Abstract
IMPORTANCE
We developed a gene transfer tool for the control of nocturnal elevated intraocular pressure (IOP).
OBJECTIVE
To demonstrate that inhibiting the trabecular meshwork RhoA pathway by delivering a mutated, dominant-negative RhoA gene (dnRhoA) carried inside a long-expressing recombinant virus would reduce nocturnal elevated IOP in a living animal.
DESIGN AND SETTING
We generated an optimized recombinant viral molecule by inserting a mutated RhoA complementary DNA with a translation enhancer-promoter into a specially designed plasmid containing mutated viral terminal repeats. We then generated the virus particle, self-complementary adeno-associated virus serotype 2 carrying the mutated gene (scAAV2.dnRhoA) and assessed its function in vitro by infecting primary human trabecular meshwork cells and in vivo by injecting living rats intracamerally with therapeutic and control viruses. Three different models of 12-hour light and dark cycles were used. Viruses were injected when animals showed the circadian dark IOP elevation. The IOP measurements were conducted with a tonometer at 2 to 4 hours after onset of the nocturnal and diurnal cycles. Values at preinjection time were used as baselines. Animals were euthanized at 4 to 8 weeks after injection.
EXPOSURES
Intraocular injection of rodent eyes with the recombinant viral vector scAAV2.dnRhoA.
MAIN OUTCOMES AND MEASURES
Nocturnal elevation of IOP blocked for prolonged periods by transferred RhoA gene.
RESULTS
By visual inspection, human trabecular meshwork cells infected with scAAV2.dnRhoA showed diminished stress fiber formation. Living rats exhibited a circadian IOP cycle that could be reset by adjusting light conditions to facilitate light and dark nocturnal IOP studies. A single-dose injection of scAAV2.dnRhoA into the rat eyes prevented elevation of IOP during the nocturnal cycle for at least 4 weeks (mean [SE], 9.2 [0.2] mm Hg light IOP and 9.6 [0.4] mm Hg dark IOP), while control eyes showed a significantly higher IOP over baseline (9.5 [0.4] mm Hg light IOP and 13.5 [0.3] mm Hg dark IOP).
CONCLUSIONS AND RELEVANCE
To our knowledge, this is the first example of a gene transfer strategy that prevents nocturnal IOP elevation in living animals for prolonged periods. Inhibiting the RhoA pathway upstream of Rho kinase with a safe gene drug could provide a new enhanced treatment for long-term management of elevated nocturnal IOP.
Glaucoma is a progressive optic neuropathy caused by the degeneration and death of the retinal ganglion cells. A major risk factor for glaucoma is elevated intraocular pressure (IOP),1 which is caused by a dysfunctional trabecular meshwork (TM). The IOP of vertebrates follows a circa-dian rhythm,2-8 in which higher and lower IOP cycling has been suggested to be associated with the diurnal, nocturnal, and crepuscular activity of the subjects.6,8 In humans, the circadian IOP nocturnal increase is confounded by postural changes, with supine positions manifesting significantly higher IOPs than those in the sitting position.3,9-11 During normal life situations (upright position during the day and supine at night), the delta nocturnal IOP in healthy individuals is higher than that in age-matched untreated patients with glaucoma.3 However, the absolute IOP at night is higher in individuals with glaucoma.3,12
The only available glaucoma drugs to date are aimed at reducing elevated IOP13,14 and have different effects on diurnal and nocturnal IOP. Therefore, brimonidine tartrate and timolol maleate do not significantly lower night IOP,15,16 while latanoprost has a similar effect during diurnal and nocturnal periods.16,17 Controlling nocturnal IOP is central to the management of glaucoma, and the availability of a night IOP drug is highly desirable.
Circadian IOP fluctuation follows the same pattern in animals, in which neither postural nor diurnal and nocturnal behavior comes into play.5,18 In conscious rats and rabbits, a true circadian rhythm has been thoroughly characterized.5,6 The circadian cycle is stimulated by light, can be reset by changing light patterns, and is influenced by anesthetic agents.5,18 A circadian high-IOP model in animals offers the unique possibility of assessing glaucoma drugs under natural conditions without the interfering effects brought by invasive or genetic models.
RhoA is a member of a small GTPase family of proteins that bind and hydrolyze guanosine triphosphate (GTP). RhoA cycles between an active (GTP bound) form and an inactive (guanine diphosphate bound) form, and the cycling between the 2 conformations activates Rho kinase (ROCK). Activation of RhoA regulates several cytoskeletal-dependent cell functions, promotes actomyosin contractility, and induces the formation of stress fibers.19 An inhibitor of ROCK, Y-27632, has been shown to be a potent smooth muscle relaxant and to correct hypertension in rats.20 Subsequently, Y-27632 was demonstrated to reduce IOP in rabbits,21 inhibit contraction of the TM,22 increase outflow facility in rabbits and perfused porcine anterior segments,23,24 and increase Schlemm Canal cellular permeability.25 Currently, great interest exists in the use of ROCK inhibitors as IOP-lowering agents, and some have already been tested in clinical trials.26,27 Inhibiting the RhoA pathway upstream of the ROCK enzyme and inactivating RhoA by adenoviral gene transfer of the dominant-negative molecule28 or the C3 transferase29 proved also to increase outflow facility in perfused human and monkey organ cultures, respectively.
To obtain extended IOP reduction in living animals, we designed a gene therapy vector carrying a GTP–binding site mutated RhoA gene (scAAV2.dnRhoA) and tested its efficacy on the nocturnal elevated IOP model in rats. We report herein that a single dose of the gene drug prevented IOP elevation for at least 1 month and propose that this approach might be useful for therapeutic control of nocturnal IOP.
Methods
All animal procedures were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee and conducted in accord with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Visual Research. Processes are described in detail in the eMethods in the Supplement.
Recombinant Viruses
The virus generated for this study was scAAV2.dnRhoA. This self-complementary adeno-associated virus is the second generation of AAV2, the highly successful virus for gene transfer and gene therapy of the eye.30,31
Culture of Outflow Pathway Cells, Viral Infection, and Actin Evaluation
Primary human TM cells were generated from residual cor-neal rims after corneal transplants at the University of North Carolina eye clinic.32 Experiments were performed with cell line HTM-137 (39 years old). For actin fluorescence, subcon-fluent cells on precoated glass coverslips were infected with 6.2 to 19 × 103 viral genomes (vg) per cell and evaluated 72 hours later. Fixed and permeabilized cells were stained with rhoda-mine-conjugated phalloidin (1:500; Sigma) and assessed by fluorescence microscopy (IX71 and DP70 camera and software; Olympus).
Experimental Animals, IOP Measurements, and Intraocular Injections
Male Wistar rats (100-150 g [4-6 weeks old]; Charles River Laboratories) were housed on arrival in 12-hour light pattern cycles. The light pattern cycles were as follows: model 1 (7 am to 7 pm light and 7 pm to 7 am dark), model 2 (9 pm to 9 am light and 9 am to 9 pm 2-lux red light), and model 3 (11 pm to 11 am light and 11 am to 11 pm dark).
Intraocular pressures were obtained on sedated rats using a tonometer (TonoLab; Colonial Medical Supply).33 For darkcycle measurements, IOPs were obtained 2 to 4 hours after cycle onset using a 2-lux light source at a distance from the operator of approximately 0.4 m (17 in).
Intracameral injections of 4 to 5 μL of the desired virus's concentration were administered manually on deeply anesthetized animals using a 30-G needle on a micrometer syringe (Gilmont; Thermo Fisher Scientific Inc). Topical antibiotic ointment was applied after injection.
Histology and Fluorescence Histochemistry
Eyes were enucleated immediately after euthanasia (at 4-8 weeks following injection) and immersed in fresh paraformaldehyde, 4%, for 30 minutes. Eyes were subsequently dissected at the equator, their lens was removed, and wedge-shaped specimens containing the anterior chamber angle region with the TM were postfixed to assess morphology and green fluorescent protein(GFP) fluorescence. For morphology, we used paraffin and plastic embedding, while for GFP fluorescence we embedded the tissue in optimal cutting temperature compound (Tissue-Tek; Sakura Finetek). Meridional sections (5-10 μm) were assessed by fluorescence microscopy.
RNA Extraction, Reverse Transcription, and Real-time Polymerase Chain Reaction
Immediately after enucleation, rat eyes were immersed in a stabilizing solution (RNAlater; Life Technologies) for 1 to 2 hours, followed by dissection. Tissue strips containing the TM were homogenized for RNA extraction (RNeasy plus kit; Qiagen). Recoveries were 0.5 to 3 μg of RNA per extraction. Complementary DNA (cDNA) was synthesized in 25 μL with a high-capacity cDNA reverse transcription kit containing MultiScribe MuLV reverse transcriptase (Applied Biosystems) and using 0.5 μg of RNA.
TaqMan probe and primer sets (Applied Biosystems) used were Hs00357608_m1 (RhoA exons 4 and 5) and Hs99999901_s1 (18S control). Reactions were performed in triplicate and run and analyzed on a real-time polymerase chain reaction system (7500; Applied Biosystems) with a software program (SDS, version 2.0.4; Applied Biosystems). Relative quantification values between treated and control samples were expressed in Fold-Change.
Statistical Analysis
The significance of experimental changes was analyzed using t test as paired or unpaired data. Confidence intervals of the means were calculated for a confidence level of 95%.
Results
Design of a Long-term, Low–Immune Response Gene Transfer Vector to Inhibit the RhoA Pathway
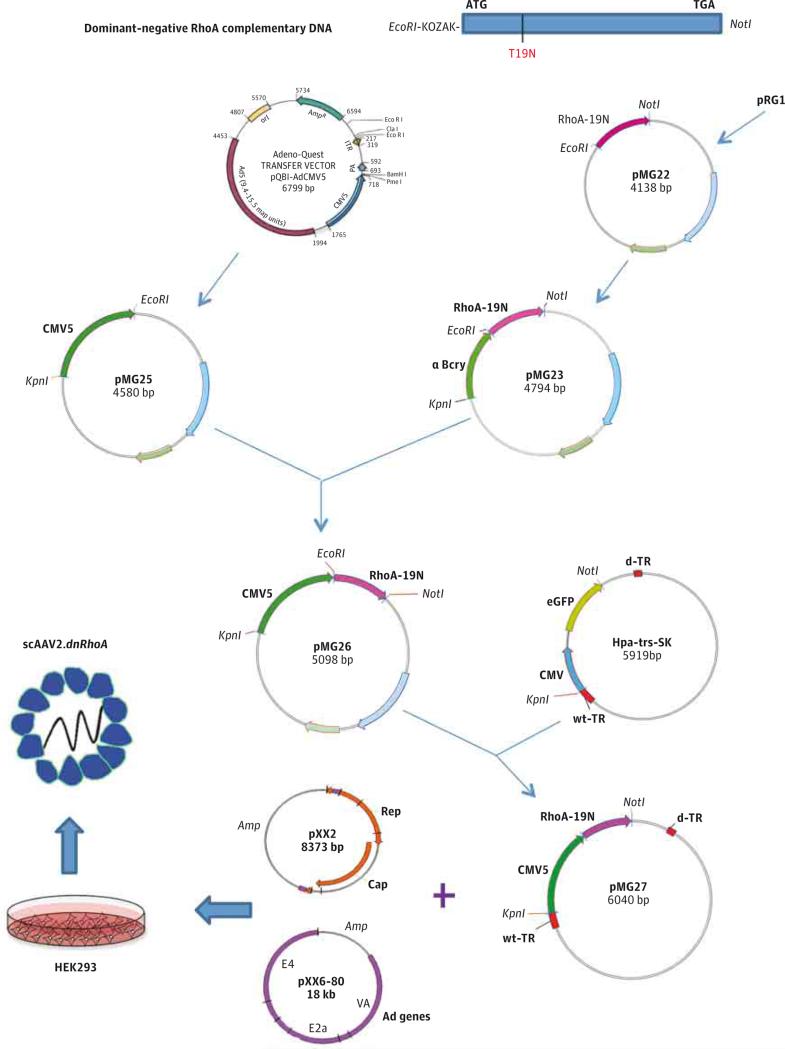
To inhibit the RhoA pathway in the TM for longer periods than conventional ROCK inhibitors, we used a gene transfer strategy. Because of the biology of GTPase RhoA, we chose to inhibit the RhoA pathway by overexpressing a dominant-negative RhoA cDNA.34 This cDNA contains a point mutation that converts the wild-type amino acid 19 of the translated protein from threonine to asparagine (T19N). The mutant renders the GTP–binding site inactive, resulting in a dominant-negative RhoA. Our group had previously generated an adenovirus vector carrying such mutated RhoA cDNA (AdhRhoA2)28 and showed that it was able to increase outflow facility in perfused anterior segment human postmortem eyes. To investigate whether this finding could be translated to IOP reduction in living animals, we generated a new long-term and low–immune response viral vector carrying the original mutated RhoA cDNA28,34 (Figure 1).
Figure 1. Diagram of the Generation of the scAAV2.dnRhoA Virus.
The scAAV2.dnRhoA virus was generated by cotransfection of plasmids pMG27 + pXX2 + pXX6-80 in HEK293 cells. The pMG27 contained the human dnRhoA complementary DNA from pRG1 in the study by Vittitow et al28 and CMV5 enhancer-promoter from pQB1-AdCMV5, both inserted into pHpa-trs-SK.35 The pAAVrep-cap (for serotype 2) was pXX2 from the study by Xiao et al,36 and pHelper was pXX6-80 from the same study. The Adeno-Quest is from Quantum Biotechnology. EcoR1, KpnI, and NotI are restriction endonucleases. pMG22, pMG23, pMG5, pMG26, pMG27, and Hpa-trs-SK are plasmids described in the eMethods in the Supplement. Ad genes indicates adenovius helper function genes; bp, base pair; d-TR, deleted terminal repeat; HEK293, human embryonic kidney 293; and wt-TR, wild-type terminal repeat.
Cellular, Morphological, and Molecular Outcomes of Delivering scAAV2.dnRhoA to Human TM Cells and Living Rats
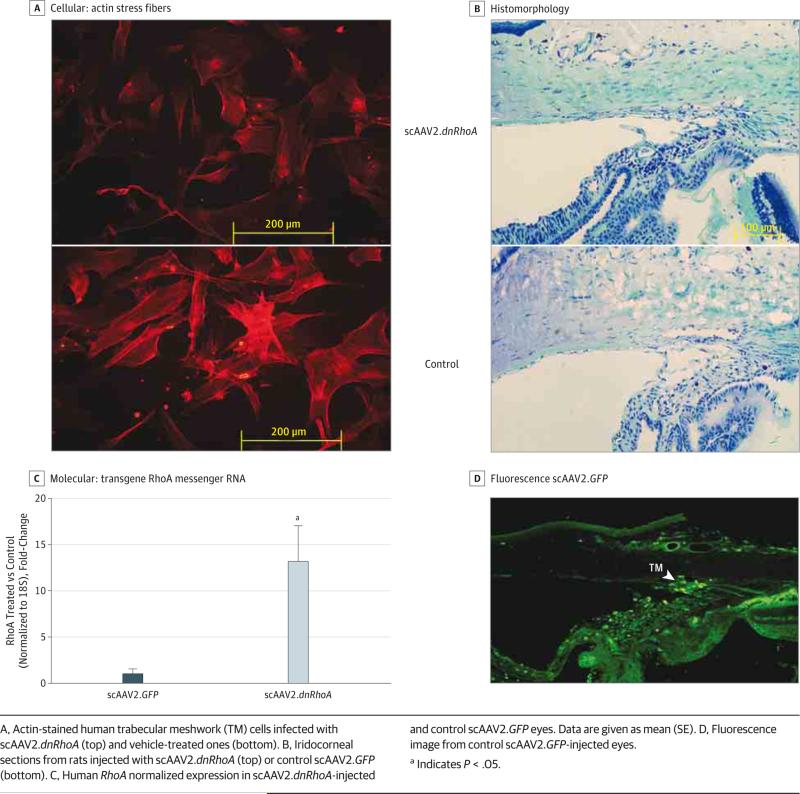
To assess the functional outcome of overexpressing dnRhoA, subconfluent human TM cells were infected with scAAV2.dnRhoA or control vehicle. Actin staining at 72 hours showed extensive stress fiber reduction and cell relaxation in the scAAV2.dnRhoA-infected cells (Figure 2A).
Figure 2. Characterization of scAAV2.dnRhoA Gene Transfer.
A, Actin-stained human trabecular meshwork (TM) cells infected with scAAV2.dnRhoA (top) and vehicle-treated ones (bottom). B, Iridocorneal sections from rats injected with scAAV2.dnRhoA (top) or control scAAV2.GFP (bottom). C, Human RhoA normalized expression in scAAV2.dnRhoA-injected and control scAAV2.GFP eyes. Data are given as mean (SE). D, Fluorescence image from control scAAV2.GFP-injected eyes.
a Indicates P < .05.
The TM morphology was examined on 4 rats from a group cycled at the model 2 light pattern and harvested during the light cycle. The treated eye received scAAV2.dnRhoA, while the contralateral control eye received scAAV2.GFP. At 42 days, tissues were plastic or paraffin embedded as meridional wedges (4 eyes each and 4 wedges per eye). Stained sections showed that the TM gross morphology was not altered; Schlemm canal and beams were well formed in treated and control eyes (Figure 2B).
Increased levels of transgene RhoA cDNA in the TM tissue were evaluated in 6 rats. Four rats (4 treated eyes and 4 control scAAV2.GFP eyes) were from a group cycled at the model 1 light pattern and harvested during the light cycle as single TMs. At 24 days, the mean (SE) Fold-Change of treated eyes vs control scAAV2.GFP eyes was 1.8 (0.4) (P = .08). Two rats (2 treated eyes and 2 control scAAV2.GFP eyes) were from a group cycled at the model 2 light pattern and harvested at 2 lux as pooled TMs. At 56 days, the mean (SE) Fold-Change of treated eyes vs control scAAV2.GFP eyes was 13.3 (3.8) (P = .004) (Figure 2C). Although a deoxyribonuclease treatment was included during RNA extraction (eMethods in the Supplement), it cannot be excluded that a small amount of the observed increase could be due to contaminant intracellular viral DNA.
Trabecular meshwork transgene delivery was further confirmed by fluorescent GFP encoded by control virus scAAV2.GFP (Figure 2D). Together, these results demonstrate a positive dnRhoA transgene delivery and show that such delivery is not detrimental to the TM structure.
Light-Cycling Models to Assess dnRhoA Effects on Nocturnal IOP
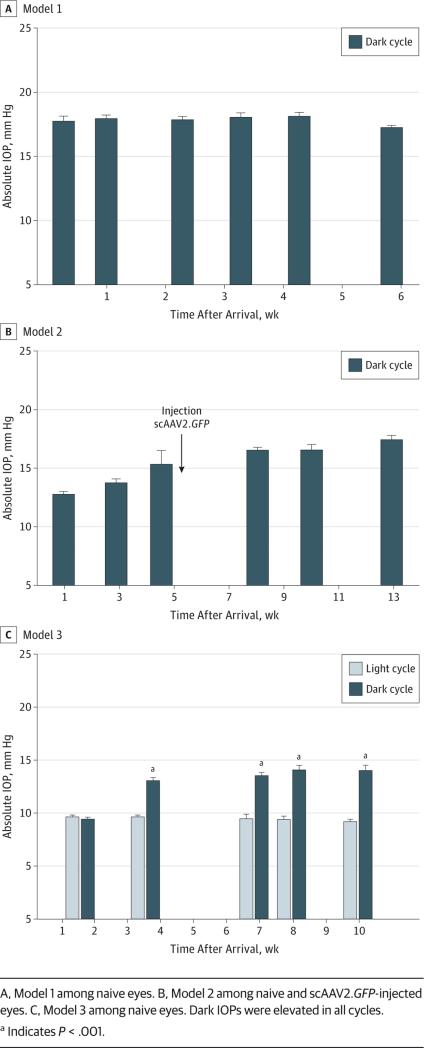
To select a feasible rat model to measure nocturnal IOP, we tested 3 different light-cycling strategies (Table). In model 1, rats were housed 7 am to 7 pm light and 7 pm to 7 am dark. Naive eye IOPs were obtained between 9 and 11 PM. Under these conditions in a first group of 8 rats, the mean (SE) dark IOP was 17.7 (0.4) mm Hg at 2 days after arrival (16 eyes, right and left; CI, 0.6) and stayed high at 17.5 (0.3) mm Hg until the end of the experiment (8 eyes, right; CI, 0.7 at 6 weeks) (Figure 3A). In a second group of 8 rats, the mean (SE) dark IOP was 16.9 (0.3) mm Hg at 1 week (16 eyes, right and left; CI, 0.6), and they also stayed high at 16.8 (0.2) mm Hg until the end of the experiment (8 eyes, right; CI, 0.4 at 4.5 weeks).
Table.
Summary of Experiments and Integral Intraocular Pressure (IOP)
| No. of Rats | Time After Treatment, d | Light-Cycling Pattern | Treatment | Dark-Cycle Integral IOP, Mean (SE), mm Hg-days | P Value |
|---|---|---|---|---|---|
| Model 1 | |||||
| 8 | 31 | 7 am to 7 pm light | Naive | 557.8 (9.2) | .04 |
| 7 pm to 7 am dark | scAAV2.dnRhoA | 547.8 (9.2) | |||
| 8 | 18 | 7 am to 7 pm light | Naive | 300.2 (5.5) | .52 |
| 7 pm to 7 am dark | Ad5.dnRhoA | 298.9 (5.0) | |||
| Model 2 | |||||
| 7 | 54 | 9 pm to 9 am light | scAAV2.GFP | 1073.6 (51.7) | <.001 |
| 9 am to 9 pm 2-lux red light | scAAV2.dnRhoA | 659.4 (30.4) | |||
| 7 | 42 | 9 pm to 9 am light | scAAV2.GFP | 698.8 (11.7) | .08 |
| 9 am to 9 pm 2-lux red light | scAAV2.dnRhoA | 686.2 (9.1) | |||
| Model 3 | |||||
| 7 | 27 | 11 pm to 11 am light | Naive | 367.3 (13.3) | <.001 |
| 11 am to 11 pm dark | scAAV2.dnRhoA | 265.9 (11.7) | |||
Figure 3. Intraocular Pressure (IOP) Profile of Representative Rat Groups From the 3 Models of Light and Dark Cycles.
A, Model 1 among naive eyes. B, Model 2 among naive and scAAV2.GFP-injected eyes. C, Model 3 among naive eyes. Dark IOPs were elevated in all cycles.
a Indicates P < .001.
In model 2, rats were housed 9 pm to 9 am light and 9 am to 9 pm 2-lux red light. In this model, eyes were naive during the first 5 weeks (first group) or 3 weeks (second group) and then injected once with control virus scAAV2.GFP. Under these conditions, rats require longer to adapt to reverse light cycling. Therefore, in the first group of 7 rats, the mean (SE) dark IOP was 12.7 (0.3) mm Hg at 1 week after arrival and rose to 15.3 (0.3) mm Hg at 5 weeks (14 eyes, right and left; CI, 0.7). Nocturnal elevation reached a mean (SE) of 16.5 (0.5) mm Hg at 10 weeks and remained high at 17.4 (0.4) mm Hg until the end of the experiment (7 eyes, right; CI, 1.0 at 13 weeks) (Figure 3B). In a second group of 7 rats, the mean (SE) dark IOP was 12.2 (0.4) mm Hg at 1 week after arrival, reached 20.5 (0.8) mm Hg at 6 weeks, and remained high at 22.0 (1.5) mm Hg until the end of the experiment (7 eyes, right; CI, 3.7 at 11 weeks).
In model 3 conditions (11 pm to 11 am light and 11 am to 11 pm dark), the light pattern conditions allow convenient times for obtaining light and dark IOP measurements in the same animal during working hours. In 7 rats, the mean (SE) naive eye IOPs were 9.6 (0.2) mm Hg (light) and 9.4 (0.2) mm Hg (dark) at 2 weeks after arrival (14 eyes, right and left; CI, 0.6-0.3 each). At 4 weeks, their mean (SE) dark IOP rose to 13.0 (0.3) mm Hg, while their light IOP remained at 9.6 (0.2) mm Hg (14 eyes). The mean (SE) nocturnal elevation continued to increase to 14.0 (0.5) mm Hg at 10 weeks when the experiment was terminated (7 eyes, right; CI, 0.1) (Figure 3C).
Together, the results indicate that rats kept at the model 1 light pattern from birth exhibit a nocturnal IOP increase of 6 to 7 mm Hg beginning at 2 to 4 hours after dark onset. Adjusting the circadian cycle to reverse time conditions at age 4 to 6 weeks results in significant increases of 3 to 4 mm Hg in 3 weeks and reaches the natural 6 to 7 mm Hg at 10 weeks. These results support the circadian model as a desirable, naturally induced, elevated IOP model. Cycle adaptation to the model 3 light pattern would enable the study of night IOP–lowering pharmaceuticals during working hours.
Single Intracameral Injection of scAAV2.dnRhoA Prevents Nocturnal IOP Elevation in Rats
The study included 37 rats (Table). After reaching nocturnal IOP elevation, the left eye of each rat was injected with 3.7 × 109 vg of scAAV2.dnRhoA, while the right eye was uninjected (naive control) or injected with 5 to 10 × 109 vg of scAAV2.GFP. In one experiment, the left eye was treated with 8.5 × 108 vg of Ad5.dnRhoA (AdhRhoA2 in the study by Vittitow et al28). In all 3 models, integral IOPs were reduced in the virus-treated eye. The IOP details for model 1 and model 2 are given in the eData in the Supplement.
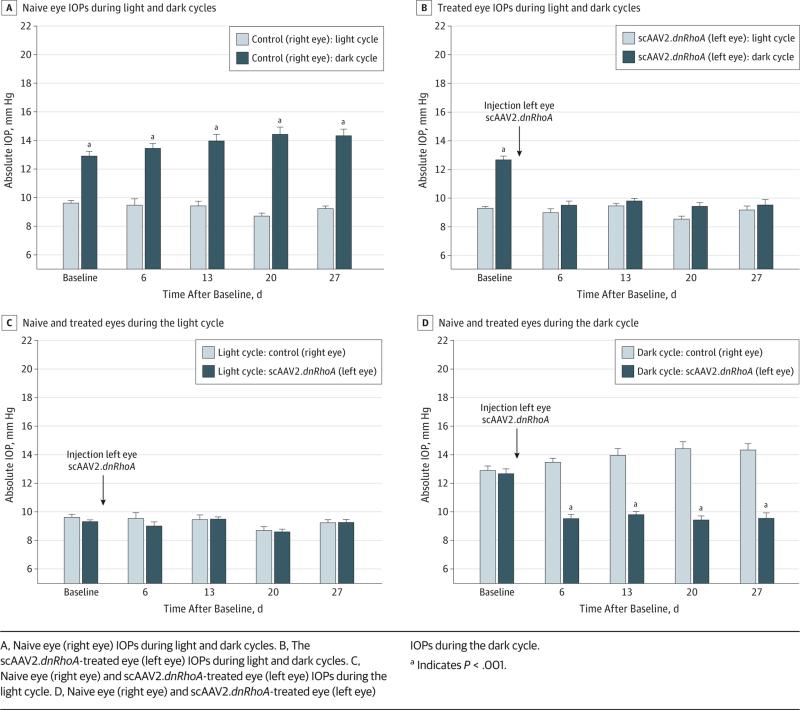
The model 3 design was chosen after the experience gained in previous models. Seven rats were injected (left eye) with scAAV2.dnRhoA after showing dark elevated IOP. The right eyes were untreated. Untreated eyes had a mean (SE) light IOP of 9.6 (0.2) mm Hg and a dark IOP of 12.9 (0.3) mm Hg (CI, 0.6-0.9) at baseline and remained the same at 9.5 (0.4) mm Hg light IOP and 13.5 (0.3) mm Hg dark IOP at 27 days (CI, 0.5-1.0) (Figure 4A). Treated eyes had a similar mean (SE) light IOP of 9.3 (0.1) mm Hg and dark IOP of 12.7 (0.3) mm Hg (CI, 0.3-1.2) at pretreatment baseline. At 6 days after treatment, treated eyes had a mean (SE) light IOP of 9.0 (0.3) mm Hg and a dark IOP of 9.5 (0.3) mm Hg (CI, 1.0-0.7) and remained the same at 9.2 (0.2) mm Hg light IOP and 9.6 (0.4) mm Hg dark IOP at 27 days (Figure 4B). Therefore, during the light cycle, untreated right eyes and treated left eyes exhibited baseline IOPs (Figure 4C). However, at dark, naive eyes manifested elevated IOPs, while scAAV2.dnRhoA treatment prevented nocturnal elevation as early as 6 days and maintained baseline levels throughout (27 days) (Figure 4D). The mean (SE) integral IOPs showed no difference between untreated and pretreated eyes during the light cycle (240.1 [8.9] vs 236.2 [9.2] mm Hg–days, P = .47), while differences were observed at dark (367.3 [13.3] vs 265.9 [11.7] mm Hg, P < .001) (Table). Results from this experiment in which each rat eye was evaluated during the light and dark cycles demonstrated that a single intracameral administration of a gene-impairing RhoA function is sufficient to prevent IOP nocturnal elevation in rats for at least 24 days.
Figure 4. Intraocular Pressure (IOP) Profiles From scAAV2.dnRhoA-Treated and Naive Rat Eyes During Light and Dark Phases of Model 3.
A, Naive eye (right eye) IOPs during light and dark cycles. B, The scAAV2.dnRhoA-treated eye (left eye) IOPs during light and dark cycles. C, Naive eye (right eye) and scAAV2.dnRhoA-treated eye (left eye) IOPs during the light cycle. D, Naive eye (right eye) and scAAV2.dnRhoA-treated eye (left eye) IOPs during the dark cycle.
a Indicates P < .001.
Discussion
Probably because of its effects on TM cell adhesion and cytoskeleton, the RhoA-ROCK-myosin light chain pathway has shown to be a strong effector of outflow facility in ex vivo eye cultures and in living animals. The ongoing pharmaceutical developments based on inhibition of this pathway are expected to produce a new generation of highly effective, IOP-lowering glaucoma drugs. Because the drugs being developed have a short duration of action, herein we investigated whether a gene drug inhibiting the Rho pathway upstream of ROCK would be able to reduce IOP for longer periods than are achieved with conventional ROCK inhibitors. Using a natural IOP elevation circadian rhythm model, we showed that a single-dose intracameral injection of gene vector scAAV2.dnRhoA prevented the nocturnal IOP elevation of living rats for at least 1 month, which was the last point evaluated.
To develop such a viral vector, we made use of our recent experience with second-generation scAAV2, whereby trans-gene delivery to a monkey's TM lasted for at least 2 years.30 For this study, we further optimized the expression cassette by switching the standard cytomegalovirus promoter to the more translationally efficient CMV5 vector.37 For the trans-gene, we chose the dominant-negative RhoA gene (T19N). Over-expression of the mutated RhoA overcomes the levels of the active protein and fills the cells with an inactive RhoA product. On Rho inactivation, the pathway is interrupted, ROCK is not activated, no cell contraction occurs, and cells and tissues relax.
The choice of the circadian elevated IOP model was based on the opportunity of having an undisturbed high-IOP eye, as well as the desire to address a clinical treatment for night-elevated IOP. We observed a circadian rhythm in all rats in the study. Although not to the same extent, Wistar rats showed elevated IOP at dark under all light-resetting conditions tested and in the presence of light anesthesia. Rats kept on the same standard light and dark cycle since birth (7 am to 7 pm) had a mean increase of 7 to 8 mm Hg during the dark phase (model 1). When the cycle was reversed at 4 to 6 weeks old to 9 am to 9 pm dark (2-lux exposure) (model 2) or to 11 am to 11 pm dark (model 3), the delta IOP increase was slightly less (5-7 mm Hg), albeit highly significant. An elevated dark-phase IOP was observed at 1 week after the cycle change and showed the greatest difference between 3 and 4 weeks after the change, when our gene transfer injections were performed. Using model 3 conditions was logistically the best model because it allowed us to measure day and night IOPs in the same animal during working hours. The delta day vs night IOP values obtained herein are somewhat lower than those reported for conscious brown Norway rats5 and are between those reported in conscious rabbits.6,18 In adult isoflurane-anesthetized mice, the delta day vs night IOP is 3 to 4 mm Hg,38 which is lower than that observed herein in Wistar rats. These results are in agreement with the fact that the IOP circadian rhythm is affected but not eliminated by anesthetic agents.5,18
It is unknown whether the mechanism by which the scAAV2.dnRhoA vector prevents IOP nocturnal elevation is by interference with the clock genes, by the inherent consequences of inhibiting the RhoA pathway,22-24,26,28 or perhaps by both. Transgenic knockout mice lacking Cry1 and Cry2 clock genes do not show circadian IOP changes.39 Furthermore, it has been reported that, in osteoblasts, unidirectional fluid flow stress activates RhoA, which then has a critical role in modulating the circadian regulatory pathway.40
The single-dose intracameral injection resulted in no undesirable clinical signs. No inflammation, tearing, or redness was observed in the eyes, and the lens and cornea remained clear. Phase microscopy morphology of the angle region of the treated rat eye was similar to that of the untreated rat eye and showed a well-preserved TM architecture. Safety of this particular viral vector in the eye is well established. Restoration of vision in patients with Leber congenital amaurosis by AAV2.RPE65 gene therapy demonstrated no toxicity in the ongoing clinical trials, with some of them lasting longer than 3 years.41 Current trials using conventional short-term ROCK inhibitor drugs appeared to be well tolerated, but no data are available on long-term toxicity to our knowledge.
Conclusions
Our findings of long-term prevention of nocturnal IOP elevation in a living animal could have important implications for the treatment of glaucoma, in which night IOPs can reach an unsafe threshold. Although expected, the use of this vector to also reduce elevated IOP during the day in living animals remains to be tested. To date, no gene drugs exist for the treatment of glaucoma. Results from this study suggest that effective transgenes carried on safe, long-duration viral vectors could become next-generation drugs not only for genetic diseases but also for acquired diseases. In particular, delivery of a dnRhoA gene to the TM for reducing nocturnal IOP could prove to be a beneficial treatment for glaucomatous and normal eyes.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded by grants EY11906 and EY13126 from the National Eye Institute (Dr Borrás) and in part by a Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology, University of North Carolina at Chapel Hill.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Borrás and Ms Buie had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Borrás, Buie. Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Borrás, Buie, Carabana. Critical revision of the manuscript for important intellectual content: Borrás, Buie, Spiga.
Statistical analysis: Borrás, Buie.
Obtained funding: Borrás.
Administrative, technical, or material support: Borrás, Buie.
Study supervision: Borrás.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none were reported.
Additional Contributions: Renekia R. Elliott, BA, Matthew H. Smith, BS, and Brandon M. Lane, PhD, members of our laboratory, provided continuous input and support during the project.
Supplemental content at jamaophthalmology.com
REFERENCES
- 1.Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol. 2011;152(3):340–344.e2.. doi: 10.1016/j.ajo.2011.05.029. doi:10.1016/j.ajo.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Drance SM. The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol. 1960;64(4):494–501. doi: 10.1001/archopht.1960.01840010496004. [DOI] [PubMed] [Google Scholar]
- 3.Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39(13):2707–2712. [PubMed] [Google Scholar]
- 4.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44(4):1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 5.Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15(2):185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- 6.Zhao M, Hejkal JJ, Camras CB, Toris CB. Aqueous humor dynamics during the day and night in juvenile and adult rabbits. Invest Ophthalmol Vis Sci. 2010;51(6):3145–3151. doi: 10.1167/iovs.09-4415. [DOI] [PubMed] [Google Scholar]
- 7.Giannetto C, Piccione G, Giudice E. Daytime profile of the intraocular pressure and tear production in normal dog. Vet Ophthalmol. 2009;12(5):302–305. doi: 10.1111/j.1463-5224.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 8.Sigle KJ, Camaño-Garcia G, Carriquiry AL, Betts DM, Kuehn MH, McLellan GJ. The effect of dorzolamide 2% on circadian intraocular pressure in cats with primary congenital glaucoma. Vet Ophthalmol. 2011;14(suppl 1):48–53. doi: 10.1111/j.1463-5224.2011.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40(12):2912–2917. [PubMed] [Google Scholar]
- 10.Nau CB, Malihi M, McLaren JW, Hodge DO, Sit AJ. Circadian variation of aqueous humor dynamics in older healthy adults. Invest Ophthalmol Vis Sci. 2013;54(12):7623–7629. doi: 10.1167/iovs.12-12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Fan S, Gulati V, et al. Aqueous humor dynamics during the day and night in healthy mature volunteers. Arch Ophthalmol. 2011;129(3):269–275. doi: 10.1001/archophthalmol.2011.4. [DOI] [PubMed] [Google Scholar]
- 12.Lee YR, Kook MS, Joe SG, et al. Circadian (24-hour) pattern of intraocular pressure and visual field damage in eyes with normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2012;53(2):881–887. doi: 10.1167/iovs.11-7846. [DOI] [PubMed] [Google Scholar]
- 13.Lichter PR, Musch DC, Gillespie BW, et al. CIGTS Study Group. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz K, Budenz D. Current management of glaucoma. Curr Opin Ophthalmol. 2004;15(2):119–126. doi: 10.1097/00055735-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Liu JH, Medeiros FA, Slight JR, Weinreb RN. Diurnal and nocturnal effects of brimonidine monotherapy on intraocular pressure. Ophthalmology. 2010;117(11):2075–2079. doi: 10.1016/j.ophtha.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of once-daily timolol and latanoprost on intraocular pressure. Am J Ophthalmol. 2004;138(3):389–395. doi: 10.1016/j.ajo.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Orzalesi N, Rossetti L, Invernizzi T, Bottoli A, Autelitano A. Effect of timolol, latanoprost, and dorzolamide on circadian IOP in glaucoma or ocular hypertension. Invest Ophthalmol Vis Sci. 2000;41(9):2566–2573. [PubMed] [Google Scholar]
- 18.Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr Eye Res. 1981;1(3):169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- 19.Lawson CD, Burridge K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases. 2014;5(1):e27958. doi: 10.4161/sgtp.27958. doi:10.4161/sgtp.27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 21.Honjo M, Tanihara H, Inatani M, et al. Effects of Rho-associated protein kinase inhibitor, Y-27632, on intraocular pressure and aqueous humor dynamics in the rabbit eye [abstract]. Invest Ophthalmol Vis Sci. 2000;41:S510. [PubMed] [Google Scholar]
- 22.Thieme H, Nuskovski M, Nass JU, Pleyer U, Strauss O, Wiederholt M. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Invest Ophthalmol Vis Sci. 2000;41(13):4240–4246. [PubMed] [Google Scholar]
- 23.Honjo M, Tanihara H, Inatani M, et al. Effects of Rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42(1):137–144. [PubMed] [Google Scholar]
- 24.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase–specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42(5):1029–1037. [PubMed] [Google Scholar]
- 25.Kameda T, Inoue T, Inatani M, et al. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Invest Ophthalmol Vis Sci. 2012;53(6):3092–3103. doi: 10.1167/iovs.11-8018. [DOI] [PubMed] [Google Scholar]
- 26.Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure–lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008;126(3):309–315. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- 27.Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013;37:1–12. doi: 10.1016/j.preteyeres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Vittitow JL, Garg R, Rowlette LL, Epstein DL, O'Brien ET, Borrás T. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol Vis. 2002;8:32–44. [PubMed] [Google Scholar]
- 29.Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- 30.Buie LK, Rasmussen CA, Porterfield EC, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010;51(1):236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 32.Comes N, Buie LK, Borrás T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma. Genes Cells. 2011;16(2):243–259. doi: 10.1111/j.1365-2443.2010.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buie LK, Karim MZ, Smith MH, Borrás T. Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2. Invest Ophthalmol Vis Sci. 2013;54(8):5441–5455. doi: 10.1167/iovs.13-11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15(11):6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 36.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72(3):2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massie B, Couture F, Lamoureux L, et al. Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette. J Virol. 1998;72(3):2289–2296. doi: 10.1128/jvi.72.3.2289-2296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zode GS, Kuehn MH, Nishimura DY, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121(9):3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeda A, Tsujiya S, Higashide T, et al. Circadian intraocular pressure rhythm is generated by clock genes. Invest Ophthalmol Vis Sci. 2006;47(9):4050–4052. doi: 10.1167/iovs.06-0183. [DOI] [PubMed] [Google Scholar]
- 40.Hamamura K, Swarnkar G, Tanjung N, et al. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect Tissue Res. 2012;53(5):398–406. doi: 10.3109/03008207.2012.671398. [DOI] [PubMed] [Google Scholar]
- 41.Testa F, Maguire AM, Rossi S, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology. 2013;120(6):1283–1291. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.