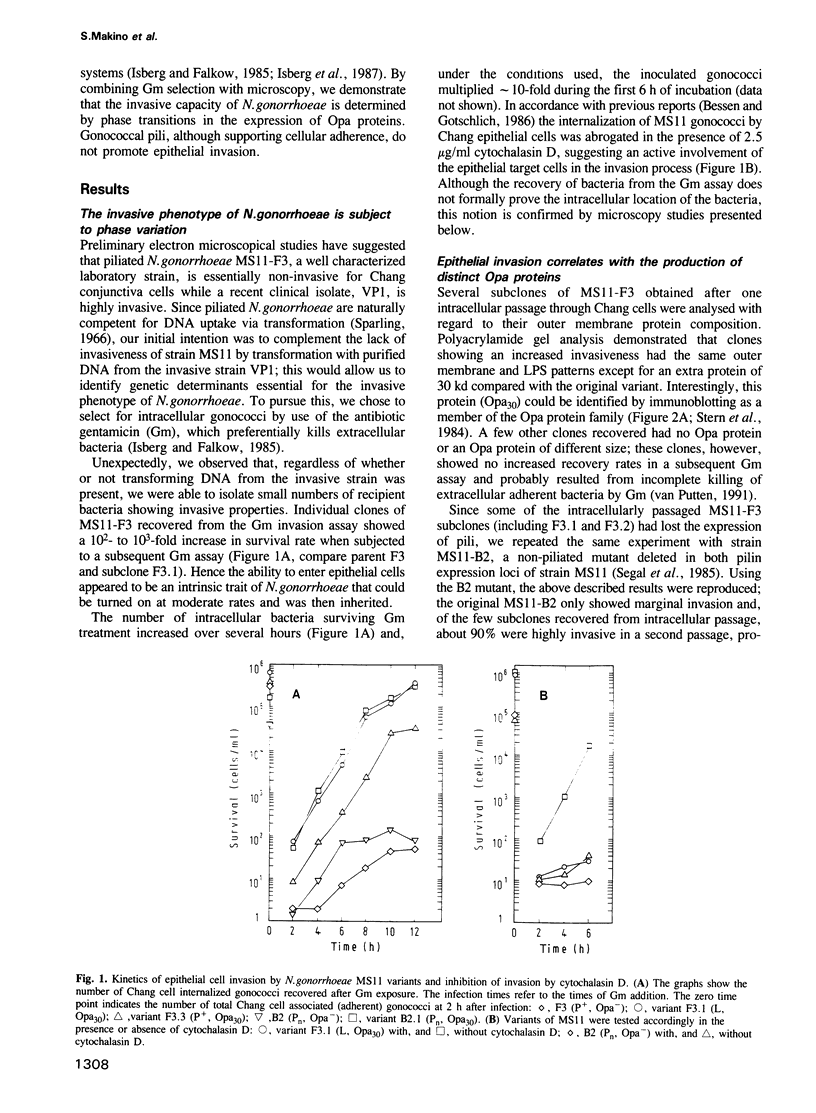
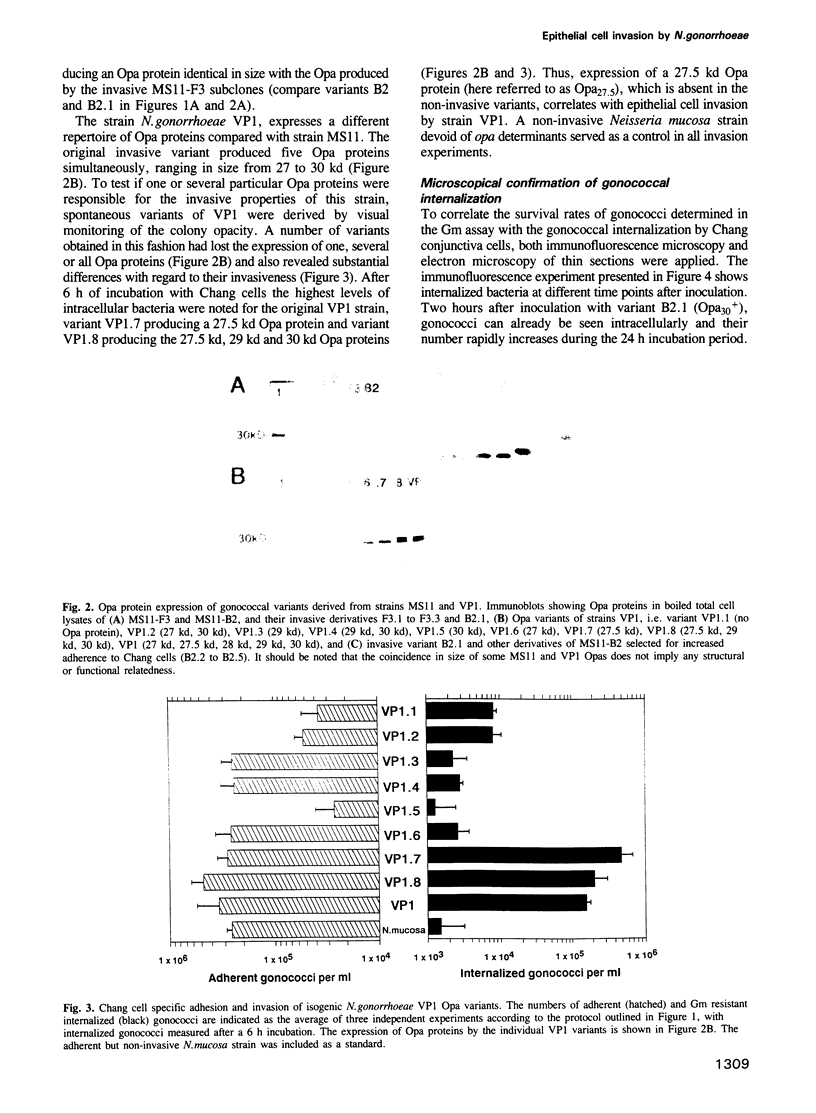
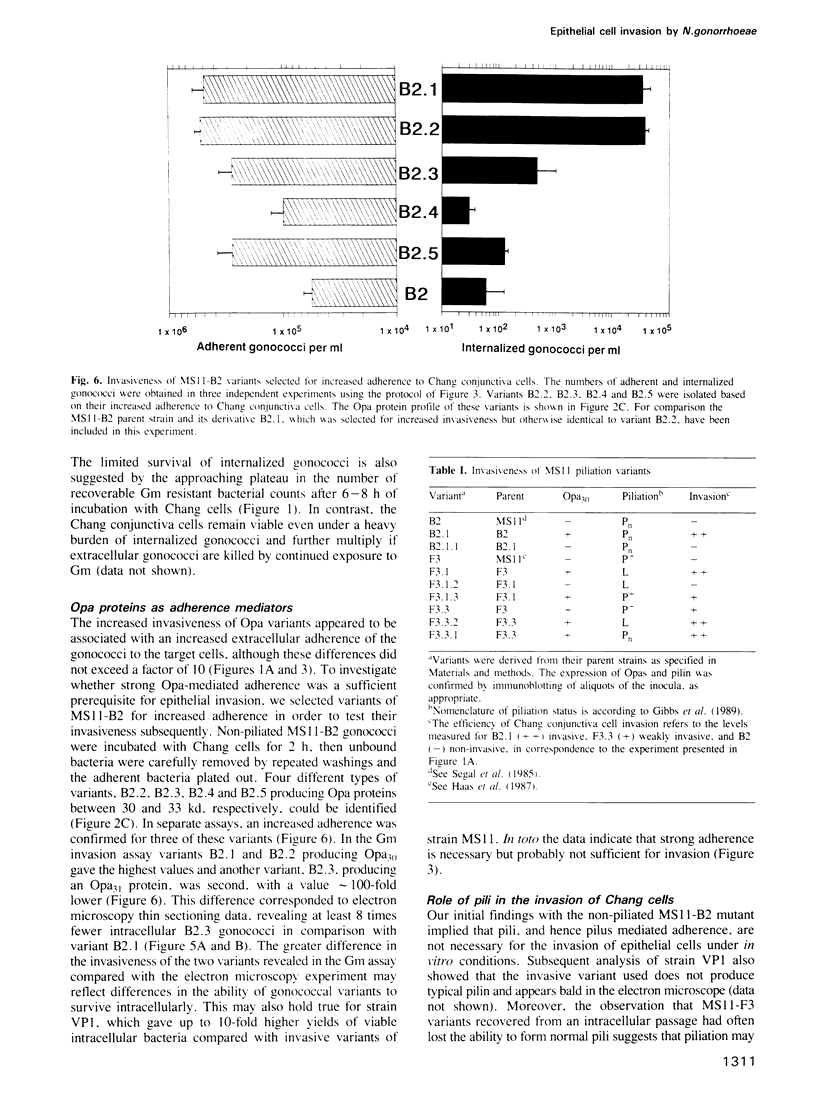
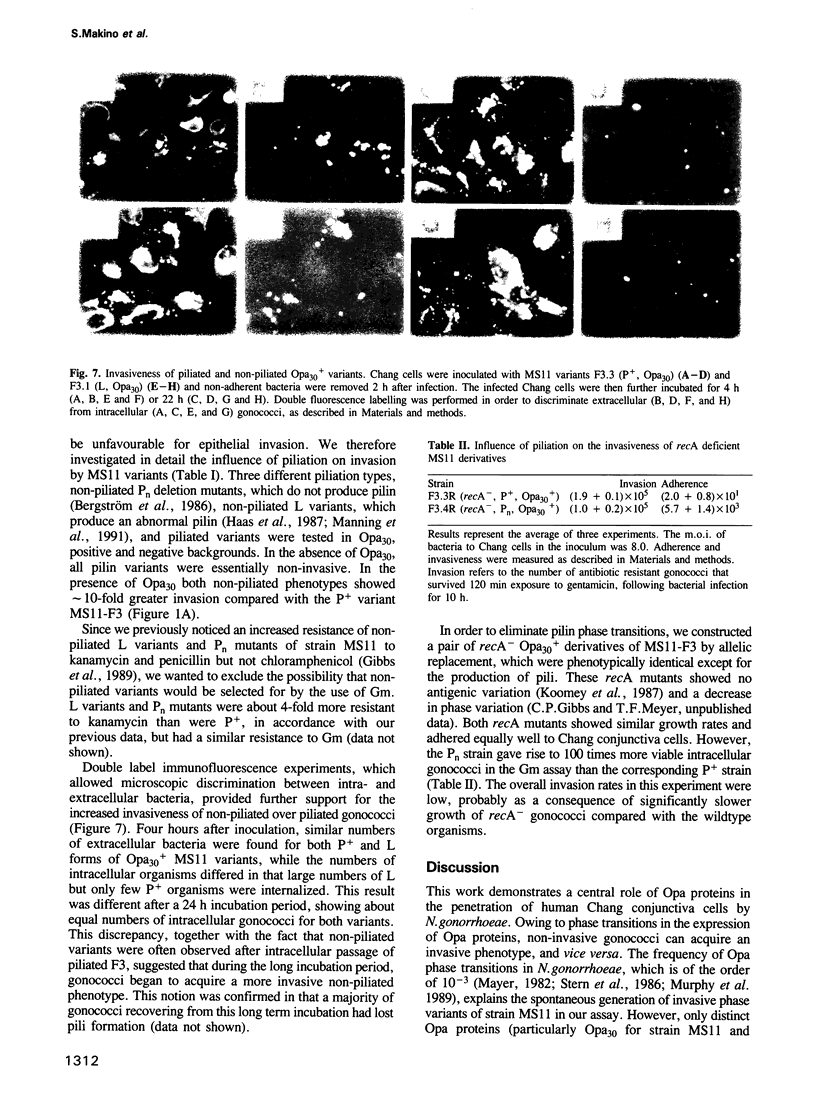
Abstract
Neisseria gonorrhoeae is a facultative intracellular bacterium capable of penetrating into certain human epithelial cell types. In order to identify gonococcal factors essential for invading Chang human conjunctiva cells, a gentamicin selection assay for the quantification of viable intracellular bacteria was used in conjunction with microscopy. The results demonstrate a correlation between the invasive behaviour of gonococci and the expression of Opa proteins, a family of variable outer membrane proteins present in all pathogenic Neisseria species. However, only particular Opa proteins supported invasion into Chang cells as indicated by the use of two unrelated gonococcal strains. Invasion was sensitive to cytochalasin D, and strong adherence mediated by the Opa proteins appeared to be essential for the internalization of gonococci. In contrast pili, which also conferred binding to Chang conjunctiva cells, did not support cellular invasion but rather were inhibitory.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström S., Robbins K., Koomey J. M., Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D., Gotschlich E. C. Interactions of gonococci with HeLa cells: attachment, detachment, replication, penetration, and the role of protein II. Infect Immun. 1986 Oct;54(1):154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins C., Rest R. F. Monoclonal antibodies to outer membrane protein PII block interactions of Neisseria gonorrhoeae with human neutrophils. Infect Immun. 1990 Apr;58(4):1078–1084. doi: 10.1128/iai.58.4.1078-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsinghorst E. A., Baron L. S., Kopecko D. J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. H., Rest R. F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988 Jun;56(6):1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. P., Reimann B. Y., Schultz E., Kaufmann A., Haas R., Meyer T. F. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989 Apr 20;338(6217):651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- Haas R., Meyer T. F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986 Jan 17;44(1):107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- Haas R., Schwarz H., Meyer T. F. Release of soluble pilin antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9079–9083. doi: 10.1073/pnas.84.24.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey M., Gotschlich E. C., Robbins K., Bergström S., Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 1987 Nov;117(3):391–398. doi: 10.1093/genetics/117.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Leong J. M., Fournier R. S., Isberg R. R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990 Jun;9(6):1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Mayer L. W. Rates in vitro changes of gonococcal colony opacity phenotypes. Infect Immun. 1982 Aug;37(2):481–485. doi: 10.1128/iai.37.2.481-485.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Stephens D. S., Hoffman L. H., Schlech W. F., 3rd, Horn R. G. Mechanisms of mucosal invasion by pathogenic Neisseria. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S708–S714. doi: 10.1093/clinids/5.supplement_4.s708. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Billyard E., Haas R., Storzbach S., So M. Pilus genes of Neisseria gonorrheae: chromosomal organization and DNA sequence. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Gibbs C. P., Haas R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., van Putten J. P. Genetic mechanisms and biological implications of phase variation in pathogenic neisseriae. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S139–S145. doi: 10.1128/cmr.2.suppl.s139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir L. L., Strugnell R. A., Davies J. K. Proteins that appear to be associated with pili in Neisseria gonorrhoeae. Infect Immun. 1988 Jul;56(7):1743–1747. doi: 10.1128/iai.56.7.1743-1747.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. L., Connell T. D., Barritt D. S., Koomey M., Cannon J. G. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989 Feb 24;56(4):539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- Nyberg G., Strömberg N., Jonsson A., Karlsson K. A., Normark S. Erythrocyte gangliosides act as receptors for Neisseria subflava: identification of the Sia-1 adhesin. Infect Immun. 1990 Aug;58(8):2555–2563. doi: 10.1128/iai.58.8.2555-2563.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Palmer L., Brooks G. F., Falkow S. Expression of gonococcal protein II in Escherichia coli by translational fusion. Mol Microbiol. 1989 May;3(5):663–671. doi: 10.1111/j.1365-2958.1989.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Parge H. E., Bernstein S. L., Deal C. D., McRee D. E., Christensen D., Capozza M. A., Kays B. W., Fieser T. M., Draper D., So M. Biochemical purification and crystallographic characterization of the fiber-forming protein pilin from Neisseria gonorrhoeae. J Biol Chem. 1990 Feb 5;265(4):2278–2285. [PubMed] [Google Scholar]
- Parsons N. J., Kwaasi A. A., Patel P. V., Nairn C. A., Smith H. A determinant of resistance of Neisseria gonorrhoeae to killing by human phagocytes: an outer membrane lipoprotein of about 20 kDa with a high content of glutamic acid. J Gen Microbiol. 1986 Dec;132(12):3277–3287. doi: 10.1099/00221287-132-12-3277. [DOI] [PubMed] [Google Scholar]
- Paruchuri D. K., Seifert H. S., Ajioka R. S., Karlsson K. A., So M. Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid-binding adhesin. Proc Natl Acad Sci U S A. 1990 Jan;87(1):333–337. doi: 10.1073/pnas.87.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrollet H., Guinet R. M. Gonococcal cell wall lectin-adhesin with vaccine potential. Lancet. 1986 May 31;1(8492):1269–1270. doi: 10.1016/s0140-6736(86)91404-2. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Fischer S. H., Ingham Z. Z., Jones J. F. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982 May;36(2):737–744. doi: 10.1128/iai.36.2.737-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Lee N., Bowden C. Stimulation of human leukocytes by protein II+ gonococci is mediated by lectin-like gonococcal components. Infect Immun. 1985 Oct;50(1):116–122. doi: 10.1128/iai.50.1.116-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Shafer W. M. Interactions of Neisseria gonorrhoeae with human neutrophils. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S83–S91. doi: 10.1128/cmr.2.suppl.s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Billyard E., So M., Storzbach S., Meyer T. F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985 Feb;40(2):293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988 Jun;56(6):1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Stromberg N., Deal C., Nyberg G., Normark S., So M., Karlsson K. A. Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4902–4906. doi: 10.1073/pnas.85.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawara R. J., Cannon J. G., Black W. J., Nachamkin I., Sweet R. L., Brooks G. F. Inhibition of Neisseria gonorrhoeae attachment to HeLa cells with monoclonal antibody directed against a protein II. Infect Immun. 1983 Dec;42(3):980–985. doi: 10.1128/iai.42.3.980-985.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Barrera O., Sola J., Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988 Dec 1;168(6):2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Barrera O., Robbins K., Corwin D. Pilus- gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985 Aug 1;162(2):729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface components affecting interactions between Neisserai gonorrhoeae and eucaryotic cells. J Infect Dis. 1977 Aug;136 (Suppl):S138–S143. doi: 10.1093/infdis/136.supplement.s138. [DOI] [PubMed] [Google Scholar]
- Taha M. K., So M., Seifert H. S., Billyard E., Marchal C. Pilin expression in Neisseria gonorrhoeae is under both positive and negative transcriptional control. EMBO J. 1988 Dec 20;7(13):4367–4378. doi: 10.1002/j.1460-2075.1988.tb03335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjia K. F., van Putten J. P., Pels E., Zanen H. C. The interaction between Neisseria gonorrhoeae and the human cornea in organ culture. An electron microscopic study. Graefes Arch Clin Exp Ophthalmol. 1988;226(4):341–345. doi: 10.1007/BF02172964. [DOI] [PubMed] [Google Scholar]
- Veale D. R., Smith H., Witt K. A., Marshall R. B. Differential ability of colonial types of Neisseria gonorrhoeae to produce infection cutaneous perforated plastic chambers in guinea-pigs and rabbits. J Med Microbiol. 1975 May;8(2):325–335. doi: 10.1099/00222615-8-2-325. [DOI] [PubMed] [Google Scholar]
- Virji M., Everson J. S. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981 Mar;31(3):965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986 Feb;132(2):503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- van Putten J. P., Hopman C. T., Weel J. F. The use of immunogold-silver staining to study antigen variation and bacterial entry into eukaryotic cells by conventional light microscopy. J Med Microbiol. 1990 Sep;33(1):35–41. doi: 10.1099/00222615-33-1-35. [DOI] [PubMed] [Google Scholar]