Abstract
Objective
Hybrid palliation is an alternative to Norwood stage 1 for the initial management of hypoplastic left heart syndrome. Contemporary multicenter hybrid use and institutional/patient factors associated with hybrid use relative to the Norwood have not been evaluated. We describe hybrid use in relation to institutional volume, patient factors, and short-term outcomes.
Methods
Infants aged 60 days or less listed in The Society of Thoracic Surgeons Congenital Heart Surgery Database (2010–2012) undergoing initial palliation of hypoplastic left heart syndrome were included. Annual institutional hybrid use rates were calculated: [hybrid procedures/(Norwood + hybrid + transplant procedures)]. In-hospital outcomes for primary hybrid and primary Norwood were compared and stratified by high (defined as ≥50%) versus low (defined as ≤10%) institutional hybrid use.
Results
Of 1728 patients (100 centers), most (n = 1496, 87%) underwent an index Norwood; 232 patients (13%) underwent an index hybrid procedure. Preoperative patient risk factors were more prevalent in patients undergoing the hybrid procedure. Only 13 of 100 institutions were high hybrid users, and these tended to have lower annual hypoplastic left heart syndrome index case volume. Unadjusted in-hospital mortality was higher for the hybrid compared with the Norwood procedure (30%vs 16%; P<.001). In-hospital mortality for the hybrid procedure was not associated with hybrid use (26%among institutions with low use vs 28%among institutions with high use). However, centers with high hybrid use had higher mortality after the Norwood (43%) compared with centers with low hybrid use (16%).
Conclusions
Few centers currently select the hybrid procedure for most infants with hypoplastic left heart syndrome. Although unadjusted in-hospital hybrid mortality is higher than Norwood mortality, potential risk factors are more prevalent among hybrid cases. Institutions with higher hybrid use have lower hypoplastic left heart syndrome case volume and higher Norwood mortality.
In cardiovascular surgery, the term “hybrid procedure” refers broadly to procedures that combine open surgical approaches with endovascular or other catheter-directed approaches. With respect to surgical palliation of hypoplastic left heart syndrome (HLHS) and related anomalies, the term “hybrid” is widely used to refer to a palliative strategy that includes placement of bilateral pulmonary artery (PA) bands, usually, although not always in combination with deployment of an intravascular stent to ensure patency of the arterial duct. The hybrid procedure for neonatal stage 1 palliation of HLHS was developed as a surgical alternative to the Norwood procedure, especially for high-risk candidates.1–11 The evolution and prevalence of use of the hybrid procedure (since its introduction in 2002), relative to other surgical strategies for single ventricle palliation, have not been studied. We sought to describe contemporary use of the hybrid procedure in relation to the Norwood procedure across a large multicenter cohort and identify institutional and patient factors associated with increased hybrid use.
MATERIAL AND METHODS
Data Source
The Society of Thoracic Surgeons Congenital Heart Surgery Database (STSCHSD) was used for this study. As of January 2014, the database contains de-identified data on more than 292,000 surgeries conducted since 2000 at 120 centers in North America, representing approximately 93% of all US centers performing congenital heart surgery and greater than 96% of all operations.12–15 Preoperative, operative, and outcomes data are collected on all patients undergoing pediatric and congenital heart surgery at participating centers. Coding for this database is accomplished by clinicians and ancillary support staff using the International Pediatric and Congenital Cardiac Code,12,13 and data are entered into the contemporary version of the STS-CHS data-collection form (version 3.0).14 The Duke Clinical Research Institute serves as the data warehouse and analysis center for all of the STS National Databases. Evaluation of data quality includes the intrinsic verification of data, along with a formal process of in-person site visits and data audits conducted by a panel of independent data quality personnel and pediatric cardiac surgeons at approximately 10%of participating institutions each year.1,5,6 This study was approved by the STSCHSD Access and Publications Committee and the Duke University institutional review board and was not considered human subjects research by the Duke University Institutional Review Boards in accordance with the Common Rule (45 CFR 46.102(f)).
Patient population
Infants (aged ≤60 days) with a primary diagnosis of HLHS with initial palliation procedures between 2010 and 2012 entered in the STSCHSD were included. Assignment to the hybrid or Norwood group was based on the index operation (first cardiovascular operation of the hospital admission). Hybrid procedures were defined using any 1 of 3 STSCHSD procedural codes: Hybrid Approach “Stage 1,” Application of RPA & LPA bands (2160), Hybrid Approach “Stage 1,” Stent placement inarterial duct (2170) or Hybrid Approach “Stage 1,” or Stent placement in arterial duct + application of RPA and LPA bands (2180). We excluded patients (N = 90) with code 1640, indicating main PA banding, because after further inspection, these patients represented a heterogeneous population who would have confounded the present analysis. Ambiguity regarding coding of PA banding in populations intended to represent patients with HLHS has been a weakness of other studies,16 and we sought to avoid any influence that such vagaries would contribute to our results and inferences. The resultant population included 1728 patients undergoing the hybrid or Norwood procedure from 100 centers.
Data collection
Data collection included demographic information, baseline characteristics, preoperative factors as defined in the STSCHSD, operative variables, and outcomes data. Center characteristics were collected, including average annualized center Norwood volume and total case volume. Center case volumes were calculated using only index cardiopulmonary bypass or non-cardiopulmonary bypass cardiovascular operations classifiable by the STS-European Association for Cardio-Thoracic Surgery mortality categories.12–15 Annual institutional rates of hybrid use were calculated as follows: [hybrid procedures/(Norwood + hybrid + transplant procedures)]. The patients receiving a transplant, because of their exceedingly small numbers, were not included in the analysis in any other respect other than for use in the denominator to calculate hybrid use rate.
Outcomes
The primary outcome measure was use of the hybrid strategy, expressed both as frequency and as percentage of all index palliative procedures for HLHS (sum of hybrid and Norwood). Additional outcome measures include in-hospital mortality (during the same hospital admission) after initial palliation and hospital length of stay after initial palliation.
Analysis
Patient characteristics and outcomes were summarized overall and stratified by initial surgical approach (hybrid vs Norwood stage I). Outcomes were compared for institutions with high hybrid use (defined as ≥50%use) versus institutions with low hybrid use (defined as ≤10%). Although there were 50 centers that did not use the hybrid procedure, these centers were still included in the analysis because they used the Norwood procedure. The 50 centers that performed zero hybrid procedures were grouped into the “low hybrid use” group. These cut points were chosen to represent sites with the clearest patterns of adoption of the hybrid strategy, recognizing that at the remaining centers the choice of approach, on a case-by-case basis, may be influenced by any number of unique circumstances. Neither multivariable modeling nor risk-adjusted comparison of both groups was performed for 2 reasons: (1) The study was meant as a descriptive report of hybrid use among adopting centers; and (2) many of the potential morphologic and intraoperative risk factors were not captured in the version of the STSCHSD used. Data analyses used frequencies and proportions for categoric variables and medians and interquartile ranges (IQRs) for continuous variables. The Wilcoxon rank-sum test was used to compare continuous variables, and the chi-square test was applied to binary outcomes. All analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, NC) and R version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient and Center Characteristics Associated With Hybrid Use
A total of 1728 infants underwent initial palliation for HLHS at 100 centers. The majority (N = 1496, 87%) underwent the Norwood operation as the index procedure, with 232 (13%) undergoing the hybrid as an index procedure and 7 (0.4%) undergoing primary transplantation. Compared with the Norwood group, the hybrid group had a higher prevalence of prematurity (23% vs 9%; P <.001) and chromosomal anomalies/syndromes (13% vs 9%, P =.04). Overall, preoperative factors defined in the STSCHSD were more frequent in the hybrid group (52% vs 37%; P <.001), including a higher prevalence of shock, sepsis, mechanical circulatory support, and stroke (P<.05 for all). Median weight at operation was lower among infants undergoing hybrid palliation versus infants undergoing Norwood palliation (3.0 vs 3.2 kg; P<.001) (Table 1).
TABLE 1.
Patient demographics stratified by groups
| Variable | Overall (1728) | Hybrid (N = 232) | Norwood (N = 1496) | P value |
|---|---|---|---|---|
| Gestational age (wk) | 39 (IQR, 38–39) | 38 (36–39) | 39 (38–39) | <.0001 |
| Premature birth (<37 wk) | 184 (10.7%) | 54 (23.2%) | 130 (8.7%) | <.0001 |
| Birth weight (kg) | 3.1 (2.8–3.4) | 3.0 (2.5–3.3) | 3.1 (2.8–3.5) | .003 |
| Female | 662 (38.3%) | 96 (41.4%) | 566 (37.8%) | .50 |
| Age at index operation (d) | 6 (4–8) | 7 (4–12) | 5 (4–7) | <.0001 |
| Weight at index operation (kg) | 3.2 (2.8–3.5) | 3.0 (2.6–3.5) | 3.2 (2.8–3.5) | .0008 |
| Any abnormalities | 178 (10.3%) | 36 (15.5%) | 142 (9.5%) | .0067 |
| Any chromosomal abnormalities | 164 (9.5%) | 31 (13.4%) | 133 (8.9%) | .036 |
| Any noncardiac anomalies | 24 (1.4%) | 5 (2.2%) | 19 (1.3%) | .307 |
| Any STS-defined preoperative factor | 669 (38.7%) | 120 (51.7%) | 549 (36.7%) | <.0001 |
| Preoperative stroke | 9 (0.5%) | 5 (2.2%) | 4 (0.2%) | .0002 |
| Preoperative shock | 149 (8.6%) | 29 (12.5%) | 120 (8.0%) | .024 |
| Necrotizing enterocolitis | 10 (0.6%) | 4 (1.7%) | 6 (0.4%) | .014 |
| Preoperative sepsis | 9 (0.5%) | 4 (1.7%) | 5 (0.3%) | .006 |
| Preoperative mechanical circulatory support | 4 (0.2%) | 2 (0.9%) | 2 (0.1%) | .032 |
| Preoperative renal dysfunction | 34 (2.0%) | 10 (4.3%) | 24 (1.6%) | .006 |
Median (IQR); no. (%). STS, Society of Thoracic Surgeons.
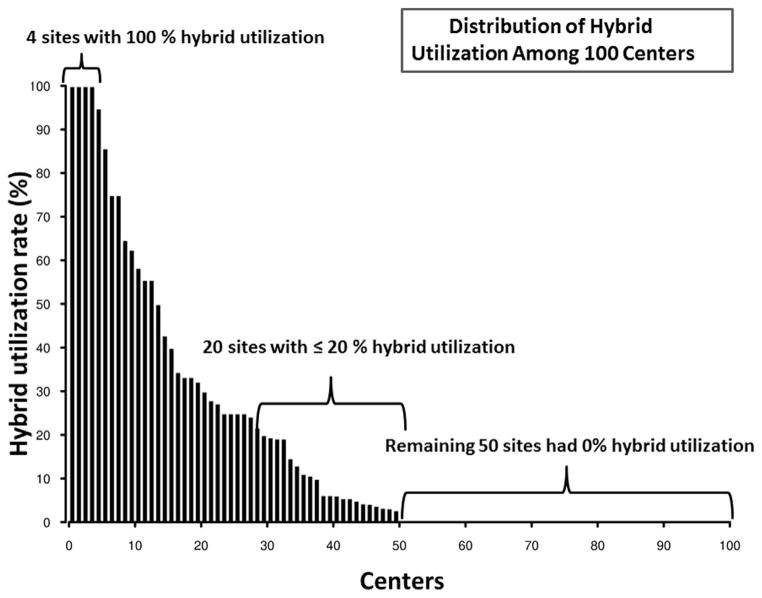
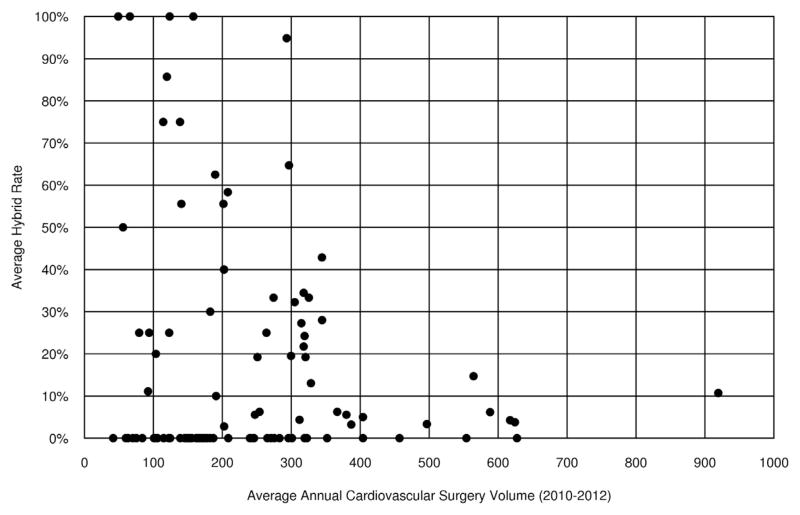
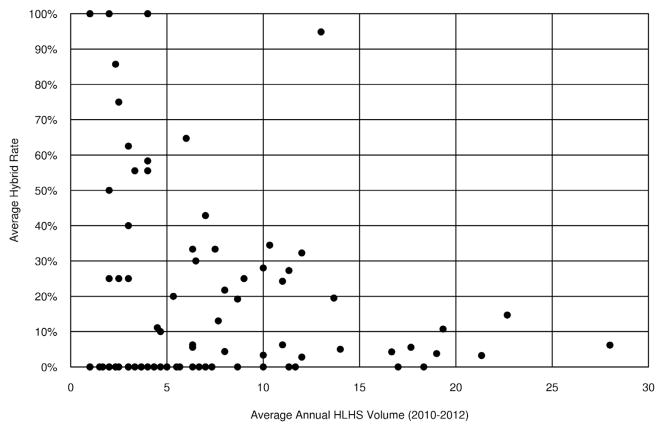
There were 50 institutions that never used the hybrid procedure, with an additional 20 institutions using the hybrid less than 20% of the time (Figure 1). Few centers (13/100) had high hybrid use rates, and these institutions tended to have lower annual index total cardiopulmonary bypass case volume (Figure 2) and lower annual HLHS volume (Figure 3). Preoperative factors of patients undergoing the Norwood were similar among institutions with high versus low hybrid use, with the exception of a higher prevalence of preoperative renal dysfunction (5% vs 2%) among patients undergoing the Norwood at the institutions with high hybrid use (Table E1).
FIGURE 1.
Bar chart demonstrating the hybrid use rate distribution (Y-axis) (as a percentage of the total index cases) among the participating 100 centers (X-axis). Note that 50 centers have zero use (bracket, inset).
FIGURE 2.
Scatter plot demonstrates the relationship of the average institutional hybrid use rate (Y axis) as a function of the annual institutional index cardiopulmonary bypass case volume from 2010 to 2012 (X-axis) for the 100 centers included in the study. Note the inverse relationship whereby institutions with higher hybrid use have lower average cardiopulmonary bypass case volume.
FIGURE 3.
Scatter plot demonstrates the relationship of the average institutional hybrid use rate (Y axis) as a function of the average annual institutional HLHS volume (X axis) for the 100 centers in the study. Note the inverse relationship whereby institutions with higher hybrid use have lower average HLHS volume. HLHS, Hypoplastic left heart syndrome.
Relationship of Hybrid Volume With Postoperative Outcomes
Overall unadjusted in-hospital mortality was 17.5%. Unadjusted in-hospital mortality was higher for the hybrid procedure (n = 69; 29.7%) compared with the Norwood procedure (n = 168; 15.7%; P<.0001). In-hospital mortality for hybrid palliation was independent of hybrid use (26.1% among institutions with low use vs 27.6% among institutions with high use; P = .88). However, institutions with high hybrid use rates had higher mortality after Norwood palliation (42.9%) compared with the institutions that rarely selected a hybrid strategy (15.7%) (P<.0001).
Among sites that performed any hybrid procedures, the log of the hybrid rate was negatively correlated with both total HLHS volume (log-transformed) (Pearson r = −0.59, P<.0001) and total case (cardiopulmonary bypass) volume (log-transformed) (r = −0.57, P<.0001).
Postoperative length of stay was not different among patients undergoing the hybrid procedure (median, 27 days; IQR, 14–57 days) compared with those undergoing the Norwood procedure (median, 28 days; IQR, 16–48 days) (P = .60).
Additional procedures during the same admission were common among both groups. Subsequent Norwood operation, occurring in 19 patients, was the most common procedure after hybrid palliation. The 19 instances of hybrid palliation followed by the Norwood were spread across 13 centers. Six patients undergoing the hybrid underwent heart transplantation during the same admission, and 1 patient required a subsequent transplant. There were no in-hospital mortalities among the 25 patients who had an initial hybrid followed by a Norwood/transplant.
DISCUSSION
The present analysis demonstrates that few centers are currently selecting the hybrid procedure for the majority of infants with HLHS. There were 50 institutions that never used the hybrid procedure. Only 13 centers used the hybrid more than 50% of the time, and we identified only 4 institutions that used the hybrid for more than 90%of neonates. Patterns of hybrid use that we identified include greater use in those institutions with lower HLHS case volume and higher Norwood mortality rates. Although determination of individual institutional factors favoring hybrid selection is beyond the scope of this study, it seems plausible that centers with higher Norwood mortality may preferentially use the hybrid as a perceived alternative to improve HLHS outcomes. Galantowicz,17 in a recent review titled “In Favor of the Hybrid Stage 1 as the Initial Palliation for Hypoplastic Left Heart Syndrome,” discussed a rationale for this possibility, “…hybrid techniques are especially useful in the following two scenarios: (1) when surgery or catheter-based interventions alone are not achieving a satisfactory result for a given problem, and (2) when the combination of the two fields results in less invasiveness and less trauma to the patient.”17 A potential advantage of the hybrid procedure that could be associated with improved outcomes in select patients relates to the perception that it is a technically less-demanding procedure and defers both cardiopulmonary bypass and aortic arch reconstruction until after the neonatal period. In addition to deferring cardiopulmonary bypass beyond the newborn period, some ascribe an advantage of the hybrid to the fact that recovery from the first major exposure to cardiopulmonary bypass is in the setting of a potentially more stable circulatory physiology (systemic and pulmonary circulations in series) subsequent to the comprehensive stage 2 after the initial hybrid. Alternatively, rather than adoption of the hybrid as a method to improve suboptimal HLHS outcomes, it is also plausible that centers with mature, well-developed Norwood programs might be comfortable exploring a new management strategy that may broaden the spectrum of “survivable” neonates. These centers may offer Norwood palliation to ideal candidates and reserve the hybrid for those patients with multiple risk factors. This paradigm could result in an improvement in the institution’s Norwood outcomes while concomitantly reducing overall institutional mortality. Other possible patterns of adoption might include the initial use of hybrids for salvage procedures that would be followed by incorporation of hybrids into more routine cases or the selective use of the hybrid as a bridge to Norwood palliation once the infant had stabilized or as a bridge to transplantation. A few individual centers outside North America have described the results of a “rapid 2-stage Norwood” strategy, in which bilateral PA banding is followed within days or weeks by Norwood stage 1 as a preferred approach.18,19 On the basis of the data in our study, approximately 8% of neonates may have been triaged to a “bridge to Norwood” strategy, whereas few neonates (3%) underwent subsequent transplantation. Unfortunately, we are unable to identify whether these subsequent procedures were part of an initial strategy or performed for hybrid failure or other reasons. The fact that 19 instances of the Norwood after the hybrid were spread across 13 centers suggests that these do not represent any institutional adoption of a “rapid 2-stage Norwood” strategy. In addition, because we have only data derived from one time period that does not circumscribe the true introduction of the hybrid procedure, we cannot clearly define the patterns of adoption in potential “early-adopting” centers or the velocity of dissemination to later adopters. Subsequent studies incorporating both the introductory period and the additional follow-up may allow elucidation of this dissemination kinetics and help characterize adopting centers and their motivations.
We did not find differences in hybrid mortality among centers with high versus low hybrid use. Although the hybrid approach is perceived by some as being less technically demanding than the Norwood procedure, there are several subtleties, especially concerning PA band assessment20 and the recognition of potential pitfalls, that may be associated with improvement in outcomes in centers with extensive hybrid experience.3,9–11 Caldarone and colleagues, from the Hospital for Sick Children in Toronto, described the potential for retrograde aortic arch obstruction after ductal stent deployment,9,19 and this group now routinely uses a reverse Blalock–Taussig shunt in patients with aortic atresia or severely restricted antegrade aortic blood flow.9,21 In addition, the timing of left atrial decompression and whether stent placement is used routinely after septostomy are modifiable procedural factors that are variably used at different centers.3,4,9,10
Multiple single-institution studies have compared Norwood outcomes with hybrid outcomes, with the potential caveat that the hybrid strategy has been used at many centers for high-risk neonates.6,7,22 Our data clearly demonstrate an important selection bias that would favor Norwood palliation compared with hybrid palliation with respect to improved early outcomes, in that patients undergoing the hybrid had more potential patient risk factors. Venugopal and colleagues22 studied 21 neonates who were triaged to a hybrid approach if they met the following criteria: birth weight less than 2.5 kg, possibility of a cerebral infarct, intact or nearly intact atrial septum, aortic stenosis with a poorly contractile left ventricle, or other associated “unusual variants.” The authors reported 29% hospital mortality, which they suggested was lower than that of the Norwood operation for patients with a similar risk profile, although the data for the comparable cohort of patients undergoing Norwood are not actually provided.22 Likewise, Pizarro and colleagues6 studied 33 “high-risk” neonates, 14 of whom were triaged to the hybrid strategy and 19 who underwent conventional Nor-wood palliation. Neonates in the hybrid group still had lower preoperative pH and a higher prevalence of preoperative organ dysfunction than the Norwood group despite the “high-risk” nature of the entire cohort. Both groups had equivalent hospital and interstage mortality (21% for the hybrid group and 23% for the Norwood group) despite the inequities mentioned. Striking in many of these reports is the notion that hybrid palliation performs equally well as conventional Norwood palliation for the highest risk patients.6,7,22 Although the objective of our present study was to describe patterns of practice and not to assess whether hybrid palliation is equivalent to Norwood palliation in similar patients, certainly a propensity-matched or risk-adjusted analysis comparing the 2 strategies in a large multicenter population would be a logical next step.
Study Limitations
The limitations of this study are primarily related to the nature of the STSCHSD. The thresholds used to designate centers into groups with high and low use were chosen to exclude the sites in the middle, which presumably reserve the hybrid procedure for the higher-risk patients and the Norwood for lower-risk patients. We acknowledge that these may not resonate with the usual assumptions regarding “high” versus “low,” although they were chosen for pragmatic reasons. Although we were able to evaluate many patient preoperative risk factors in our analysis of patient risk status, at the time of this study, not all potential risk factors and intraoperative factors were collected in the database, for example, the presence of an intact atrial septum, pulmonary venous obstruction, the size of the ascending aorta, and the full spectrum of perfusion techniques and temperatures. This, in part, precluded a detailed multivariable analysis comparing outcomes in those undergoing the hybrid versus the Norwood. Incorporation of many of these variables into the database in the last update in January may enable these types of analysis in the future. In addition, the database contains specific definitions for all variables, but cannot exclude variation in coding across centers. We acknowledge that patients undergoing hybrid palliation had a higher proportion of preoperative risk factors than patients undergoing the Norwood operation, but we were unable, because of limitations regarding sample size and available variables, to perform a risk-adjusted analysis to reduce selection bias.
After careful deliberation, we chose to exclude 90 patients who were coded as having main PA banding, mainly to avoid confounding due to miscoding. On the basis of a sensitivity analysis that included the PA band subset, it is unlikely that exclusion of this subset has informed the results in a meaningful way. The mortality among the subset of patients with PA bands was 28.9%, which was similar to the in-hospital mortality of the hybrid cases (29.7%). In regard to whether sites could have misclassified hybrid cases as PA band cases (therefore altering our assignment of a low vs high hybrid use site and changing our center-level interpretation), this is also unlikely given that only one center would move from the low hybrid to the high hybrid groups if they were indeed misclassifying hybrid cases as PA bands.
Finally, although the database currently captures information regarding some process measures, information regarding personnel or hospital structure or process measures were unavailable for this analysis; therefore, we are unable to evaluate the relationship of these factors to center volume or outcome.
CONCLUSIONS
Few centers are currently selecting hybrid palliation for the majority of infants with HLHS. Although unadjusted in-hospital hybrid mortality rates are higher than after Nor-wood palliation, potential patient risk factors are more prevalent among neonates undergoing hybrid procedures. Institutions with higher hybrid use have lower annual HLHS case volume and higher Norwood mortality. Future studies describing the introduction of the hybrid procedure and additional follow-up could elucidate hybrid dissemination kinetics and help characterize adopting centers and their motivations.
Supplementary Material
Abbreviations and Acronyms
- HLHS
hypoplastic left heart syndrome
- IQR
interquartile range
- PA
pulmonary artery
- STSCHSD
Society of Thoracic Surgeons Congenital Heart Surgery Database
Footnotes
Read at the 94th Annual Meeting of The American Association for Thoracic Surgery, Toronto, Ontario, Canada, April 26–30, 2014.
Disclosures: Dr Pasquali receives support from the National Heart, Lung, and Blood Institute (K08HL103631, Principal Investigator: Dr Pasquali). All other authors have nothing to disclose with regard to commercial support.
References
- 1.Akintuerk H, Michel-Behnke I, Valeske K, Mueller M, Thul J, Bauer J, et al. Stenting of the arterial duct and banding of the pulmonary arteries: basis of a combined Norwood stage I and II repair in hypoplastic left heart. Circulation. 2002;105:1099–103. doi: 10.1161/hc0902.104709. [DOI] [PubMed] [Google Scholar]
- 2.Michel-Behnke I, Akintuerk H, Marquardt I, Mueller M, Thul J, Bauer J, et al. Stenting of the ductus arteriosus and banding of the pulmonary arteries: basis for various surgical strategies in newborns with multiple left heart obstructive lesions. Heart. 2003;89:645–50. doi: 10.1136/heart.89.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol. 2005;26:190–9. doi: 10.1007/s00246-005-8962-6. [DOI] [PubMed] [Google Scholar]
- 4.Galantowicz M, Cheatham JP, Phillips A, Cua CL, Hoffman TM, Hill SL, et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–71. doi: 10.1016/j.athoracsur.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd FA, Cutler L, Tibby SM, Vimalesvaran S, Qureshi SA, Rosenthal E, et al. Analysis of preoperative condition and interstage mortality in Norwood and hybrid procedures for hypoplastic left heart syndrome using the Aristotle scoring system. Heart. 2014;100:775–80. doi: 10.1136/heartjnl-2013-304759. [DOI] [PubMed] [Google Scholar]
- 6.Pizarro C, Derby CD, Baffa JM, Murdison KA, Radtke WA. Improving the outcome of high-risk neonates with hypoplastic left heart syndrome: hybrid procedure or conventional surgical palliation? Eur J Cardiothorac Surg. 2008;33:613–8. doi: 10.1016/j.ejcts.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Baba K, Kotani Y, Chetan D, Chaturvedi RR, Lee KJ, Benson LN, et al. Hybrid versus Norwood strategies for single-ventricle palliation. Circulation. 2012;126(Suppl 1):S123–31. doi: 10.1161/CIRCULATIONAHA.111.084616. [DOI] [PubMed] [Google Scholar]
- 8.Caldarone CA, Benson L, Holtby H, Li J, Redington AN, Van Arsdell GS. Initial experience with hybrid palliation for neonates with single-ventricle physiology. Ann Thorac Surg. 2007;84:1294–300. doi: 10.1016/j.athoracsur.2007.04.127. [DOI] [PubMed] [Google Scholar]
- 9.Honjo O, Benson LN, Mewhort HE, Predescu D, Holtby H, Van Arsdell GS, et al. Clinical outcomes, program evolution, and pulmonary artery growth in single ventricle palliation using hybrid and Norwood palliative strategies. Ann Thorac Surg. 2009;87:1885–92. doi: 10.1016/j.athoracsur.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 10.Honjo O, Tran KC, Hua Z, Sapra P, Alghamdi AA, Russell JL, et al. Impact of evolving strategy on clinical outcomes and central pulmonary artery growth in patients with bilateral superior vena cava undergoing bilateral bidirectional cavopulmonary shunt. J Thorac Cardiovasc Surg. 2010;140:522–8. doi: 10.1016/j.jtcvs.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Caldarone CA, Honjo O, Benson LN, Van Arsdell GS. Modification of stage II procedure after hybrid palliation (bilateral pulmonary artery banding and ductal stenting) for hypoplastic left heart syndrome: modified arch reconstruction with retained stented ductus patch. J Thorac Cardiovasc Surg. 2007;134:1588–9. doi: 10.1016/j.jtcvs.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs ML, Daniel M, Mavroudis C, Morales DL, Jacobs JP, Fraser CD, Jr, et al. Report of the 2010 Society of Thoracic Surgeons congenital heart surgery practice and manpower survey. Ann Thorac Surg. 2011;92:762–9. doi: 10.1016/j.athoracsur.2011.03.133. [DOI] [PubMed] [Google Scholar]
- 13.Clarke DR, Breen LS, Jacobs ML, Franklin RC, Tobota Z, Maruszewski B, et al. Verification of data in congenital cardiac surgery. Cardiol Young. 2008;18(Suppl 2):177–87. doi: 10.1017/S1047951108002862. [DOI] [PubMed] [Google Scholar]
- 14.Society of Thoracic Surgeons. [Accessed December 1, 2013];Society of Thoracic Surgeons National Database. Available at: www.sts.org/sections/stsnationaldatabase/
- 15.Jacobs ML, O’Brien SM, Jacobs JP, Mavroudis C, Lacour-Gayet F, Pasquali SK, et al. An empirically based tool for analyzing morbidity associated with operations for congenital heart disease. J Thorac Cardiovasc Surg. 2013;145:1046–57. e1041. doi: 10.1016/j.jtcvs.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roeleveld PP, de Wilde R, Hazekamp M, Rycus PT, Thiagarajan RR. Extracorporeal membrane oxygenation in single ventricle lesions palliated via the hybrid approach. World J Pediatr Congenit Heart Surg. 2014;5:393–7. doi: 10.1177/2150135114526420. [DOI] [PubMed] [Google Scholar]
- 17.Galantowicz M. In favor of the hybrid stage 1 as the initial palliation for hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2013;16:62–4. doi: 10.1053/j.pcsu.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Gomide M, Furci B, Mimic B, Brown KL, Hsia TY, Yates R, et al. Rapid 2-stage Norwood I for high-risk hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2013;146:1146–51. doi: 10.1016/j.jtcvs.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 19.Ota N, Muramata M, Tosaka Y, Ide Y, Tachi M, Ito H, et al. Is routine rapid-staged bilateral pulmonary artery banding before stage 1 Norwood a viable strategy? J Thorac Cardiovasc Surg. 2014;148:1519–25. doi: 10.1016/j.jtcvs.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 20.Zampi JD, Hirsch JC, Goldstein BH, Armstrong AK. Use of a pressure guidewire to assess pulmonary artery band adequacy in the hybrid stage I procedure for high-risk neonates with hypoplastic left heart syndrome and variants. Congenit Heart Dis. 2013;8:149–58. doi: 10.1111/chd.12005. [DOI] [PubMed] [Google Scholar]
- 21.Baba K, Honjo O, Chaturvedi R, Lee KJ, Van Arsdell G, Caldarone CA, et al. “Reverse Blalock-Taussig shunt”: application in single ventricle hybrid palliation. J Thorac Cardiovasc Surg. 2013;146:352–7. doi: 10.1016/j.jtcvs.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Venugopal PS, Luna KP, Anderson DR, Austin CB, Rosenthal E, Krasemann T, et al. Hybrid procedure as an alternative to surgical palliation of high-risk infants with hyoplastic left heart syndrome and its variants. J Thorac Cardiovasc Surg. 2010;139:1211–5. doi: 10.1016/j.jtcvs.2009.11.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.