Abstract
Background
Geographic variations associated with surgical intervention for congenital heart disease are ill defined. This study uses a large clinical registry to assess frequency of surgical intervention for various infant congenital heart diseases overall and across US geographic regions.
Methods
Patients younger than 1 year of age in the Society of Thoracic Surgeons Congenital Heart Surgery Database (January 2010 through June 2012) were included. Index operations were classified on the basis of seven major diagnostic groups and 10 specific diagnoses and were compared across geographic regions using a χ2 test. Region was defined by patient residence.
Results
The study included 23,379 patients (94 centers). Septal defects (26.2%) were the most frequently reported diagnostic group, and tetralogy of Fallot (10.6%) was the most frequent specific diagnosis. Significant geographic variation was noted for all seven major diagnostic groups. The proportion of patients undergoing surgery for septal defects varied from 23.9% to 30.2% (p = 0.001); pulmonary venous anomalies, 2.8% to 4.5% (p = 0.03); right heart lesions, 15.7% to 21.4% (p < 0.0001); left heart lesions, 22.7% to 30.4% (p = 0.0002); single-ventricle lesions, 7.3% to 11.4% (p < 0.0001); transposition of the great arteries and double-outlet right ventricle, 9.0% to 15.3% (p < 0.0001); and coronary artery anomalies, 0.4% to 1.4% (p = 0.04). Significant regional variation was also observed for 7 of the 10 specific diagnoses examined.
Conclusions
These data demonstrate significant variation in congenital heart disease diagnostic groups requiring surgery before 1 year of age across US geographic regions.
Congenital heart disease (CHD) is the most common birth defect, and hospital costs for infants with CHD exceed those of all other birth defects combined [1]. Therefore it is critical to understand, on both a national and regional level, the prevalence of various CHD diagnoses. Such estimates guide programmatic planning, resource allocation, and other strategic initiatives.
Inherent difficulties exist in estimating overall prevalence of any condition. These difficulties become more pronounced when evaluating regional variation. Probability analyses rely on incomplete datasets using nonstandardized methodologies of analysis and report from either individual centers or specific geographic areas [2, 3]. In the United States, regional studies have based their analysis on either birth defect registries or regional administrative databases [4, 5]. Recent studies have noted the limitations of such data sources [6, 7]. It is possible to overcome many of these limitations by evaluating treatment stratified according to diagnostic cohorts. From a resource allocation perspective, assessment of treatment cohorts provides an equally important assessment of disease burden.
Surgical treatment for infant CHD is likely the most important driver of CHD resource utilization. Defining overall frequency of surgical operations for various CHD diagnostic groups, on both a national and regional level, can be used to guide strategic and other resource allocation initiatives. Therefore, the purpose of this study was to use a large clinical registry to evaluate the frequency of surgical operations and the relative proportions of various diagnostic groups undergoing surgery among infants (<1 year) in the United States. We also sought to determine whether regional differences exist in frequency of surgical operations for various diagnoses and whether these differences might be substantial enough to impact programmatic planning.
Patients and Methods
Data Source
The Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD), a voluntary registry that contains preoperative, operative, and outcomes data for all patients undergoing congenital and pediatric cardiovascular operations at participating centers, was used for this study. As of January 2014, the STS-CHSD contains data on more than 292,000 surgeries conducted since 2000 at 120 hospitals in North America, representing approximately 95% of all US centers performing congenital heart surgery [8]. Database coding is accomplished by clinicians and support staff using the International Pediatric and Congenital Cardiac Code [9, 10] and is entered into the STS-CHSD data collection form (version 3.0) [11]. Evaluation of data quality includes the intrinsic verification of data, along with a formal process of site visits and data audits at approximately 10% of participating centers each year [12]. The Duke Clinical Research Institute serves as the analytic center for all STS National Databases. This study was approved by the STS-CHSD Access and Publications Committee and was not considered human subjects research by the Duke University Institutional Review Board (45 CFR 46.102(f)).
Study Population
One hundred four centers submitted data for 55,415 cardiovascular operations performed on patients younger than 18 years of age from January 1, 2010, to June 29, 2012, using STS-CHSD version 3.0. Those undergoing operation at greater than 1 year of age were excluded, leaving 30,102 operations. Patients undergoing isolated surgical closure of a patent ductus arteriosus (n = 3,534), operations performed on patients whose region was documented as being outside the United States (defined in the STS-CHSD as region “other”; n = 2,808 at 9 centers), and operations on patients with missing data for region of residence (n = 381 at 1 center) were also excluded. The final study population therefore included 23,379 operations performed at 94 centers.
Data Collection
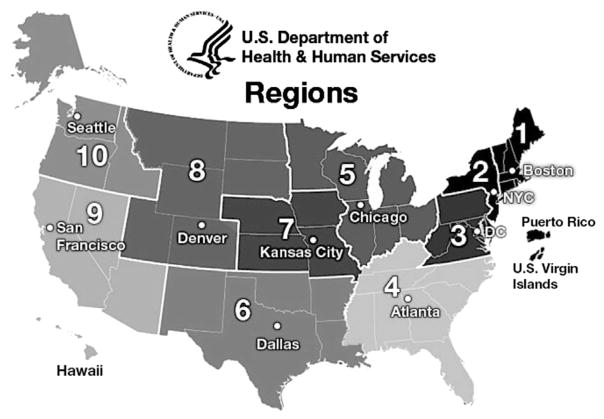
Data collected included patient age and patient region (based on location of patient residence before surgery), as well as fundamental diagnosis and primary diagnoses at the time of operation. The fundamental diagnosis is defined by the STS-CHSD as the most complex cardiac anomaly (congenital or acquired) or condition of the patient at the time of the patient’s birth or initial presentation for cardiac care; the fundamental diagnosis is a diagnosis that is associated with the patient throughout the life of the patient, through all operations and hospitalizations. The primary diagnosis is defined as the diagnosis that is the principal diagnostic reason for performing the operation. The primary diagnosis may or may not be the same as the fundamental diagnosis. Isolated ductal ligations were thus excluded to ensure data analysis focused on the seven major diagnostic groups and 10 primary diagnoses. Patient regions were categorized into 10 health care regions as defined by the US Department of Health and Human Services (USDHHS). By convention, each of the 10 USDHHS regions is identified with the name of a major city within that region (Fig 1).
Fig 1.
- Boston region (CT, ME, MA, NH, RI, VT)
- New York region (NJ, NY, PR, VI)
- DC (District of Columbia) region (DE, DC, MD, PA, VA, WV)
- Atlanta region (AL, FL, GA, KY, MS, NC, SC, TN)
- Chicago region (IL, IN, MI, MN, OH, WI)
- Dallas region (AR, LA, NM, OK, TX)
- Kansas City region (IA, KS, MO, NE)
- Denver region (CO, MT, ND, SD, UT, WY)
- San Francisco region (AZ, CA, HI, NV, AS, MP, FM, GU, MH, PW)
- Seattle region (AK, ID, OR, WA)
Data Analysis
All eligible operations were categorized into groups on the basis of the patient’s STS-CHSD fundamental diagnosis (or primary diagnosis in instances when the fundamental diagnosis was missing). Cases were assigned to one of seven major diagnostic groups (Table 1). In addition, a subset of cases, made up of those that could be assigned to 1 of 10 more specific diagnoses of interest, were considered (Table 2). After stratification of cases according to these diagnostic criteria and assignment of each case to 1 of the 10 standard USDHHS health care regions used in government reporting, the frequency of each diagnostic group across the 10 regions was estimated (along with 95% confidence intervals). The proportion of patients in each diagnostic category (of all patients <1 year of age undergoing cardiac surgery in the region) was compared across regions using a χ2 test. The USDHHS regional classification system assigns a number and a major city to each region. The city names, which correspond to USDHHS contact sites within each region, are retained in this report only to facilitate recognition of regions. It should not, however, be inferred that cases correspond to that particular city any more than any others within that region. All analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, NC). A probability value of less than 0.05 was considered statistically significant.
Table 1.
Seven Major Diagnostic Groupsa
|
The numbers within parenthesis are the individual diagnostic codes in the STS-CHSD Version 3.0 (2010–2013).
Table 2.
Ten Specific Diagnosesa
|
The numbers within parenthesis are the individual diagnostic codes in the STS-CHSD Version 3.0 (2010–2013).
Results
Overall Frequency of Surgical Intervention
Evaluation of the overall frequency of surgical intervention within the first year of life with regard to major diagnostic groups demonstrated septal defects (26.2%) and left heart lesions (24.7%) as being the most common (Table 3). In terms of specific diagnoses, tetralogy of Fallot (10.6%) and hypoplastic left heart syndrome (10.4%) were the most frequently reported (Table 4).
Table 3.
Frequency of Seven Major Diagnostic Groups Requiring Surgical Intervention in the First Year of Life in the Overall Study Cohort
| Diagnostic Group | Overall (n = 23,379) |
|---|---|
| Septal defects, n (%) | 6,116 (26.2) |
| Pulmonary venous anomalies, n (%) | 860 (3.7) |
| Other right heart lesions, n (%) | 4,507 (19.3) |
| Left heart lesions, n (%) | 5,780 (24.7) |
| Single ventricle, n (%) | 2,285 (9.8) |
| Transposition of the great arteries and double-outlet ventricles, n (%) | 2,760 (11.8) |
| Coronary artery anomalies, n (%) | 139 (0.6) |
Table 4.
Frequency of Ten Specific Diagnoses Requiring Surgical Intervention in the First Year of Life in the Overall Study Cohort
| Specific Diagnosis | Overall (n = 23,379) |
|---|---|
| Atrioventricular canal, n (%) | 2,126 (9.1) |
| Truncus arteriosus, n (%) | 426 (1.8) |
| Total anomalous pulmonary venous return, n (%) | 826 (3.5) |
| Tetralogy of Fallot, n (%) | 2,486 (10.6) |
| Pulmonary atresia/intact ventricular septum, n (%) | 462 (2.0) |
| Tricuspid atresia, n (%) | 633 (2.7) |
| Unbalanced atrioventricular canal, n (%) | 248 (1.1) |
| Hypoplastic left heart syndrome, n (%) | 2,432 (10.4) |
| Interrupted aortic arch, n (%) | 404 (1.7) |
| Transposition of the great arteries, n (%) | 1,657 (7.1) |
Geographic Variation for Seven Major Diagnostic Groups
Significant variation was observed for all seven major diagnostic groups across the USDHHS health care regions (Table 5). The proportion of patients undergoing surgery for septal defects ranged from 23.9% to 30.2% (p = 0.001); pulmonary venous anomalies, 2.8% to 4.5% (p = 0.03); right heart lesions, 15.7% to 21.4% (p < 0.0001); left heart lesions, 22.7% to 30.4% (p = 0.0002); single-ventricle lesions, 7.3% to 11.4% (p < 0.0001); transposition of the great arteries and double-outlet right ventricle, 9.0% to 15.3% (p < 0.0001); and coronary artery anomalies, 0.4% to 1.4% (p = 0.04). For each diagnostic group there were regions for which the 95% confidence intervals did not overlap. When compared with the region with the highest lower limit confidence interval, there was absence of overlap for 10% to 60% of regions, depending on the diagnostic cohort (Table 5).
Table 5.
Frequency and Relative Prevalence of Seven Major Diagnostic Groups Across US Department of Health and Human Services Health Care Regionsa
| Diagnostic Group | Boston (n = 872) | New York (n = 1,391) | DC (n = 2,312) | Atlanta (n = 5,100) | Chicago (n = 4,444) | Dallas (n = 2,509) | Kansas City (n = 1,120) | Denver (n = 1,281) | San Francisco (n = 3,149) | Seattle (n = 1,201) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Septal defects, n (%) | 263 (30.2) | 333 (23.9) | 565 (24.4) | 1,305 (25.6) | 1,147 (25.8) | 667 (26.6) | 305 (27.2) | 369 (28.8) | 810 (25.7) | 352 (29.3) | 0.001 |
| 95% CI |
|
21.7–26.2 | 22.7–26.2 | 24.4–26.8 | 24.5–27.1 | 24.9–28.3 | 24.6–29.8 | 26.3–31.3 | 24.2–27.2 | 26.7–31.9 | |
| Pulmonary venous anomalies, n (%) | 28 (3.2) | 44 (3.2) | 64 (2.8) | 181 (3.5) | 152 (3.4) | 104 (4.1) | 42 (3.8) | 48 (3.7) | 143 (4.5) | 54 (4.5) | 0.03 |
| 95% CI | 2.0–4.4 | 2.2–4.1 | 2.1–3.4 | 3.0–4.1 | 2.9–4.0 | 3.4–4.9 | 2.6–4.9 | 2.7–4.8 |
|
3.3–5.7 | |
| Other right heart lesions, n (%) | 142 (16.3) | 298 (21.4) | 456 (19.7) | 1,038 (20.4) | 827 (18.6) | 465 (18.5) | 202 (18.0) | 201 (15.7) | 674 (21.4) | 204 (17.0) | <0.0001 |
| 95% CI | 13.8–18.7 |
|
18.1–21.3 | 19.2–21.5 | 17.5–19.8 | 17.0–20.1 | 15.8–20.3 | 13.7–17.7 | 20.0–22.8 | 14.9–19.1 | |
| Left heart lesions, n (%) | 208 (23.9) | 363 (26.1) | 555 (24.0) | 1,262 (24.7) | 1,108 (24.9) | 602 (24.0) | 273 (24.4) | 389 (30.4) | 714 (22.7) | 306 (25.5) | 0.0002 |
| 95% CI | 21.0–26.7 | 23.8–28.4 | 22.3–25.7 | 23.6–25.9 | 23.7–26.2 | 22.3–25.7 | 21.9–26.9 |
|
21.2–24.1 | 23.0–27.9 | |
| Single ventricle, n (%) | 70 (8.0) | 147 (10.6) | 228 (9.9) | 579 (11.4) | 468 (10.5) | 224 (8.9) | 90 (8.0) | 116 (9.1) | 275 (8.7) | 88 (7.3) | <0.0001 |
| 95% CI | 6.2–9.8 | 9.0–12.2 | 8.6–11.1 |
|
9.6–11.4 | 7.8–10.0 | 6.4–9.6 | 7.5–10.6 | 7.7–9.7 | 5.9–8.8 | |
| TGA and double-outlet ventricles, n (%) | 118 (13.5) | 150 (10.8) | 291 (12.6) | 560 (11.0) | 552 (12.4) | 274 (10.9) | 171 (15.3) | 115 (9.0) | 383 (12.2) | 146 (12.2) | <0.0001 |
| 95% CI | 11.3–15.8 | 9.2–12.4 | 11.2–13.9 | 10.1–11.8 | 11.5–13.4 | 9.7–12.1 |
|
7.4–10.5 | 11.0–13.3 | 10.3–14.0 | |
| Coronary artery anomalies, n (%) | 5 (0.6) | 19 (1.4) | 9 (0.4) | 30 (0.6) | 21 (0.5) | 18 (0.7) | 7 (0.6) | 6 (0.5) | 17 (0.5) | 7 (0.6) | 0.04 |
| 95% CI | 0.1–1.1 |
|
0.1–0.6 | 0.4–0.8 | 0.3–0.7 | 0.4–1.0 | 0.2–1.1 | 0.1–0.8 | 0.3–0.8 | 0.2–1.0 |
For each diagnostic group, the region with the highest 5% CI is boxed and those regions for which there is no overlap with this CI are italicized.
CI = confidence interval; TGA = transposition of the great arteries.
Geographic Variation for Ten Specific Diagnoses
Significant regional variation was also observed among the cases assigned to 7 of the 10 specific diagnoses selected for evaluation. More specifically, atrioventricular canal defect was the highest frequency in region 1 (Boston; p = 0.02), tetralogy of Fallot was the highest frequency in regions 2 and 3 (New York City and Washington, DC; p = 0.0006), pulmonary atresia and intact ventricular septum was the highest frequency in region 9 (San Francisco; p < 0.0001), tricuspid atresia was the highest frequency in region 1 (Boston; p = 0.009), unbalanced atrioventricular canal defect was the highest in region 5 (Chicago; p < 0.0001), hypo-plastic left heart syndrome was the highest frequency in region 7 (Kansas City; p = 0.03), and transposition of the great arteries was the highest frequency in the region 7 (Kansas City; p = 0.001); all displayed significance (Table 6). For all of the specific diagnoses with the exception of truncus arteriosus, there were regions for which the 95% confidence intervals did not overlap. When compared with the region with the highest lower limit confidence interval, there was absence of overlap for 10% to 60% of these regions depending on the diagnostic cohort (Table 6).
Table 6.
Frequency and Relative Prevalence of Ten Specific Diagnoses Across US Department of Health and Human Services Health Care Regionsa
| Specific Diagnosis | Boston (n = 872) | New York (n = 1,391) | DC (n = 2,312) | Atlanta (n = 5,100) | Chicago (n = 4,444) | Dallas (n = 2,509) | Kansas City (n = 1,120) | Denver (n = 1,281) | San Francisco (n = 3,149) | Seattle (n = 1,201) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AV canal, n (%) | 103 (11.8) | 118 (8.5) | 191 (8.3) | 505 (9.9) | 407 (9.2) | 210 (8.4) | 102 (9.1) | 123 (9.6) | 256 (8.1) | 111 (9.2) | 0.02 |
| 95% CI |
|
7.0–9.9 | 7.1–9.4 | 9.1–10.7 | 8.3–10.0 | 7.3–9.5 | 7.4–10.8 | 8.0–11.2 | 7.2–9.1 | 7.6–10.9 | |
| Truncus arteriosus, n (%) | 13 (1.5) | 24 (1.7) | 36 (1.6) | 99 (1.9) | 74 (1.7) | 52 (2.1) | 19 (1.7) | 28 (2.2) | 64 (2.0) | 17 (1.4) | 0.70 |
| 95% CI | 0.7–2.3 | 1.0–2.4 | 1.1–2.1 |
|
1.3–2.0 | 1.5–2.6 | 0.9–2.5 | 1.4–3.0 | 1.5–2.5 | 0.7–2.1 | |
| Total anomalous pulmonary venous return, n (%) | 28 (3.2) | 44 (3.2) | 64 (2.8) | 172 (3.4) | 146 (3.3) | 100 (4.0) | 40 (3.6) | 44 (3.4) | 138 (4.4) | 50 (4.2) | 0.08 |
| 95% CI | 2.0–4.4 | 2.2–4.1 | 2.1–3.4 | 2.9–3.9 | 2.8–3.8 | 3.2–4.8 | 2.5–4.7 | 2.4–4.4 |
|
3.0–5.3 | |
| Tetralogy of Fallot, n (%) | 93 (10.7) | 171 (12.3) | 285 (12.3) | 582 (11.4) | 454 (10.2) | 237 (9.4) | 120 (10.7) | 105 (8.2) | 327 (10.4) | 112 (9.3) | 0.0006 |
| 95% CI | 8.6–12.7 | 10.6–14.0 |
|
10.5–12.3 | 9.3–11.1 | 8.3–10.6 | 8.9–12.5 | 6.7–9.7 | 9.3–11.5 | 7.7–11.0 | |
| Pulmonary atresia/intact ventricular septum, n (%) | 10 (1.1) | 21 (1.5) | 24 (1.0) | 104 (2.0) | 92 (2.1) | 47 (1.9) | 23 (2.1) | 33 (2.6) | 94 (3.0) | 14 (1.2) | <−.0001 |
| 95% CI | 0.4–1.9 | 0.9–2.2 | 0.6–1.5 | 1.7–2.4 | 1.7–2.5 | 1.3–2.4 | 1.2–2.9 | 1.7–3.4 |
|
0.6–1.8 | |
| Tricuspid atresia, n (%) | 29 (3.3) | 36 (2.6) | 70 (3.0) | 155 (3.0) | 131 (2.9) | 60 (2.4) | 31 (2.8) | 40 (3.1) | 67 (2.1) | 14 (1.2) | 0.009 |
| 95% CI | 2.1–4.5 | 1.8–3.4 | 2.3–3.7 |
|
2.5–3.4 | 1.8–3.0 | 1.8–3.7 | 2.2–4.1 | 1.6–2.6 | 0.6–1.8 | |
| Unbalanced AV canal, n (%) | 2 (0.2) | 12 (0.9) | 25 (1.1) | 68 (1.3) | 78 (1.8) | 15 (0.6) | 5 (0.4) | 2 (0.2) | 35 (1.1) | 6 (0.5) | <−.0001 |
| 95% CI | 0.1–0.5 | 0.4–1.3 | 0.7–1.5 | 1.0–1.6 |
|
0.3–0.9 | 0.1–0.8 | 0.1–0.4 | 0.7–1.5 | 0.1–0.9 | |
| HLHS, n (%) | 92 (10.6) | 150 (10.8) | 235 (10.2) | 560 (11.0) | 451 (10.1) | 293 (11.7) | 133 (11.9) | 120 (9.4) | 287 (9.1) | 111 (9.2) | 0.03 |
| 95% CI | 8.5–12.6 | 9.2–12.4 | 8.9–11.4 | 10.1–11.8 | 9.3–11.0 |
|
10.0–13.8 | 7.8–11.0 | 8.1–10.1 | 7.6–10.9 | |
| Interrupted aortic arch, n (%) | 8 (0.9) | 30 (2.2) | 35 (1.5) | 84 (1.6) | 80 (1.8) | 36 (1.4) | 18 (1.6) | 18 (1.4) | 70 (2.2) | 25 (2.1) | 0.14 |
| 95% CI | 0.3–1.6 | 1.4–2.9 | 1.0–2.0 | 1.3–2.0 | 1.4–2.2 | 1.0–1.9 | 0.9–2.3 | 0.8–2.1 |
|
1.3–2.9 | |
| TGA, n (%) | 82 (9.4) | 101 (7.3) | 179 (7.7) | 317 (6.2) | 324 (7.3) | 138 (5.5) | 111 (9.9) | 80 (6.2) | 230 (7.3) | 95 (7.9) | <−.0001 |
| 95% CI | 7.5–11.3 | 5.9–8.6 | 6.7–8.8 | 5.6–6.9 | 6.5–8.1 | 4.6–6.4 |
|
4.9–7.6 | 6.4–8.2 | 6.4–9.4 |
For each diagnostic group, the region with the highest 5% CI is boxed and those regions for which there is no overlap with this CI are italicized.
AV = atrioventricular; CI = confidence interval; HLHS = hypoplastic left heart syndrome; TGA = transposition of the great arteries.
Comment
This study addresses a knowledge gap concerning the overall frequency and regional variability of surgery for CHD within the first year of life. Operations performed on patients within the first year of life account for approximately two thirds of all procedures in the STS-CHSD. Our analysis revealed significant regional variation in the frequency and relative proportions of both the seven major diagnostic groups and the 10 specific diagnoses across USDHHS health care regions.
The results of the present study add a new dimension in relation to previous studies, which described the prevalence of CHD within certain specific regions in the United States, but not rates of surgical intervention [13]. In these prior analyses, septal defects (including atrial septal defect, ventricular septal defect, and atrioventricular canal defect) are the most common diagnostic group [14]. Our analysis demonstrates that they are also the most common lesion undergoing surgical intervention in neonates and infants. However, the frequency of surgery for more complex diagnostic groups is proportionately much greater than the overall prevalence of these complex lesions. For example, hypoplastic left heart syndrome, although 25 times less prevalent than septal defects in population estimates, accounts for almost half as many surgeries in the first year of life. Similar trends are seen for other complex lesions in our analysis; collectively tetralogy of Fallot and single-ventricle defects including hypoplastic left heart syndrome, atrioventricular canal defect, and transposition of the great arteries account for almost half of all index surgery cases performed. There are several relatively intuitive explanations for these noted discrepancies between overall prevalence and surgical frequency. First, less complex but more prevalent forms of CHD may not require any surgical intervention, or more specifically not within the first year of life. In contrast, more complex lesions sometimes require multiple surgical interventions during the first year of life. Because in our analysis every new hospital admission for cardiac surgery was noted as a separate database entry, a particular patient could be counted several times in prevalence estimates. For these reasons, our data regarding frequency of surgical intervention provides a useful estimate of overall lesion-related resource utilization and may be of particular utility for programmatic strategic planning and resource allocation.
Anecdotal theories regarding a higher frequency of surgical intervention for certain forms of CHD within specific US geographic regions are often discussed. This study provides objective data of significant variation across regions. Given the large sample size, it is not surprising that statistically significant differences were observed. Of greater overall utility is the quantification of these differences, which are perhaps best represented by the confidence intervals provided in Tables 5 and 6. There is expected overlap; however, evidence also demonstrates that there are differences in the relative proportions of various diagnostic groups among patients with CHD who undergo operative intervention.
Several questions arise when analyzing these data. Are these differences meaningful from a public health perspective? Is there a need to better investigate the impact of environmental and socioeconomic factors, which may be associated with geographic regions? Could economic or payer mix variables impact approval for surgical intervention within certain regions? Although previous studies have investigated the possible associations of environment with CHD [15], further investigation focused on answering these questions may lead to a significant transformation in how we understand the mechanisms by which we can improve our delivery of surgical care.
Strengths of this analysis include a study cohort representing greater than 95% of the pediatric heart surgery centers in the United States, and use of standardized nomenclature as applied to clinical data from the STS-CHSD, with its high degree of data entry completeness for the variables being analyzed. Given the nature of the STS-CHSD, the scope of the study was intentionally limited to an examination of the frequency of surgical intervention for these lesions within the first year of life. Although it is a comprehensive analysis of the surgical population, there is no intent to represent these results as pertaining to prevalence of all CHD. Such an evaluation would of necessity include patients who either expire without treatment or undergo nonsurgical intervention.
In summary, this study analyzed 23,379 operations performed within the first year of life at 94 centers across the United States and found significant regional variation in the frequency of both the seven major diagnostic groups and the 10 specific diagnoses across USDHHS regions. A better understanding of these patterns may allow for more informed approaches toward regional and national programmatic planning and resource allocation. This may direct initiatives to better address administrative and economic challenges evolving within this highly resource-intensive surgical subspecialty.
Acknowledgments
Dr Pasquali receives support from the National Heart, Lung, and Blood Institute (K08HL103631, PI: Pasquali).
Footnotes
Presented at the Poster Session of the Fiftieth Annual Meeting of The Society of Thoracic Surgeons, Orlando, FL, Jan 25–29, 2014.
References
- 1.Russo CA, Elixhauser A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville, MD: U.S. Agency for Healthcare Research and Quality; Hospitalizations for Birth Defects, 2004. Statistical Brief #24. 2007. [PubMed] [Google Scholar]
- 2.Gillum RF. Epidemiology of congenital heart disease in the United States. Am Heart J. 1994;127:919–27. doi: 10.1016/0002-8703(94)90562-2. [DOI] [PubMed] [Google Scholar]
- 3.Dolk H, Loane M, Garne E European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000–2005. Circulation. 2011;123:841–9. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PD, Correa-Villaseñor A, Loffredo CA, Ferencz C. Temporal trends in prevalence of cardiovascular malformations in Maryland and the District of Columbia, 1981–1988. The Baltimore-Washington Infant Study Group. Epidemiology. 1993;4:259–65. [PubMed] [Google Scholar]
- 5.Ferencz C, Rubin JD, McCarter RJ, et al. Congenital heart disease: prevalence at livebirth the Baltimore-Washington Infant Study. Am J Epidemiol. 1985;121:31–6. doi: 10.1093/oxfordjournals.aje.a113979. [DOI] [PubMed] [Google Scholar]
- 6.Pasquali SK, Peterson ED, Jacobs JP, et al. Differential case ascertainment in clinical registry versus administrative data and impact on outcomes assessment for pediatric cardiac operations. Ann Thorac Surg. 2013;95:197–203. doi: 10.1016/j.athoracsur.2012.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cronk CE, Malloy ME, Pelech AN, et al. Completeness of state administrative databases for surveillance of congenital heart disease. Birth Defects Res A Clin Mol Teratol. 2003;67:597–603. doi: 10.1002/bdra.10107. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs ML, Daniel M, Mavroudis C, et al. Report of the 2010 Society of Thoracic Surgeons Congenital Heart Surgery Practice and Manpower Survey. Ann Thorac Surg. 2011;92:762–9. doi: 10.1016/j.athoracsur.2011.03.133. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed December 30, 2013];International Paediatric and Congenital cardiac Code. Available at: http://www.ipccc.net.
- 10.Franklin RC, Jacobs JP, Krogmann ON, et al. Nomenclature for congenital and pediatric cardiac disease: historical perspectives and the International Pediatric and Congenital Cardiac Code. Cardiol Young. 2008;(18 Suppl 2):70–80. doi: 10.1017/S1047951108002795. [DOI] [PubMed] [Google Scholar]
- 11. [December 30, 2013];STS Congenital Heart Surgery Database v3.0. http://www.sts.org/sites/defaultfiles/pdf/congenitaldataspecficationsV3_0_20090904.pdf.
- 12.Clarke DR, Breen LS, Jacobs ML, et al. Verification of data in congenital cardiac surgery. Cardiol Young. 2008;(18 Suppl 2):177–87. doi: 10.1017/S1047951108002862. [DOI] [PubMed] [Google Scholar]
- 13.Mai CT, Riehle-Colarusso T, O’Halloran A, et al. National Birth Defects Prevention Network. Selected birth defects data from population-based birth defects surveillance programs in the United States, 2005–2009: featuring critical congenital heart defects targeted for pulse oximetry screening. Birth Defects Res A Clin Mol Teratol. 2012;94:970–83. doi: 10.1002/bdra.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153:807–13. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoll C, Alembik Y, Roth MP, Dott B, De Geeter B. Risk factors in congenital heart disease. Eur J Epidemiol. 1989;5:382–91. doi: 10.1007/BF00144842. [DOI] [PubMed] [Google Scholar]