingrowth in surrounding organs and/or tumour size was available.
If a report presented two different malignant tumours, the one described as a chance finding was excluded. If a malignant and a benign tumour were reported on the same specimen, the malignant tumour was recorded.
The quality of the pathology reports was assessed by measuring the percentage of missing data, such as the pTN status, tumour diameter, differentiation and radicality of the resection in the summaries of the pathology reports.
During this study period, neoadjuvant treatment (chemotherapy or chemoradiotherapy) was used in less than 1% of patients.
Definitions
The PD volume of each hospital was assessed per year; centres with ≥ 20 PDs per year were defined as high-volume hospitals.5 The old definition of resection margins were used as that was the definition during the study period. According to the old definition an R0 resection is the absence of cancer cells in the pancreatic or circumferential resection margin, and an R1 is the presence of microscopic tumour invasion at the resection margin. If there was any doubt as to the radicality of the excision or if this was not stated in the report an unknown resection margin was recorded. Reports using the new definition were removed.
Tumours were divided according to histological type. Carcinomas in situ, and adenomas or neoplasms with high-grade dysplasia (which were defined as carcinoma in situ), IPMNs (without invasive growth) and low-grade or moderate dysplasia, pseudo-papillary tumours, and mucinous cystadenomas were considered were considered to be premalignant. All neuroendocrine tumours, indifferent of grade were (arbitrarily) included in the malignant tumour group. If the histological type was not named, but the description was that of a malignant tumour, this was marked as unknown. Specimens with unknown histological type were only included in the calculations of the trends.
For data analysis, the various histology types of malignant tumours were pooled according to the anatomic origin of the tumour: pancreatic head, peri-ampullary and distal bile duct tumours. Pancreatic head tumours included pancreatic adenocarcinoma, invasive IPMN=s and neuroendocrine tumours of the pancreatic head. Peri-ampullary tumours included all papilla and duodenum tumours, such as adenocarcinomas, neuro-endocrine tumours and any remaining histological types such as sarcomas and lymphomas. Distal bile duct tumours included adenocarcinomas and neuro-endocrine tumours of the distal bile ducts. Pancreatic head tumours were analysed together with distal bile duct tumours. This was based on the assumption that when distal bile duct tumours invade the pancreas it is often difficult to assess the origin of the primary tumour (i.e. the pancreas or in the distal bile duct) and as a result of this patients will often undergo similar surgical procedures.
The pT stage was pooled for all further analysis into four categories: high stage, low stage, unknown stage (Tx) and NA (not applicable). The high stage included all T3/T4 tumours, the low stage included all T1/T2s tumours and NA included several tumours for which no pTNM staging system was available, such as lymphomas and gastro-intestinal stroma cell tumours (GIST).
The differentiation of malignant tumours was graded as poor, moderate, well and unknown. If more than one differentiation degree was stated in the report (i.e. a well to moderately differentiated tumour), the worst differentiation degree was registered.
Statistical analysis
Continuous non-normally distributed variables were compared using the Mann–Whitney U-test while normally distributed variables were compared using the t-test. Categorical variables were compared with the Chi-squared test. Resectability rates were calculated by dividing the total number of PDs (R0, R1/R2 and unknown resection margins) by the total number of tumours per anatomic localization per year as provided by the Comprehensive Cancer Centre The Netherlands (CCCNL). R0 resectability rates were calculated by dividing the number of R0 resection margins by the total number of tumours per anatomic localization per year. In order to analyse any changes throughout the study period, the difference between the total and R0 resection rates in 2004 and 2009 were calculated. This was achieved by applying the Z-test for the difference between two percentage means. A two-tailed P-value of < 0.05 was considered statistically significant. All calculations were carried out in IBM SPSS Statistics (version 16.0, SPSS Inc, Chicago, IL, USA).
Results
A total of 2180 pathology reports were obtained, out of which 444 (20%) reports were excluded for the following reasons: 81 premalignant tumours (4%), 174 pancreatitis (8%), 65 benign tumours (3%), 26 reports in which no histological abnormalities were observed (0.01%), 19 metastases (0.9%), 13 pancreatic tail tumours (0.6%), 2 gallbladder carcinomas (0.09%), 4 reports that did not mention the tumour type or type of surgery (0.2%), 60 reports (3%) that stated the resection margin only according to the new definition, thus leaving a total of 1736 pathology reports for analysis.
Centralization and trends
During the study period, centralization of pancreatic surgery occurred: the number of hospitals performing PDs decreased from 39 to 23 (out of a total of 95 hospitals in the Netherlands; 16.7 million inhabitants), whereas the number of high-volume hospitals increased from 3 to 9. The total volume of PDs for malignant tumours increased from [250 in 2004 to 389 in 2009 (56% relative increase)]. According to data from CCCNL, this increase is partly explained by increased overall resection rates of pancreatic head and distal bile duct tumours (11.2% in 2004 versus 17.5% in 2009, P < 0.001). During this period, the overall reported R0 resection rate also increased from 6.3% in 2004 to 10.5% in 2009, P < 0.001 (Fig.1).
Figure 1.
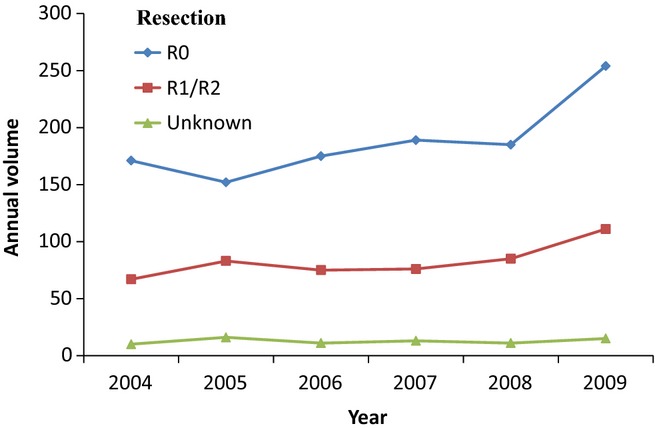
Annual trends in volume of pancreatoduodenectomy in all malignant tumors per resection margin in the Netherlands (2004–2009)
Comparison of patient and tumour characteristics by hospital volume are shown in Tables1 and 2.
Table 1.
Characteristics of pancreatoduodenectomies (2004–2009) for malignant tumours
| High-volume (N = 747) | Low-volume (N = 989) | P-value | |
|---|---|---|---|
| Age, mean (SD) | 64.3 (10.39) | 64.4 (9.6) | 0.831 |
| Gender, male (%) | 435 (58.1) | 596 (60.1) | 0.386 |
| Pancreatic and distal bile duct tumours, number (%) | 500 (66.9) | 653 (66.0) | 0.653 |
| Peri-ampullary tumours, number (%) | 229 (30.7) | 317 (32.1) | 0.535 |
| Unknown tumour type, number (%) | 18 (2.4) | 19 (1.90) | 0.485 |
| T1/T2, number (%) | 144 (19.3) | 203 (20.5) | 0.519 |
| T3/T4, number (%) | 491 (65.7) | 538 (54.4) | <0.001 |
| Unknown pT Stage, number (%) | 111 (14.9) | 245 (24.8) | <0.001 |
| Not applicable, number (%) | 1 (0.1) | 3 (0.3) | 0.466 |
| N0, number (%) | 302 (40.4) | 450 (45.5) | 0.038 |
| N1, number (%) | 424 (56.8) | 471 (47.6) | <0.001 |
| Unknown pN Stage, number (%) | 21 (2.8) | 68 (6.9) | <0.001 |
| R0, number (%) | 512 (68.5) | 641 (64.8) | 0.103 |
| R1/R2, number (%) | 208 (27.8) | 297 (30.0) | 0.343 |
| Unknown resection margin, number (%) | 27 (3.6) | 51 (5.2) | 0.125 |
| Poorly differentiated, number (%) | 235 (31.5) | 243 (24.6) | 0.001 |
| Moderately differentiated, number (%) | 307 (41.1) | 470 (47.5) | 0.008 |
| Well differentiated, number (%) | 73 (9.8) | 114 (11.5) | 0.243 |
| Unknown, number (%) | 132 (17.7) | 162 (16.4) | 0.478 |
Table 2.
Tumour stage and differentiation in subtypes of malignant tumours
| Tumour stage in subtypes of malignant tumours | |||
|---|---|---|---|
| Pancreatic head and distal bile duct tumours | High-volume (N = 500) | Low-volume (N = 653) | P-value |
| High stage, number (%) | 360 (72.0) | 380 (58.2) | <0.001 |
| Low stage, number (%) | 49 (9.8) | 82 (12.6) | 0. |
| Unknown pT Stage, number (%) | 90 (18.0) | 190 (29.1) | <0.001 |
| NA, number (%) | 1 (0.2) | 1 (0.2) | 0.850 |
| Peri-ampullary tumours | High-volume (N = 229) | Low-volume (N = 317) | P-value |
|---|---|---|---|
| High stage, number (%) | 123 (53.7) | 153 (48.3) | 0.209 |
| Low stage, number (%) | 95 (41.5) | 118 (37.2) | 0.144 |
| Unknown pT stage, number (%) | 11 (4.8) | 44 (13.9) | 0.001 |
| NA, number (%) | 0 (0) | 2 (0.6) | 0.229 |
| Tumour differentiation in all subtypes of malignant tumors | |||
|---|---|---|---|
| Pancreatic head and distal bile duct tumours | High-volume (N = 500) | Low-volume (N = 653) | P-value |
| Poorly differentiated, number (%) | 173 (34.6) | 163 (25.0) | <0.001 |
| Moderately differentiated, number (%) | 186 (37.2) | 308 (47.2) | 0.001 |
| Well differentiated, number (%) | 46 (9.2) | 85 (13.0) | 0.043 |
| Unknown differentiation, number (%) | 95 (19.0) | 97 (14.9) | 0.061 |
| Peri-ampullary tumours | High-volume (N = 229) | Low-volume (N = 317) | P-value |
|---|---|---|---|
| Poorly differentiated, number (%) | 56 (24.5) | 76 (24.0) | 0.897 |
| Moderately differentiated, number (%) | 112 (48.9) | 152 (47.2) | 0.825 |
| Well differentiated, number (%) | 25 (10.9) | 29 (9.1) | 0.494 |
| Unknown differentiation, number (%) | 36 (15.7) | 60 (18.9) | 0.331 |
NA, not applicable.
High-volume hospitals reported to resect more lymph nodes per procedure [median (IQR, interquartile range): 10 (6.00–14.00) versus 7 (4.00–11.00), P < 0.001]. High-volume hospitals operated on significantly more N1 cancers than low-volume hospitals (Table1). This was due to more N1 pancreatic head and distal bile duct tumours (61.2% versus 52.2%, P = 0.002), but not in peri-ampullary tumours.
The resection margin status by hospital type and tumour subtype are shown in Table3.
Table 3.
Resection margins for subtypes of malignant tumours
| Pancreatic head and distal bile duct tumours | High-volume (N = 500) | Low-volume (N = 653) | P-value |
|---|---|---|---|
| R0, number (%) | 299 (59.8) | 350 (53.6) | 0.035 |
| R1/R2, number (%) | 183 (36.6) | 268 (41.0) | 0.126 |
| Unknown R margin, number (%) | 18 (3.6) | 35 (5.4) | 0.157 |
| Peri-ampullary | High-volume (N = 229) | Low-volume (N = 317) | P-value |
|---|---|---|---|
| R0, number (%) | 200 (87.3) | 277 (87.4) | 0.987 |
| R1/R2, number (%) | 20 (8.7) | 26 (8.2) | 0.825 |
| Unknown R margin, number (%) | 9 (3.9) | 14 (4.4) | 0.780 |
The frequency of missing data from pathology reports by hospital type is shown in Table4.
Table 4.
Missing data in pathology reports for all malignant tumours
| High-volume (N = 747) | Low-volume (N = 989) | Total (N = 1736) | P-value | |
|---|---|---|---|---|
| Unknown R margin, number (%) | 19 (2.5) | 29 (2.9) | 48 (2.8) | 0.625 |
| Unknown pT stage, number (%) | 111 (14.9) | 245 (24.8) | 356 (20.5) | <0.001 |
| Unknown diameter, number (%) | 128 (17.1) | 254 (25.7) | 382 (22.0) | <0.001 |
| Unknown differentiation, number (%) | 132 (17.2) | 163 (16.2) | 295 (16.6) | 0.596 |
| Unknown pN stage, number (%) | 21 (2.8) | 68 (6.9) | 89 (5.1) | <0.001 |
Discussion
This study has shown that during a period where centralization of pancreatic surgery took place both the overall and reported R0 resection rates of pancreatic head and distal bile duct tumours increased. High-volume hospitals more frequently reported R0 resections in tumours of the pancreatic head and distal bile duct, even although they operated on more advanced (T3/T4) tumours. Additionally, high-volume hospitals reported more resected lymph nodes and the pathology reports were less often incomplete.
These findings are probably best explained by the additional surgical expertise in high-volume hospitals, despite operating on more advanced cancers. With additional experience, surgeons can resect more tumours and possibly also achieve higher R0 resection rates. It has previously been shown that high-volume hospitals are more likely to resect tumours in pancreatic cancer.20–22 Additionally, Michalski et al.16 showed that 42% of the patients who were given an initial diagnosis of unresectability as a result of venous involvement in lower volume hospitals were resected at a large tertiary referral centre with a 55% R0 resection rate. This phenomenon probably also occurred in the Netherlands. Because the pathology reports in the current study provided too little information no trends in portomesenteric vein resections could be performed.
In the present study, high-volume hospitals did not report more R0 resections in peri-ampullary tumours. Peri-ampullary tumours usually present at an earlier stage and are less bulky than pancreatic head tumours. It might, therefore, be easier to remove radically them than it is to remove pancreatic head tumours. This is reflected in the higher percentage of R0 resections in papilla and duodenum, as compared with pancreatic head and distal bile duct tumours, respectively, 82–100%,23–27 46–90%,25,27 41–88%24,25,27,28 and 22–80%.24,25,27,29 It should be taken in to account that the reported R0 resection rate in the present study is clearly influenced by the (old) definition used, not requiring a 1-mm-free margin.
Lastly, in this study most of the distal bile duct tumours were either a T3/T4 or an unknown stage, therefore, pancreatic head and distal bile duct tumours were analysed as one group. This was based on the assumption that when distal bile duct tumours invade the pancreas it is often difficult to assess the origin of the primary tumour (i.e. the pancreas or in the distal bile duct) and therefore patients will often undergo similar surgical procedures.
The effect of centralization on the reported R0 resection rates has been scarcely analysed: a recent systematic review10 has shown that institutions with a minimum of 12 PDs per year achieve higher R0 rates in pancreatic adenocarcinoma than in centres with lower volumes. This study did, however, not assess overall resection rates. The achievement of an R0 resection margin is one of the most important prognostic factors in the treatment of pancreatic head tumours,13–15 thus the current results suggest that patients treated in high-volume hospitals may have a better long-term survival in patients with pancreatic head tumours than those treated in low-volume hospitals. This is confirmed by two recent studies8,11 that showed that centralization improves the 2- and 3-year survival after surgery for pancreatic head tumours.
This study does not show any improvement in the resection rates of peri-ampullary tumours. To the author=s knowledge, no other study has analysed the effect of PD volume on the achievement of R0 resections of peri-ampullary tumours. One study, however, has shown that centralization improves the 2-year survival after surgery in peri-ampullary tumours.9 Besides a negative resection margin,26,27,30 other quoted prognostic factors include: lymph node invasion,23,24,27,30 the use of adjuvant chemotherapy,28 tumour recurrence24 and histopathological factors, such as invasion in the pancreas,23 poor differentiation28 and perineural invasion.15 Birkmeyer et al.12 showed that patients undergoing pancreatic surgery at high-volume hospitals were more likely to have pre-operative stress tests, invasive peri-operative monitoring and consultation with medical or radiation oncologists compared with patients in low-volume hospitals. Previous studies also showed that high-volume hospitals were more likely to apply multi-modality therapy in pancreatic adenocarcinoma.21–23 It is, therefore, possible that the better supportive care and the use of adjuvant chemotherapy may play a role in the improvement of the long-term prognosis of peri-ampullary as pancreatic head tumours in high-volume institutions.
The present study also shows that pathologist in high-volume hospitals report more lymph nodes during a PD than low-volume institutions do and that pathology reports in low-volume institutions lack essential data, such as the pT-, pN stage and tumour diameter. The optimal histopathological staging of regional lymph nodes for pancreatic and ampullary cancer should include at least 10 lymph nodes, for distal bile duct tumours this number is 3.14 Previous literature has suggested that the analysis of more lymph nodes is an independent prognostic factor in the long-term survival.31–34 Two randomized controlled trials29,35 have shown that extended retroperitoneal lymphadenectomy does not prolong survival but increases morbidity. It is unclear whether the higher mean number of lymph nodes detected per PD in high-volume hospitals reflects better surgical or better pathology techniques in these hospitals (or both): surgeons potentially remove more lymph nodes per procedure and pathologists potentially identify more lymph nodes in resection specimens.
This does also reflect the importance of ‘high-volume pancreatic pathologists’. It is unlikely that pathologists will gain sufficient experience when they only occasionally assess a pancreatoduodenectomy specimen. One suggested solution to this is central reviewing of pathology samples from several hospitals as in this way experience can be bundled and expertise obtained.
The results of this study should be interpreted in light of some limitations, which are mainly the high percentage of missing data in the pathology reports, such as tumour stage (20%) and size (23%). The distribution of the data was also skewed: there are more missing data for tumour stage and size in low- than in high-volume hospitals (25% versus 15%, P < 0.001 and 26% versus 17%, P < 0.001, respectively). Importantly, a recent study36 has shown that owing to an improved, standardized pathological staging system the R1 resection rate increased from 14% to 76%, suggesting that an improvement in experience leads to the detection of more positive resection margins. Additionally, the Leeds protocol37 that includes multicolour margin staining, axial slicing and extensive tissue sampling also improves the reporting of R1 resection margins. It is, therefore, possible that some of the observed differences in the resection rates of high and low-volume hospitals may be in fact be underestimated. This study underlines the importance of centralization of pancreatic surgery: it improves not only surgical expertise but also that of supporting specializations.
Another limitation of this study is the lack of correlation with post-operative mortality and survival data. However, multiple previous Dutch studies have already shown that throughout the same period (2005–2009) post-operative mortality decreased (from 9.8% to 5.1%) whereas the 2-year survival increased.7,8
In conclusion, in a period where centralization of pancreatic surgery occurred, the annual volume of PDs carried out for malignant tumours in the Netherlands increased by 56%. This is largely explained by higher resection rates. Although high-volume hospitals operated on more advanced and poorly differentiated pancreatic tumours, they reported more R0 resections in tumours of the pancreatic head. The exact impact of this finding on overall survival should be assessed in future studies. Finally, pathology reports of low-volume hospitals more often lacked essential data. Thus pathology reports should be standardized in order to minimize missing data.
Acknowledgments
Both the CCNL and PALGA are acknowledged for providing data for analysis.
Funding sources
No specific funding was obtained for this work.
Conflicts of interest
None declared.
References
- International Agency for Research on Cancer; World Health Organisation. Available at http://eco.iarc.fr/eucan/Cancer.aspx?Cancer=15#block-table-a (last accessed 23 October 2014)
- Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, et al. Pancreatic ductal adenocarcinoma. is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731–736. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- Gooiker GA, van Gijn W, Wouters MWJM, Post PN, van de Velde CJH, Tollenaar RAEM. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485–494. doi: 10.1002/bjs.7413. [DOI] [PubMed] [Google Scholar]
- Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99–132. [PubMed] [Google Scholar]
- De Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, et al. Impact of nationwide centralization of pancreaticoduodenectomy on mortality. Br J Surg. 2012;99:404–410. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]
- Nienhuijs SW, van den Akker SA, de Vries E, de Hingh IH, Visser O, Lemmens VE. Nationwide improvement of only short-term survival after resection for pancreatic cancer in the Netherlands. Pancreas. 2012;41:1063–1066. doi: 10.1097/MPA.0b013e31824c3dbf. [DOI] [PubMed] [Google Scholar]
- Birkmeyer JD, Warshaw AL, Finlayson SR, Grove MR, Tosteson AN. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- Lemmens VE, Bosscha K, van der Schelling G, Brenninkmeijer S, Coebergh JW, de Hingh IH. Improving outcome for patients with pancreatic cancer through centralization. Br J Surg. 2011;98:1455–1462. doi: 10.1002/bjs.7581. [DOI] [PubMed] [Google Scholar]
- Gooiker GA, van der Geest LG, Wouters MW, Vonk M, Karsten TM, Tollenaar RA, et al. Quality improvement of pancreatic surgery by centralization in the Western part of the Netherlands. Ann Surg Oncol. 2011;18:1821–1829. doi: 10.1245/s10434-010-1511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Torre M, Nigri G, Ari LF, Cosenza G, Ravailoli M, Ramacciato G. Hospital volume, margin status, and long-term survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2012;78:225–229. [PubMed] [Google Scholar]
- Parks RW, Bettschart V, Frame S, Stockton DL, Brewster DH, Garden OJ. Benefits of specialisation in the management of pancreatic cancer: results of a Scottish population-based study. Br J Cancer. 2004;91:459–465. doi: 10.1038/sj.bjc.6601999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Jang JY, Kim SW, Kim WH, Lee KU, Park YH. Analysis of long-term survivors after surgical resection for pancreatic cancer. Pancreas. 2006;32:271–275. doi: 10.1097/01.mpa.0000202953.87740.93. [DOI] [PubMed] [Google Scholar]
- Shimada K, Sakamoto Y, Nara S, Esaki M, Kosuge T, Hiraoka N. Analysis of 5-year survivors after a macroscopic curative pancreatectomy for invasive ductal adenocarcinoma. World J Surg. 2010;34:1908–1915. doi: 10.1007/s00268-010-0570-9. [DOI] [PubMed] [Google Scholar]
- Van Roest MH, Gouw AS, Peeters PM, Porte RJ, Slooff MJ, Fidlrer V, et al. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: perineural growth more important prognostic factor than tumor localization. Ann Surg. 2008;248:97–103. doi: 10.1097/SLA.0b013e31817b6609. [DOI] [PubMed] [Google Scholar]
- Michalski CW, Kleeff J, Bachmann J, Alkhatib J, Erkan M, Esposito I, et al. Second-look operation for unresectable pancreatic ductal adenocarcinoma at a high-volume center. Ann Surg Oncol. 2008;15:186–192. doi: 10.1245/s10434-007-9535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathologisch-Anatomisch Landelijk Geautomatiseerd Archief (PALGA database)
- Netherlands Cancer Registry managed by the Comprehensive Cancer Centre Netherlands (CCCNL)
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th edn. New York, Dordrecht, Heidelberg, London: Springer; 2010. [Google Scholar]
- Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–1234. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MO, Alderson D, Peters TJ, Bedford C, Edwards D, Wotton S, et al. Influence of specialization on the management and outcome of patients with pancreatic cancer. Br J Surg. 2003;90:171–177. doi: 10.1002/bjs.4028. [DOI] [PubMed] [Google Scholar]
- de Castro SM, van Heek NT, Kuhlmann KF, Busch OR, Offerhaus GJ, van Gulik TM, et al. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery. 2004;136:994–1002. doi: 10.1016/j.surg.2004.03.010. [DOI] [PubMed] [Google Scholar]
- de Castro SM, Kuhlmann KF, van Heek NT, Busch OR, Offerhaus GJ, van Gulik TM, et al. Recurrent disease after microscopically radical (R0) resection of periampullary adenocarcinoma in patients without adjuvant therapy. J Gastrointest Surg. 2004;8:775–784. doi: 10.1016/j.gassur.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718–725. doi: 10.1001/archsurg.139.7.718. [DOI] [PubMed] [Google Scholar]
- Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the Ampulla of Vater. Experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526–532. doi: 10.1001/archsurg.134.5.526. [DOI] [PubMed] [Google Scholar]
- Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228:87–94. doi: 10.1097/00000658-199807000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Matsumoto T, Sasaki A, Morii Y, Aramaki M, Kitano S. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69–73. doi: 10.1001/archsurg.137.1.69. [DOI] [PubMed] [Google Scholar]
- Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenecomty of periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229:613–622. doi: 10.1097/00000658-199905000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamini MA, Moesinger RC, Pitt HA, Sohn TA, Hruban RH, Lillemoe KD, et al. Adenocarcinoma of the Ampulla of Vater. A 28-year experience. Ann Surg. 1997;225:590–599. doi: 10.1097/00000658-199705000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- Tomlinson JS, Jain S, Bentrem DJ, Sekeris EG, Maggard MA, Hines OJ, et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg. 2007;142:767–774. doi: 10.1001/archsurg.142.8.767. [DOI] [PubMed] [Google Scholar]
- Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–517. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]


