Abstract
The remarkable mechanical behavior of bone is attributed to its complex nanocomposite structure that, in addition to mineral and collagen, comprises a variety of non-collagenous matrix proteins or NCPs. Traditionally, NCPs have been studied as signaling molecules in biological processes including bone formation, resorption and turnover. Limited attention has been given to their role in determining the mechanical properties of bone. Recent studies have highlighted that NCPs can indeed be lost or modified with aging, diseases and drug therapies. Homozygous and heterozygous mice models of key NCP provide a useful approach to determine the impact of NCPs on bone morphology as well as matrix quality, and to carry out detailed mechanical analysis for elucidating the pathway by which NCPs can affect the mechanical properties of bone. In this article, we present a systematic analysis of a large cohort of NCPs on bone’s structural and material hierarchy, and identify three principal pathways by which they determine bone’s mechanical properties. These pathways include alterations of bone morphological parameters crucial for bone’s structural competency, bone quality changes in key matrix parameters (mineral and collagen), and a direct role as load bearing structural proteins.
Keywords: non-collagenous proteins, bone quality, bone morphology, mineralization, mechanical properties, fracture
Introduction
The complex hierarchical structure and composition of bone provides it with exceptional resistance to fracture. However, the unique contribution of each material component in the prediction of bone fracture, is not fully elucidated. Bone mineral density (BMD) is most commonly used to predict fracture risk, but fracture and non-fracture populations show an overlap in BMD values [1–2]. Therefore, inclusion of other factors such as bone quality may help improve the determination of fracture risk [3–5]. Non-collagenous proteins (NCPs), an integral component of bone’s organic matrix, have been implicated to play a role in bone quality. For example, in osteoporotic bone, NCPs are modified [6] and their amount in bone matrix is reduced [7]. Further understanding of the role of NCPs in bone quality and fragility may improve the determination of fracture risk.
NCPs exhibit multifunctional roles in bone that are critical for the determination of bone quality and fracture resistance. They have been shown to affect bone modeling and subsequently alter bone geometry through regulation of osteoblast and osteoclast activity [8–9]. The changes that occur in cortical (diameter and thickness) and trabecular (number and thickness) bone structure by removal of NCPs from bone matrix can impact bone strength and alter its resistance to fracture. Similarly, NCPs are involved in bone remodeling, thereby affecting bone matrix microarchitecture, also through their influence on osteoblast and osteoclast activity [10–11]. In that regard, NCP mediated alterations in microarchitectural features such as porosity, connectivity and anisotropy are important considerations in bone strength.
The mineralization of bone matrix, a key determinant of matrix quality and mechanical properties, is also influenced by NCPs [12–14]. Studies have shown that NCPs impact hydroxyapatite crystallinity [15], formation of collagen fibrils [16] and coordinate cell-matrix interactions [13,17]. Therefore, alterations in NCPs can affect bone material properties such as hardness through their effect on mineralization [18–19].
Recent evidence suggests that NCPs also play a direct structural role in bone. Various conceptual models postulate that the spatial arrangement of NCPs within bone matrix, places them as structural elements that may determine bone’s propensity to fracture. These models propose that NCPs act as “glue” at the collagen-mineral interface to resist the separation of the mineralized fibrils and consequently enhance toughness [17, 20–22]. The removal of NCPs at this interface translates through the hierarchical scales in bone and impairs its mechanical properties including toughness [22].
The contribution of NCPs to bone mechanical properties has been most commonly studied using genetically modified mouse models devoid of one or more NCPs. Complete removal of NCPs from bone matrix can elucidate the role of individual NCPs and their relationship with other NCPs that may impact bone strength and fracture. While there are several NCPs in bone, only a few have been investigated in regard to their impact on bone phenotype and structural properties. More importantly, several clinical studies have shown the utility of a small number of NCPs as potential biomarkers for identifying fracture and non-fracture groups [23–24]. This review summarizes the mechanistic roles of various NCPs in bone mechanics and fracture. We discuss the role of NCPs on bone mechanical properties through their impact on bone structure, mineralization and as key structural components of bone’s extracellular matrix, and identify areas of future research.
NCPs Impact Whole Bone Morphology
As discussed previously, the size and shape of whole bone is critical to its mechanical properties and a major predictor of fracture risk. It has been shown that the outer diameter of long bones can predict ~55% of the variation in bone strength [25], and bones with thinner cortices are more susceptible to fracture [26]. In addition, the geometry of bone affects the distribution of its mass and BMD, which are known to play a role in bone strength [27–28]. Bone size and shape is determined by bone formation and resorption at different surfaces and rates through bone modeling. NCPs have been demonstrated to act as biological stimuli, influencing these processes, thereby determining bone structure and contributing to bone biomechanics (Table 1)
Table 1.
Summary of morphological changes and structural properties of NCP mouse models.
| Mouse Model | Morphological Traits (compared to WT) | Type of Mechanical Test | Structural Mechanical Properties (compared to WT) | Ref |
|---|---|---|---|---|
| Osteocalcin (OC−/−) | Increased bone mass and cortical thickness | Strength | Increased ultimate load | [8] |
| Biglycan (Bgn−/−) | Reduced bone length, bone mass and cortical thickness | Strength | Reduced bone strength and ductility | [31] |
| Fibrillin-2 (Fbn2−/−) | Decreased length and BMD, thick cortex | Strength | Decreased in stiffness, maximum load and total work | [32] |
| Osteonectin (SPARC) | Decreased cortical and trabecular bone mass | Strength | Decreased stiffness and maximum load | [34] |
| Periostin (Postn−/−) | Decreased cortical and trabecular bone mass | Strength | Reduced stiffness, ultimate force and strength | [35,36] |
| Bone Sialoprotein (BSP−/−) | Reduced length, BMD and thinner cortices | NA | NA | [38] |
| Matrix Extracellular Protein (OF45−/−) | Increase endosteal and periosteal circumference, increased trabecular bone content | NA | NA | [37] |
| Osteopontin (OPN−/−) | NA | Strength | Increased elastic modulus, decreased maximum load, work to fracture and post yield deformation | [40] |
Osteocalcin (OC) is one of the most abundant NCPs in bone and is produced exclusively by osteoblasts [29]. Mice deficient in OC expressed a phenotype of increased bone mass and cortical thickness compared to wild type controls (WT) [8]. The substantial increase in bone size and shape was attributed to the osteoblastic cells in OC−/− mice producing up to 50% more matrix than in WTs. The increase in cortical thickness and bone mass of OC−/− mice coincided with higher failure loads. It was therefore concluded that OC when present, acts as an inhibitor of bone formation, and consequently a determinant of bone structure.
Unlike OC, other NCPs such as biglycan, fibrillin-2 and osteonectin impair bone formation when they are absent from the matrix. Biglycan (Bgn), a highly expressed small leucine rich proteoglycan (SLRP) in bone matrix [30] severely affects bone structure. Bgn−/− mouse bones exhibited reduced bone mass and length, with thinner cortices [31]. The change in bone structure was attributed to a net reduction in bone formation in the Bgn−/− mice, due to inhibition of osteoblastic activity and no effect on the activity of osteoclasts. The consequence of biglycan’s deletion from bone matrix and concomitant alterations in bone structure, was a reduction in bone strength and ductility compared to WT controls [31] (Figure 1).
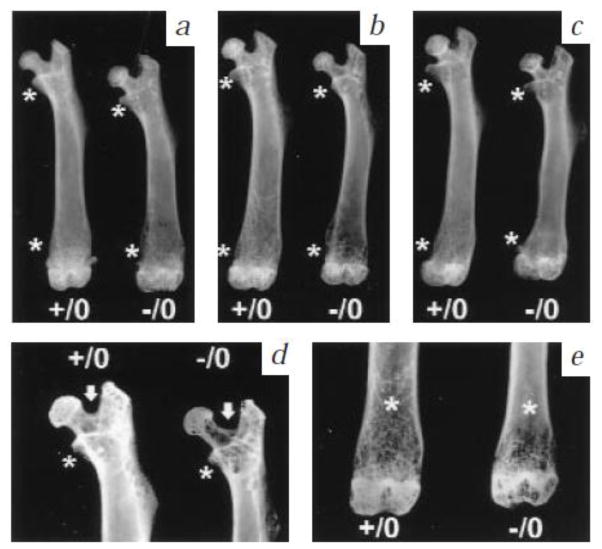
Figure 1.
Radiological analysis of wild type (+/0) and bgn knockout (−/0) bone morphology. Femoral length, bone mass and cortical thickness decreased with age at three (a), six (b) and nine months (c). Detailed high resolution radiographs of 6 months old (d, e) with * indicating regions of reduced trabecular bone mass, and white arrow highlighting the wider angle between the femoral neck and greater trochanter in the mutants. From Xu et al [31] with permission obtained from Nature Genetics.
Decorin (Dcn), like biglycan, is a member of the SLRP family. However, unlike Bgn mutants, mice deficient in Dcn exhibit no overt skeletal changes. Since Bgn and Dcn are structurally related NCPs, the interplay between these SLRPs was assessed using a biglycan/decorin double knockout mouse model (Bgn-Dcn−/−;−/−). The long bones of Bgn-Dcn−/−;−/− were shorter and wider compared to WTs and single mutants (Bgn−/− and Dcn−/−). The cortical and trabecular bone mass in this model was also significantly reduced [43]. However, the impact of changes in bone morphology on whole bone mechanical properties in Bgn-Dcn−/−;−/− mice has not been investigated.
A similar phenotype was observed in Fibrillin-2 (Fbn2) mutant mice. Fbn2 is expressed by differentiating osteoblasts and plays a role in bone formation through the signaling of transforming growth factor -β (TGF-β) and bone morphogenetic proteins (BMP). The femora of Fbn2−/− mice were 4.5% shorter with smaller endosteal and periosteal diameters compared to WTs. The slender morphology of the mutant group when assessed mechanically, exhibited a 30% decrease in stiffness, 29% decrease in maximum load and 25% decrease in total work [32]. However, the mechanism by which Fbn2 influences bone mechanical properties is not understood as osteoblast and osteoclast activities were not quantified.
Osteonectin, also known as Secreted Protein Acidic and Rich in Cysteine (SPARC) exhibits multiple functions in mineralized bone matrix [33]. Although no change in cortical thickness was observed in SPARC−/− mice, a rapid decrease in bone mass was observed with age [34]. Consequently, the mechanical properties of SPARC−/− mice were age dependent and a lower stiffness and maximum load were observed in SPARC−/− mice compared to WT controls.
The NCP periostin is preferentially localized in the periosteum of bone and is critical for skeletal growth and development [35]. Mice deficient in the periostin gene (Postn) exhibited dwarfism [35]. These mutants also expressed altered cortical and cancellous bone microarchitecture and lower BMD [36]. The reduction in cortical and trabecular bone mass impaired the mechanical properties (decreased stiffness and ultimate strength) in mice deficient in periostin [36].
Bone Sialoprotein (BSP) and Matrix Extracellular Protein (MEPE) are other NCPs expressed by osteoblasts, and both of these are known to play a functional role in bone formation [37–38]. In vivo, the femora of BSP−/− mice, like Bgn mutants, are shorter, have thinner cortices and exhibit lower BMD as compared to WT. Similar to BSP, MEPE is restricted to mineralized tissues and clusters with BSP on human chromosome 4 [9]. Mice deficient in MEPE showed increased trabecular bone content with larger endosteal and periosteal circumferences compared to controls but no changes were noted in cortical thickness [37]. The net increase in trabecular bone volume observed in MEPE knockout mice was attributed to an increase in osteoblast-mediated bone formation. The number of osteoblasts and the percentage of bone surface occupied by osteoblasts were significantly larger in the knockouts than in controls. Bone formation and mineral apposition rates were also higher in MEPE−/− mice [37]. The changes observed in bone structure and the associated mechanical properties in MEPE and BSP deficient mice are unknown at this point.
It is noteworthy that the removal of NCPs from bone matrix, does not always result in alteration of bone geometry. For example, the gross skeletal phenotype of osteopontin deficient mice (OPN−/−) is normal [11]. Measures of BMD, cortical thickness or porosity of the mutant femora do not significantly differ from controls [39]. However, Duvall et al demonstrated via whole bone testing, a significant increase in elastic modulus of 10-week-old OPN−/− femora. Furthermore, there was a reduction in maximum load, work to fracture and post yield deformation [40]. Thus, it appears that OPN increase bone strength and reduces its ductility. Since the lack of changes in geometric distribution or bone mass observed for OPN−/− mice cannot account for the changes in mechanical properties observed, these results suggest that factors other than bone morphology and structure could influence whole bone fracture. These factors are discussed in sections below.
NCPs Impact Bone Material Quality
The material quality of bone matrix takes into account the composition and organization of its organic and mineral constituents, the prevalence of matrix microdamage and cortical/trabecular microarchitecture. Bone remodeling alters the material quality of bone matrix, and is critical for maintaining the mechanical integrity of bone. NCPs such as OC, OPN and SPARC are involved in remodeling by participating in the recruitment and attachment of cells to bone matrix [10, 41–42]. Removal of NCPs from bone matrix is associated with changes in the degree of matrix mineralization [14], collagen fibril geometry [43], and microdamage accumulation (Table 2) [44]. The degree of mineralization is associated with bone’s stiffness such that with increasing mineralization, bone becomes brittle and its resistance to fracture is lowered [45–46]. Furthermore, structural modification of collagen fibrils has been shown to impact bone strength [5,47–48]. By participating in bone remodeling and matrix organization, NCPs can affect bone material properties and determine bone fragility.
Table 2.
Summary of bone matrix microarchitectural changes and mechanical properties of NCP mouse models.
| Mouse Model | Material Traits (compared to WT) | Type of Mechanical Test | Matrix Mechanical Properties (compared to WT) | Ref |
|---|---|---|---|---|
| Osteocalcin (OC−/−) | Smaller and less mature crystals; increased crystallinity; decreased type B carbonate substitution; reduction in diffuse damage | Fracture toughness, Nanoindentation | Increased hardness, reduced fracture toughness | [15,22,54] |
| Biglycan (Bgn−/−) | Reduced trabecular number, thickness and poor trabecular connectivity | NA | NA | [31,43] |
| Decorin (Dcn−/−) | Irregular fibril geometry, decreased fibril diameter | NA | NA | [43,61] |
| Fibrillin-2 (Fbn2−/−) | Decreased type B carbonate substitution | Nanoindentation | Decreased hardness and elastic modulus | [32] |
| Osteonectin (SPARC) | Increased mineral content and crystallinity | NA | NA | [68,69] |
| Periostin (Postn−/−) | Decreased collagen crosslinks, increased crack number and surface | Fatigue, Nanoindentation | Reduced hardness, stiffness | [44] |
| Osteopontin (OPN−/−) | Reduced mineral: protein ratio (4 weeks); reduction in diffuse damage; increased mineral content and crystal size (12–16 weeks) | Fracture Toughness, Nanoindentation | Reduced elastic modulus, hardness and fracture toughness | [18,19,22, 39] |
The lack of morphological changes in the OPN knockout model suggests that this NCP may affect mechanical properties of bone by altering bone remodeling. Yoshitake et al demonstrated that OPN−/− mice are resistant to ovariectomy-induced bone loss and show only a modest reduction in bone volume compared to ovariectomized controls. Bone formation was also normal in these animals [49]. At the mineral level, OPN is involved in matrix mineralization. It inhibits the formation of hydroxyapatite crystals through the binding of phosphate and carboxylate groups binding to mineral [50]. Mouse bones deficient in OPN (12–16 weeks old) showed increased mineral content with larger, more perfect crystals [18]. Nanoindentation tests do not show changes in hardness or elastic modulus of these OPN−/− mouse bones. However, both properties were significantly decreased in younger animals (<12 weeks) compared to controls [19, 39], and suggests that OPN is involved in the earlier stages of mineralization. The reduction in material properties may be explained by previous studies [51–52] that show a decrease in fracture properties such as strength and ductility with increase number of large crystals within the bone matrix. In addition, whole bone fracture toughness in OPN−/− mice was reduced by 30% compared to WT. The loss in toughness of the OPN−/− bones, however, did not correlate with changes in crystallinity or mineral concentration (calcium variability), suggesting that other factors such as matrix heterogeneity may vary with loss of OPN [39] or OPN may itself play a direct role in fracture [20,22].
Similar to OPN, OC is involved in matrix mineralization by binding to hydroxyapatite through its three Gla residues [12,53]. More specifically, the affinity of OC to hydroxyapatite allows it to participate in mineral maturation. Boskey et al. demonstrated that OC−/− mouse femora have mineral crystals that are smaller, less mature/crystalline and contain lower type B carbonate substitutions compared to WTs [15]. In contrast, Kavukcuoglu et al. showed that the mineral crystallinity in OC−/− mice femora is higher and is accompanied by increased intra bone variations [54]. The effects of increased crystallinity was associated with an increase in bone hardness but had little effect on elasticity [54]. Further work is required to address the discrepancy between the two studies. More recently, Poundarik et al showed that the absence of OC creates a ~35% reduction in fracture toughness compared to controls [22]. These findings support the argument that OC and OPN are important for bone quality and mechanics, but their effects on bone fracture cannot be explained by altered mineralization. It is noteworthy that fracture properties of bone are quantitatively more dependent on post-yield deformation and crack propagation [55–56] and these properties are determined by the organic matrix [57–58]. Thus, similar to OPN, OC may also play a direct structural role in bone fracture as a constituent of bone’s organic matrix.
Other NCPs like Fbn2 also affect mineral maturation and matrix composition. Although Fbn2−/− mouse femora exhibited a non-significant increase in crystallinity and mineral: organic ratio over WT [32,59], there was a significant decrease in elastic modulus and hardness across the entire bone cross-section compared to WTs [59]. In addition, intra-bone variations in mechanical properties and measures of mineral quality (crystallinity and carbonate substitutions) were noted. The absence of Fbn-2 had the greatest influence in the mid-cortical section, where crystallinity increased and mechanical properties decreased. Because the mid-cortical section contains more mature bone, Fbn-2 may play a more active role in the mechanical properties of a fully mineralized matrix [59]. The lower fracture resistance observed in the knockouts is unlikely due to changes in the mineral phase of bone as no significant differences were noted in mineral crystallinity and mineral: organic ratio of Fbn2−/− mouse bones. As such Fbn-2 knockout mouse bones can provide additional insight into the role of this protein and the organic matrix in bone fragility.
In addition to alteration of mineral composition and crystallinity, NCPs also affect bone quality through their impact on collagen maturity. As previously mentioned, removal of Dcn from bone matrix, caused no overt changes on whole bone morphology. However, Dcn’s impact on bone quality is linked to its effect on the organic matrix where it acts as a regulator of collagen fibrillogenesis [60]. More specifically, its protein core binds non-covalently to the triple helical domain of type I collagen and retards the degree and rate of collagen formation [61]. Additional evidence was seen in Dcn−/− mice where the average fibril diameter was decreased and fibrils possessed irregular cross-sectional profiles compared to WTs [43]. Dcn also acts as an inhibitor of matrix mineralization where it prevents the mineralization of collagen fibrils [62] and crystal growth by binding to hydroxyapatite and calcium ions [63–64]. Therefore, it is possible that Dcn (and/or Bgn) can negatively affect bone material properties, however, no information on either model is currently available.
Periostin is a γ-carboxylated Gla NCP like OC but unlike OC, it contributes to bone matrix quality through a different mechanism. In particular the effect of Postn on bone microarchitecture and organization is linked to altered bone turnover and its effect on collagen crosslinking [65]. The amount of mature and immature collagen crosslinks observed in Postn−/− mouse tibiae are indeed lower than controls [44] and both of these are known to alter bone fragility and affect the mechanical competence of bone [48]. Indeed, low levels of enzymatic crosslinks in Postn knockouts were associated with reduced local hardness and elastic modulus [44].
SPARC and Bgn are two NCPS that affect the matrix quality of, both, collagen and mineral. SPARC binds strongly to collagen and hydroxyapatite, and plays a direct role in bone mineralization [68]. The SPARC-deficient mouse tibiae also showed significant changes in mineral content, crystallinity (crystal size and perfection) and collagen maturity [69] (Figure 2). As the animals matured (36-weeks) the crystallinity began to increase compared to WT controls. The observed increase in crystal size and perfection in the older SPARC-deficient mice is consistent with their reduced ability to remodel. The lack of remodeling accounts for the observed increase in collagen maturity (crosslinks) and brittleness [69]. Altered levels of collagen crosslinks have been shown to be detrimental to bone mechanical properties [70–71] but their impact has not been evaluated in SPARC-deficient mice.
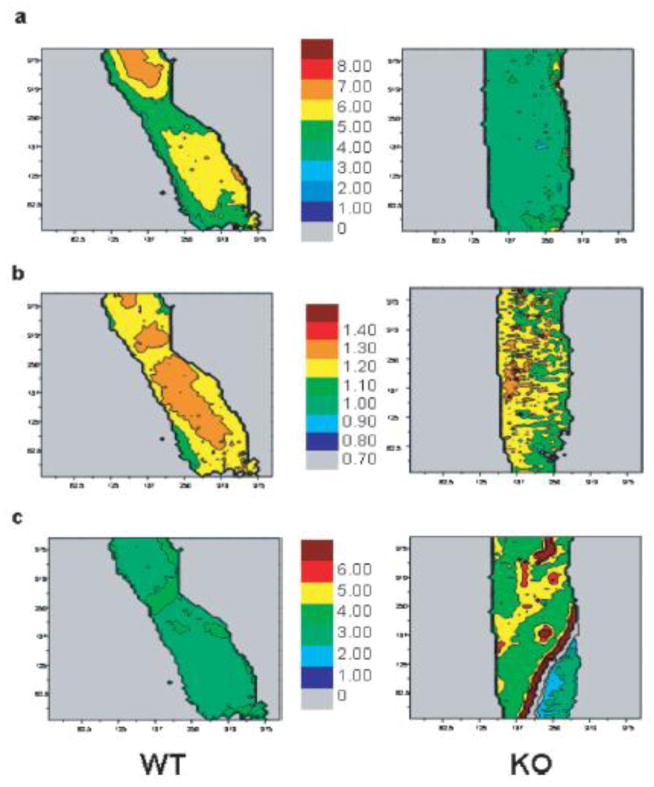
Figure 2.
FTIR imaging of 11-week-old wild type (WT) and SPARC-deficient (KO) mouse femora. a) mineral: organic ratio and b) mineral crystallinity (1030/1020 intensity ratios) are lower in KO while c) collagen maturity is higher compared to WT. From Boskey et al [69] with permission obtained from Journal of Bone and Mineral Research.
Biglycan (Bgn) binds strongly to hydroxyapatite, in vitro, through its glycosaminoglycan (GAG) side chain, and inhibits crystal growth [63–64]. However, it has been shown that Bgn’s interaction with collagen in vitro reduces its inhibitory role [63]. X-ray diffraction analysis of hydroxyapatite in Bgn−/− mouse bones revealed that crystal size and shape were unaffected [31] but there is a significant reduction in the mineral: organic ratio. It is therefore possible that Bgn deficiency has a local effect on mineral formation. Bgn is also involved in collagen fibrillogenesis, and the loss of Bgn from bone matrix results in irregular collagen fibrils with increased average fibril diameter [43]. Moreover, the abnormal collagen structure was also seen in the Bgn-Dcn−/−;−/− mouse model. The basic circular fibril geometry of type I collagen was completely lost and fibrils appeared serrated (Figure 3) [43]. It is widely known that type I collagen has several functions in bone including serving as a template for mineral deposition [72], absorbing energy during fracture [47,58] and providing viscoelasticity [73]. Consequently, the reduced mineral content in Bgn−/− mice and abnormal collagen fibrils in both Bgn−/− and Bgn-Dcn−/−;−/− can have severe impact on tissue hardness and elastic modulus as well as on other mechanical properties of bone. However, no information is currently available on either models.
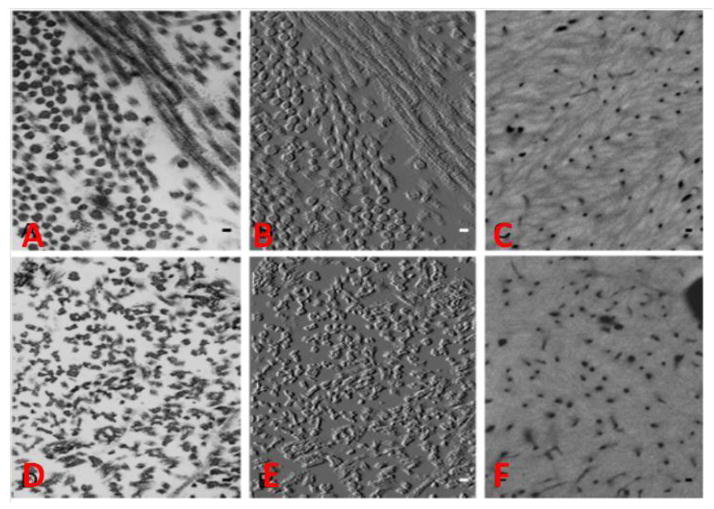
Figure 3.
Electron Microscopy images of 2-month-old wild type (A–C) and Bgn-Dcn−/−;−/− (D–F) mouse tibiae. (B) and (F) are TEM images of (A) and (D) modified for improved visualization of collagen fibril profiles. In Bgn-Dcn−/−;−/− mice the collagen fibrils appear serrated and lack circular cross-sectional profile. The typical collagenous texture seen in WT (C) from quantitative backscattered electron imaging was completely loss in the mutant (F) and replaced by a uniform glassy mineralized matrix. From Corsi et al [43] with permission obtained from Journal of Bone and Mineral Research.
Thus, the effect of certain NCPs at the matrix level, as manifested through changes in collagenous or mineral components, significantly impacts local and whole bone mechanical properties. However as noted in this section, in addition to their contribution to bone fracture through modeling (or morphological) and remodeling (or matrix level), NCPs may directly partake as mechanical elements in bone matrix and affect bone fragility. This particular aspect is reviewed in the section below.
Direct Structural Role of NCPs
The spatial arrangement of nanoscale elements and their material properties, are finely tuned to create complex hierarchical materials with superior mechanical performance [74]. In natural and biological materials, the addition small amount of protein inclusions [75] within the nanoscale elements have been shown to improve fracture resistance [20,76–77]. NCPs comprise ~10% of the organic matrix in bone and have recently been demonstrated to act as structural molecules to enhance bone toughness [22,78].
As previously discussed, OC and OPN interact with both collagen and mineral, and hence have been postulated to play a seminal role at the organic-mineral interface. Both OC and OPN, form a high-affinity complex with each other through calcium bridges [79], and Solid State Nuclear Magnetic Resonance (SSNMR) has been used to elucidate their structural role at the interface. Nikel et al. [17] demonstrated that deletion of OC and OPN alters the organic-inorganic interface in bone. In particular, less abundant amino acids including lysine move in closer proximity to the inorganic component, hydroxyapatite, while preserving the organic matrix and mineral. Thus NCPs, including but not limited to OC and OPN, can indeed be important components of the organic-inorganic interface in bone and consequently influence its propensity to fracture.
Studies have shown that crack formation occurs between the mineralized fibrils when they separate under loading [80–82]. During separation, energy is initially dissipated through the sacrificial bonding mechanism. Sacrificial bonds are hidden random networks of bonds between mineralized fibrils [82]. In sacrificial bonding, NCPs like OPN interact with divalent Ca2+ cations forming and reforming bonds, thereby dissipating energy and increasing bone’s fracture toughness [83]. These bonds are weak, and break before the stronger bonds that hold the structure together. However, the energy required to break the sacrificial bonds, increases the total energy (or toughness) that is required to fracture bone.
In addition to sacrificial bonding, NCPs like OC and OPN have a mechanical function in bone matrix, through dilatational band formation. The interaction between OC and OPN has been proposed to impact the mechanical properties across the levels of hierarchy in bone. During separation of mineralized fibrils, ellipsoidal voids called dilatational bands are formed between fused mineral aggregates. These voids are ~100 nm and result due to extension and failure of the OC-OPN complex [22]. Microdamage accumulates in regions of dilatational band formation, as diffuse damage, and colocalizes with both OC and OPN. In the absence of either OC, OPN, or both, from bone matrix, diffuse damage formation is significantly reduced. Since diffuse damage allows bone to dissipate large amounts of energy [67,84] the lack of diffuse damage formation compromises the ability of the bone to dissipate energy and increases its propensity to fracture.
A comparison between knock-out mice gives insight into the dilatational band mechanism. In the OC-OPN−/−;−/− mice bone, the fracture toughness was significantly reduced compared to WT and there were no differences between OC−/−, OPN−/− and OC-OPN−/−;−/− bones. This indicated that the interaction between OC and OPN is akin to “links in a chain” whereby removal of OC, OPN, or both will result in altered microdamage morphology and reduced crack propagation toughness [22] (Figure 4). An examination of damage mechanisms revealed that the reason for bone’s inability to resist failure was due to the inability of the single and double knock-out bones to form diffuse damage. The resistance to failure was ultimately translated through the hierarchical scales in bone (Figure 5).
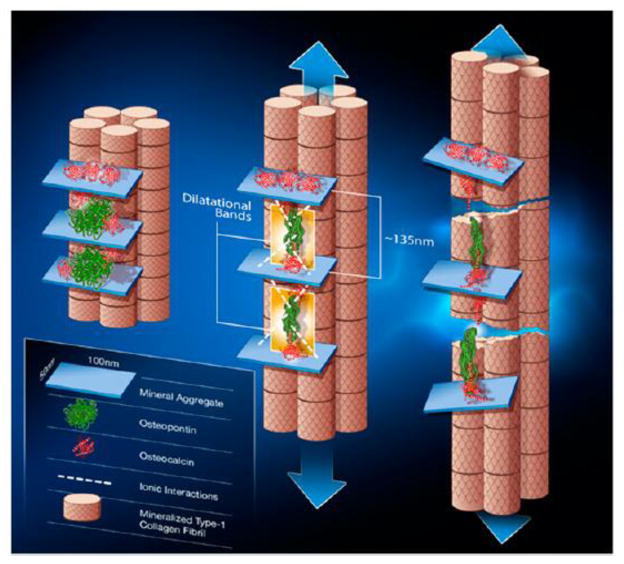
Figure 4.
Schematic of the OC-OPN-OC complex at the organic-mineral interface in bone. The first stage (left) illustrates two sites of dilatational band formation. Each site comprises two OC molecules (red) and an OPN molecule (green). The OC and OPN molecules are sandwiched between two fused mineral aggregates. Application of a load (center) causes the OC-OPN-OC protein complex to unfold. Dilatational bands (highlighted in yellow) form and extend until the maximum extension of 135 nm is reached. Continuous loading causes OC and OPN to separate (right). The separation of OC and OPN dictates the subsequent rupture and shear of collagen fibrils. Shear is shown by the difference in longitudinal displacement of the fibrils. From Poundarik et al [22] with permission obtained from PNAS.
Figure 5.
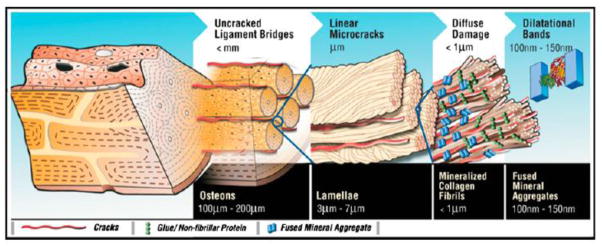
Hierarchical model of bone toughness showing that dilatational bands and diffuse damage are linked to higher-level toughening mechanisms in bone. Removal of OC and OPN translates through the different scales of hierarchy and reduces bone matrix toughness. From Poundarik et al [22] with permission obtained from PNAS.
It is possible that other NCPs besides OC and OPN may together play a structural role in bone. For example, the morphological and material quality changes observed in Bgn-Dcn−/−;−/− knockout suggests that Bgn and Dcn could potentially act in synergy. As discussed previously, these NCPs also play a role in mineral formation individually but how they affect bone mineral quality, when both absent, is unknown. The impact of altered skeletal phenotype, abnormal collagen and impaired mineral quality on bone mechanical properties in this mouse model requires further investigation.
Summary
In conclusion, non-collagenous proteins in bone matrix contribute to bone mechanical properties and bone fracture through multiple mechanisms including alterations of bone morphological parameters crucial for bone’s structural competency, bone quality changes in key matrix parameters (mineral and collagen), and a direct role as load bearing proteins. There has been an increase in literature studying the effect of NCPs on bone quality and mechanics. However, much needs to be addressed, as highlighted throughout this review, to further expand the understanding of NCPs and their role in bone fracture.
For example, many investigations on NCPs focus only on morphological or bone material quality changes. Using a plethora of tools from nano-indentation and AFM to microCT and whole bone three-point bending tests can allow for a comprehensive evaluation of protein knockout genotypes and provide a better understanding of how NCPs transcend the scales of hierarchy in bone, and ultimately affect bone fracture.
Additionally, increased use of heterozygotes (mice lacking one allele of a particular gene encoding the protein), conditional knockouts (mice lacking a specific gene associated with an NCP but only in specific organ system) and double knockouts (mice lacking in two separate proteins) mouse models will further our current understanding of NCPs in bone. Heterozygotes can allow investigators to assess the effect of dose response of implicated proteins like osteocalcin, osteopontin and SPARC, on bone fracture. Conditional knockouts can be useful where the NCP under investigation is vital for other physiological functions, and its global absence can lead to several systemic changes and potentially death. Double knockouts on the other hand can provide greater insight into the role of protein interactions and their independent contributions to bone quality and fracture.
Understanding the effects of NCPs on skeletal mechanical properties in humans through the use of mouse models has proven to be useful because bone matrix proteins are common between the two systems. Such research, in many instances, has allowed increased understanding and modulation of bone morphology and material quality in humans. However, due to inherent differences in microstructure and bone turnover mechanisms between human and mouse bones, the results from mouse models should be explored in humans through the use of aging or pathological tissues obtained from cadaveric or surgical models. Well-designed in vitro and in vivo studies should enhance the current understanding and further elucidate the role and pathway by which NCPs affect bone mechanical properties.
Acknowledgments
This work has been funded by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases AR49635.
Footnotes
Conflict of Interest:
Ms. Stacyann Morgan reports no biomedical financial interest or potential conflict of interest
Dr. Atharva Poundarik reports no biomedical financial interest or potential conflict of interest
Dr. Deepak Vashishth reports no biomedical financial interest or potential conflicts of interest
References
- 1.Mccreadie BR, Goldstein SA. Biomechanics of fracture: is bone mineral density sufficient to assess risk? Journal of bone and mineral research. 2000;15(12):2305–2308. doi: 10.1359/jbmr.2000.15.12.2305. [DOI] [PubMed] [Google Scholar]
- 2.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Bmj. 1996;312(7041):1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouxsein ML. Bone quality: where do we go from here? Osteoporosis international. 2003;14(5):118–127. doi: 10.1007/s00198-003-1489-x. [DOI] [PubMed] [Google Scholar]
- 4.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone Fragility and Collagen Cross-Links. Journal of Bone and Mineral Research. 2004;19(12):2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sroga GE, Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Current osteoporosis reports. 2012;10(2):141–150. doi: 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris BD, Klenerman L, Dodds RA, Bitensky L, Chayen J. Altered organization of non-collagenous bone matrix in osteoporosis. Bone. 1987;8(5):285–288. doi: 10.1016/8756-3282(87)90003-2. [DOI] [PubMed] [Google Scholar]
- 7.Grynpas MD, Tupy JH, Sodek J. The distribution of soluble, mineral-bound, and matrix-bound proteins in osteoporotic and normal bones. Bone. 1994;15(5):505–513. doi: 10.1016/8756-3282(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 8.Ducy P, Desbois C, Boyce B, Pinero G, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382(6590):448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 9.Gundberg CM. Matrix proteins. Osteoporosis international. 2003;14(5):37–42. doi: 10.1007/s00198-003-1471-7. [DOI] [PubMed] [Google Scholar]
- 10.Glowacki J, Rey C, Glimcher MJ, Cox KA, Lian J. A role for osteocalcin in osteoclast differentiation. Journal of cellular biochemistry. 1991;45(3):292–302. doi: 10.1002/jcb.240450312. [DOI] [PubMed] [Google Scholar]
- 11.Rittling SR, Matsumoto HN, Mckee MD, et al. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. Journal of Bone and Mineral Research. 1998;13(7):1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- 12.Romberg RW, Werness PG, Riggs BL, Mann KG. Inhibition of hydroxyapatite-crystal growth by bone-specific and other calcium-binding proteins. Biochemistry. 1986;25(5):1176–1180. doi: 10.1021/bi00353a035. [DOI] [PubMed] [Google Scholar]
- 13.Price PA. Gla-containing proteins of bone. Connective tissue research. 1989;21(1–4):51–60. doi: 10.3109/03008208909049995. [DOI] [PubMed] [Google Scholar]
- 14.Boskey AL. Noncollagenous matrix proteins and their role in mineralization. Bone and mineral. 1989;6(2):111–123. doi: 10.1016/0169-6009(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 15.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23(3):187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 16.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12(9):107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 17.Nikel O, Laurencin D, McCallum SA, Gundberg CM, Vashishth D. NMR Investigation of the Role of Osteocalcin and Osteopontin at the Organic–Inorganic Interface in Bone. Langmuir. 2013;29(45):13873–13882. doi: 10.1021/la403203w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcified tissue international. 2002;71(2):145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 19.Kavukcuoglu NB, Denhardt DT, Guzelsu N, Mann AB. Osteopontin deficiency and aging on nanomechanics of mouse bone. Journal of Biomedical Materials Research Part A. 2007;83(1):136–144. doi: 10.1002/jbm.a.31081. [DOI] [PubMed] [Google Scholar]
- 20.Hansma PK, Fantner GE, Kindt JH, et al. Sacrificial bonds in the interfibrillar matrix of bone. Journal of Musculoskeletal and Neuronal Interactions. 2005;5(4):313. [PubMed] [Google Scholar]
- 21.Gupta HS, Wagermaier W, Zickler GA, et al. Nanoscale deformation mechanisms in bone. Nano Letters. 2005;5(10):2108–2111. doi: 10.1021/nl051584b. [DOI] [PubMed] [Google Scholar]
- 22.Poundarik A, Diab T, Sroga, et al. Dilatational band formation in bone. Proceedings of the National Academy of Sciences. 2012;109(47):19178–19183. doi: 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garnero P, Delmas PD. Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. Journal of Musculoskeletal and Neuronal Interactions. 2004;4(1):50. [PubMed] [Google Scholar]
- 24.Seibel MJ. Biochemical markers of bone turnover part I: biochemistry and variability. The Clinical biochemist. Reviews/Australian Association of Clinical Biochemists. 2005;26(4):97. [PMC free article] [PubMed] [Google Scholar]
- 25.Ammann P, Rizzoli R, Meyer JM, Bonjour JP. Bone density and shape as determinants of bone strength in IGF-I and/or pamidronate-treated ovariectomized rats. Osteoporosis international. 1996;6(3):219–227. doi: 10.1007/BF01622738. [DOI] [PubMed] [Google Scholar]
- 26.Crabtree N, Loveridge N, Parker M, Rushton N, et al. Intracapsular Hip Fracture and the Region-Specific Loss of Cortical Bone: Analysis by Peripheral Quantitative Computed Tomography. Journal of Bone and Mineral Research. 2001;16(7):1318–1328. doi: 10.1359/jbmr.2001.16.7.1318. [DOI] [PubMed] [Google Scholar]
- 27.Cole JH, van der Meulen MC. Whole bone mechanics and bone quality. Clinical Orthopaedics and Related Research. 2011;469(8):2139–2149. doi: 10.1007/s11999-011-1784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Meulen MCH, Jepsen KJ, Mikić B. Understanding bone strength: size isn’t everything. Bone. 2001;29(2):101–104. doi: 10.1016/s8756-3282(01)00491-4. [DOI] [PubMed] [Google Scholar]
- 29.Wolf G. Function of the bone protein osteocalcin: definitive evidence. Nutrition reviews. 1996;54(10):332–333. doi: 10.1111/j.1753-4887.1996.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 30.Ingram RT, Clarke BL, Fisher LW, Fitzpatrick LA. Distribution of noncollagenous proteins in the matrix of adult human bone: evidence of anatomic and functional heterogeneity. Journal of Bone and Mineral Research. 1993;8(9):1019–1029. doi: 10.1002/jbmr.5650080902. [DOI] [PubMed] [Google Scholar]
- 31.Xu T, Bianco P, Fisher LW, Longenecker G, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nature genetics. 1998;20(1):78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 32.Arteaga-Solis E, Sui-Arteaga L, Kim M, Schaffler MB, et al. Material and mechanical properties of bones deficient for fibrillin-1 or fibrillin-2 microfibrils. Matrix Biology. 2011;30(3):188–194. doi: 10.1016/j.matbio.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Termine JD, Kleinman HK, Whitson SW, Conn KM, et al. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26(1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 34.Delany AM, Amling M, Priemel M, et al. Osteopenia and decreased bone formation in osteonectin-deficient mice. Journal of Clinical Investigation. 2000;105(7):915. doi: 10.1172/JCI7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rios H, Koushik SV, Wang H, Wang J, et al. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Molecular and Cellular Biology. 2005;25(24):11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnet N, Standley KN, Bianchi EN, Stadelmann V, et al. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. Journal of Biological Chemistry. 2009;284(51):35939–35950. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gowen LC, Petersen DN, Mansolf AL, Qi H, et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. Journal of Biological Chemistry. 2003;278(3):1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 38.Malaval L, Wade-Guéye NM, Boudiffa M, Fei J, et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. The Journal of experimental medicine. 2008;205(5):1145–1153. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thurner PJ, Chen CG, Ionova-Martin S, et al. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone. 2010;46(6):1564–1573. doi: 10.1016/j.bone.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired Angiogenesis, Early Callus Formation, and Late Stage Remodeling in Fracture Healing of Osteopontin-Deficient Mice. Journal of Bone and Mineral Research. 2007;22(2):286–297. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 41.Sodek J, Ganss B, McKee MD. Osteopontin. Critical Reviews in Oral Biology & Medicine. 2000;11(3):279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 42.Delany AM, Hankenson KD. Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. Journal of cell communication and signaling. 2009;3(3–4):227–238. doi: 10.1007/s12079-009-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corsi A, Xu T, Chen XD, Boyde A, et al. Phenotypic Effects of Biglycan Deficiency Are Linked to Collagen Fibril Abnormalities, Are Synergized by Decorin Deficiency, and Mimic Ehlers-Danlos-Like Changes in Bone and Other Connective Tissues. Journal of Bone and Mineral Research. 2002;17(7):1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 44.Bonnet N, Gineyts E, Ammann P, Conway SJ, Garnero P, Ferrari S. Periostin deficiency increases bone damage and impairs injury response to fatigue loading in adult mice. PloS one. 2013;8(10):e78347. doi: 10.1371/journal.pone.0078347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Philosophical Transactions of the Royal Society of London. B, Biological Sciences. 1984;304(1121):509–518. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 46.Schaffler MB, Burr DB. Stiffness of compact bone: effects of porosity and density. Journal of biomechanics. 1988;21(1):13–16. doi: 10.1016/0021-9290(88)90186-8. [DOI] [PubMed] [Google Scholar]
- 47.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporosis International. 2006;17(3):319–336. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 48.Vashishth D. The role of the collagen matrix in skeletal fragility. Current osteoporosis reports. 2007;5(2):62–66. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- 49.Yoshitake H, Rittling SR, Denhardt DT, Noda M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proceedings of the National Academy of Sciences. 1999;96(14):8156–8160. doi: 10.1073/pnas.96.14.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez DE, Thula-Mata T, Toro EJ, Yeh YW, Holt C, Holliday LS, Gower LB. Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta biomaterialia. 2014;10(1):494–507. doi: 10.1016/j.actbio.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boskey A. Bone mineral crystal size. Osteoporosis international. 2003;14(5):16–21. doi: 10.1007/s00198-003-1468-2. [DOI] [PubMed] [Google Scholar]
- 52.Augat P, Schorlemmer S. The role of cortical bone and its microstructure in bone strength. Age and ageing. 2006;35(suppl 2):ii27–ii31. doi: 10.1093/ageing/afl081. [DOI] [PubMed] [Google Scholar]
- 53.Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proceedings of the National Academy of Sciences. 1976;73(5):1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kavukcuoglu NB, Patterson-Buckendahl P, Mann AB. Effect of osteocalcin deficiency on the nanomechanics and chemistry of mouse bones. Journal of the mechanical behavior of biomedical materials. 2009;2(4):348–354. doi: 10.1016/j.jmbbm.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Jepsen KJ, Davy DT, Krzypow DJ. The role of the lamellar interface during torsional yielding of human cortical bone. Journal of biomechanics. 1999;32(3):303–310. doi: 10.1016/s0021-9290(98)00179-1. [DOI] [PubMed] [Google Scholar]
- 56.Vashishth D. Rising crack-growth-resistance behavior in cortical bone:: implications for toughness measurements. Journal of biomechanics. 2004;37(6):943–946. doi: 10.1016/j.jbiomech.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Burstein AH, Zika JM, Heiple KG, Klein L. Contribution of collagen and mineral to the elastic-plastic properties of bone. The Journal of Bone & Joint Surgery. 1975;57(7):956–961. [PubMed] [Google Scholar]
- 58.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 59.Kavukcuoglu NB, Arteaga-Solis E, Lee-Arteaga S, Ramirez F, Mann AB. Nanomechanics and Raman spectroscopy of fibrillin 2 knock-out mouse bones. Journal of Materials Science. 2007;42(21):8788–8794. [Google Scholar]
- 60.Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. The Journal of cell biology. 1997;136(3):729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoshi K, Kemmotsu S, Takeuchi Y, Amizuka N, Ozawa H. The primary calcification in bones follows removal of decorin and fusion of collagen fibrils. Journal of bone and mineral research. 1999;14(2):273–280. doi: 10.1359/jbmr.1999.14.2.273. [DOI] [PubMed] [Google Scholar]
- 63.Sugars RV, Milan AM, Brown JO, Waddington RJ, et al. Molecular interaction of recombinant decorin and biglycan with type I collagen influences crystal growth. Connective tissue research. 2002;44:189–195. doi: 10.1080/713713596. [DOI] [PubMed] [Google Scholar]
- 64.Boskey AL, Spevak L, Doty SB, Rosenberg L. Effects of bone CS-proteoglycans, DS-decorin, and DS-biglycan on hydroxyapatite formation in a gelatin gel. Calcified tissue international. 1997;61(4):298–305. doi: 10.1007/s002239900339. [DOI] [PubMed] [Google Scholar]
- 65.Garnero P. The contribution of collagen crosslinks to bone strength. BoneKEy reports. 2012;1:182. doi: 10.1038/bonekey.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burr DB, Turner CH, Naick P, Forwood MR, et al. Does microdamage accumulation affect the mechanical properties of bone? Journal of biomechanics. 1998;31(4):337–345. doi: 10.1016/s0021-9290(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 67.Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005;37(1):96–102. doi: 10.1016/j.bone.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 68.Termine JD, Kleinman HK, Whitson SW, et al. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26(1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 69.Boskey AL, Moore DJ, Amling M, Canalis E, Delany AM. Infrared Analysis of the Mineral and Matrix in Bones of Osteonectin-Null Mice and Their Wildtype Controls. Journal of Bone and Mineral Research. 2003;18(6):1005–1011. doi: 10.1359/jbmr.2003.18.6.1005. [DOI] [PubMed] [Google Scholar]
- 70.Oxlund H, Barckman M, Ortoft G, Andreassen TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17(4):S365–S371. doi: 10.1016/8756-3282(95)00328-b. [DOI] [PubMed] [Google Scholar]
- 71.Vashishth D. Collagen glycation and its role in fracture properties of bone. Journal of Musculoskeletal and Neuronal Interactions. 2005;5(4):316. [PubMed] [Google Scholar]
- 72.Landis WJ, Hodgens KJ, Song MJ, Arena J, et al. Mineralization of collagen may occur on fibril surfaces: evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. Journal of structural biology. 1996;117(1):24–35. doi: 10.1006/jsbi.1996.0066. [DOI] [PubMed] [Google Scholar]
- 73.Sasaki N, Nakayama Y, Yoshikawa M, Enyo A. Stress relaxation function of bone and bone collagen. Journal of biomechanics. 1993;26(12):1369–1376. doi: 10.1016/0021-9290(93)90088-v. [DOI] [PubMed] [Google Scholar]
- 74.Tai K, Dao M, Suresh S, Palazoglu A, Ortiz C. Nanoscale heterogeneity promotes energy dissipation in bone. Nature materials. 2007;6(6):454–462. doi: 10.1038/nmat1911. [DOI] [PubMed] [Google Scholar]
- 75.Gao H, Ji B, Jäger IL, Arzt E, Fratzl P. Materials become insensitive to flaws at nanoscale: lessons from nature. Proceedings of the national Academy of Sciences. 2003;100(10):5597–5600. doi: 10.1073/pnas.0631609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson AP, Vincent JFV, Turner RM. The mechanical design of nacre. Proceedings of the Royal society of London. Series B. Biological sciences. 1988;234(1277):415–440. [Google Scholar]
- 77.Barthelat F, Rabiei R. Toughness amplification in natural composites. Journal of the Mechanics and Physics of Solids. 2011;59(4):829–840. [Google Scholar]
- 78.Maruyama N, Shibata Y, Mochizuki A, Yamada A, et al. Bone micro-fragility caused by the mimetic aging processes in α-klotho deficient mice: In situ nanoindentation assessment of dilatational bands. Biomaterials. 2015;47:62–71. doi: 10.1016/j.biomaterials.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Ritter NM, Farach-Carson MC, Butler WT. Evidence for the formation of a complex between osteopontin and osteocalcin. Journal of Bone and Mineral Research. 1992;7(8):877–885. doi: 10.1002/jbmr.5650070804. [DOI] [PubMed] [Google Scholar]
- 80.Thompson JB, Kindt JH, Drake B, Hansma HG, et al. Bone indentation recovery time correlates with bond reforming time. Nature. 2001;414(6865):773–776. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- 81.Hassenkam T, Fantner GE, Cutroni JA, Weaver JC, et al. High-resolution AFM imaging of intact and fractured trabecular bone. Bone. 2004;35(1):4–10. doi: 10.1016/j.bone.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 82.Fantner GE, Hassenkam T, Kindt JH, et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nature materials. 2005;4(8):612–616. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 83.Fantner GE, Adams J, Turner P, Thurner PJ, et al. Nanoscale ion mediated networks in bone: osteopontin can repeatedly dissipate large amounts of energy. Nano letters. 2007;7(8):2491–2498. doi: 10.1021/nl0712769. [DOI] [PubMed] [Google Scholar]
- 84.Diab T, Vashishth D. Morphology, localization and accumulation of in vivo microdamage in human cortical bone. Bone. 2007;40(3):612–618. doi: 10.1016/j.bone.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]