Abstract
Encapsulated in vitro follicle growth (eIVFG) has great potential to provide an additional fertility preservation option for young women and girls with cancer or other reproductive health threatening diseases. Currently, follicles are cultured for a defined period of time and analyzed as a cohort. However, follicle growth is not synchronous, and culturing follicles for insufficient or excessive times can result in compromised gamete quality. Our objective is to determine whether the selection of follicles based on size, rather than absolute culture time, better predict follicle maturity and oocyte quality. Multilayer secondary mouse follicles were isolated and encapsulated in 0.25% alginate. Follicles were cultured individually either for defined time periods or up to specific follicle diameter ranges, at which point several reproductive endpoints were analyzed. The metaphase II (MII) percentage after oocyte maturation on day 6 was the highest (85%) when follicles were cultured for specific days. However, if follicles were cultured to a terminal diameter of 300–350 μm irrespective of absolute time in culture, 93% of the oocytes reached MII. More than 90% of MII oocytes matured from follicles with diameters of 300–350 μm showed normal spindle morphology and chromosome alignment, 85% of oocytes showed 2 pronuclei after in vitro fertilization (IVF), 81% developed into the 2-cell embryo stage, and 38% developed to the blastocyst stage, all significantly higher than the percentages in the other follicle size groups. Our study demonstrates that size-specific follicle selection can be used as a non-invasive marker to identify high quality oocytes and improve reproductive outcomes during eIVFG.
Keywords: fertility preservation, follicle culture, folliculogenesis, meiosis, fertilization
Introduction
Young cancer patients undergoing chemotherapy and radiation treatments may experience threatened fertility as an unintended consequence of these life-saving medical interventions (Jeruss & Woodruff 2009). This issue has been at the forefront of reproductive research activities over the past 10 years and many new technologies have been developed to help these patients preserve their fertility (De Vos et al. 2014). In addition to cancer, there are also non-malignant diseases and conditions, as well as their treatments, which can negatively affect reproductive function (Hirshfeld-Cytron et al. 2011, Purcell & Moley 2011). In addition to fertility concerns, loss of endocrine support of hormonally responsive tissues can cause a cascade of medical and quality-of-life problems and must be addressed as part of the initial comprehensive plan of care for young women.
To address the fertility needs of young women and girls with any fertility-threatening condition or treatment, we have developed an alginate hydrogel-based encapsulation system that supports the growth, development, and maturation of gamete-containing follicles outside the context of the ovary (Xu et al. 2006a). This culture method maintains follicle architecture and the spatial relationship of the oocyte and its supporting somatic cells. This method is significant because it provides a potential alternative to ovarian tissue transplantation for preserving fertility and does not have the inherent risk of reintroducing cancer cells because follicles develop completely in vitro (Woodruff 2007). Encapsulated in vitro follicle growth (eIVFG) has successfully resulted in live births in mice (Xu et al. 2006a) in addition to follicle growth, oocyte development, and preimplantation embryo development in other large mammalian species (Xu et al. 2009a, Xu et al. 2009b, Xu et al. 2010, Songsasen et al. 2011, Xu et al. 2011a). Thus, eIVFG is among one of several other systems that has been successful in supporting the in vitro growth and development of ovarian follicles (Smitz et al. 2010, Telfer & McLaughlin 2012). However, despite the promise of this technology, there is significant room for improvement, as the efficiency of the technique in terms of IVF success and live birth outcomes remains low in the mouse (Xu et al. 2006a). Moreover, there are unique challenges in translating this work from mouse to primates because of distinct species differences in follicle growth patterns and requirements (Xu et al. 2011a).
Hydrogel-based methods of eIVFG permit the growth and development of follicles and oocytes in vitro followed by hormone induced oocyte maturation to stimulate coordinated ovulation and meiotic resumption in the oocyte (Xu et al. 2006a). During eIVFG, follicles are typically isolated and cultured for a defined period of time and analyzed as a cohort before performing the oocyte maturation. However, follicle growth in culture is not synchronous, which means that at any given point there may only be a fraction of follicles that are ready to mature. We hypothesize that this asynchrony combined with our inability to select follicles that contain a fully-grown oocyte may contribute to the reduced efficiency of eIVFG. To improve the eIVFG system and produce fully mature, high-quality oocytes that are competent to be fertilized and produce viable embryos, it is critical to define the point at which cultured follicles are fully-grown and oocytes have achieved full developmental potential. The primary objective of the present study was to monitor mouse follicles individually to determine whether size-specific follicle selection, rather than absolute culture time, can be used as a non-invasive marker to identify follicles during eIVFG to ultimately improve reproductive outcomes using this technique. This is particularly important for human eIVFG, where follicles take longer periods of time (>30 days) to reach maturity (Xu et al. 2009a).
Materials and Methods
Animals
Immature follicles were isolated from ovaries harvested from 16-day-old CD-1 female mice. For control for IVF, mature eggs were collected from the oviducts of hyper-stimulated adult CD-1 female mice. Sperm were collected from the cauda epipdidymi of proven CD-1 male breeders. All mice were housed in polypropylene cages and provided food and water ad libitum. Animals were kept on a 12-hour light/dark cycle (7:00 AM to 7:00 PM) at 23±1°C with 30–50% relative humidity. Animals were fed Teklad Global irradiated 2919 or 2916 chow (Madison, WI, USA), which does not contain soybean or alfalfa meal to minimize exposure to phytoestrogens. All methods used in this study were approved by the Northwestern University Institutional Animal Care and Use Committee (IACUC) and correspond to the National Institutes of Health guidelines and public law.
Follicle isolation, encapsulation, and culture
Multilayer secondary follicles (150–180 μm, type 5b) were isolated from 16-day-old CD-1 female mice as previously described (Xu et al. 2006a). Only follicles that displayed intact morphology were selected for encapsulation and culture. Follicles were placed in maintenance media containing 50% minimal essential medium (αMEM Glutamax) and 50% Nutrient Mixture (F-12 with Glutamax) with 1% fetal bovine serum (FBS, Life Technology, Grand Island, NY, USA) for 2 h before encapsulation. Selected follicles were then encapsulated individually in 0.25% alginate (NovaMatrix, Sandvika, Norway) as previously described (Xu et al. 2006a, Xu et al. 2006b). Alginate beads were placed in 96-well plates, with each well containing 100 μl growth media (50% αMEM Glutamax and 50% F-12 Glutamax supplemented with 3 mg/ml bovine serum albumin [BSA] [Sigma-Aldrich, St. Louis, MO, USA], 10 mIU/ml recombinant follicle-stimulating hormone [rFSH; from A. F. Parlow, National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA], 1 mg/ml bovine fetuin [Sigma-Aldrich, St. Louis, MO, USA], 5 μg/ml insulin, 5 μg/ml transferrin, and 5 μg/ml selenium [Sigma-Aldrich, St. Louis, MO, USA]). For all experiments, follicles were maintained at 37°C. Encapsulated follicles were cultured at 37°C in 5% CO2 in air for specific periods of time (2, 4, 6, 8, 10 days) or until reaching specific follicle diameters of 200–250 μm, 250–300 μm, 300–350 μm, and 350–400 μm. Half of the growth media (50 μl) was replaced every other day. Follicles were imaged at each media change using an inverted Leica DM IRB microscope with 4x and 20x objectives (Leica Microsystems, Buffalo Grove, IL, USA). Follicle growth curves were obtained by plotting the average follicle diameter, which was calculated by averaging two perpendicular measurements from basement membrane to basement membrane of each follicle in ImageJ software (National Institutes of Health, Bethesda, MD, USA). Follicles were considered dead if they had unhealthy appearing oocytes and/or granulosa cells, or if the integrity of the oocyte and somatic cell interface was visibly compromised. Dead and unhealthy follicles were removed from the culture, and only surviving follicles were included in the analysis. The follicle survival rate is more than 90% and less than 10% of follicles showed unhealthy follicle morphology and degenerated oocyte during the culture.
Analysis of oocyte chromatin configuration
Follicles cultured for specific periods of time were removed from alginate beads using 10 IU/ml alginate lyase from flavobacterium multivorum (Sigma-Aldrich, St. Louis, MO, USA). Oocytes were mechanically isolated from follicles cultured for specific days or when follicles reached specific diameters. Oocytes were then immediately incubated in L15 media with 0.1% milrinone (Sigma-Aldrich, St. Louis, MO, USA) and 1 μg/ml Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA) for 15 minutes, and immediately analyzed by confocal microscopy (Leica Microsystems, Buffalo Grove, IL, USA). During oocyte development and maturation, oocyte chromatin can be classified by three types configurations (Zuccotti et al. 1998, Bouniol-Baly et al. 1999). Here we use the following criteria to assign oocytes to these three classes: First, the non-surrounded nucleolus (NSN) was characterized by a cloud of euchromatin occupying the entire nucleoplasm, which contains some chromatin foci. The second configuration is the surrounded nucleolus (SN) which was characterized by condensed heterochromatin surrounding the nucleolus and a nucleoplasm with minimal patches of euchromatin. Finally, the intermediate stage (I) configuration is between NSN and SN and was characterized by a partial rim of perinucleolar chromatin around the nucleolus.
Oocyte maturation, superovulation, fertilization, and embryo culture
Oocyte maturation was performed on specific days of follicle culture or when the follicles reached a specific diameter ranges. Follicles were removed from alginate beads and incubated for 16 h at 37°C in 5% CO2 in air in maturation media (αMEM with 10% fetal bovine serum, 1.5 IU/ml human chorionic gonadotropin (hCG), 10 ng/ml epidermal growth factor [EGF] [BD Biosciences, Franklin Lakes, NJ, USA], and 10 mIU/ml rFSH). Oocytes were then denuded from the surrounding cumulus cells using 0.3% hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA). Oocytes were considered to be arrested at prophase I in the germinal vesicle (GV) stage if the nucleus was intact, but were considered to have undergone germinal vesicle breakdown (GVBD) if the nucleus was not visible. If a polar body was present in the perivitelline space, the oocytes were classified as metaphase II (MII). Fragmented or shrunken oocytes were classified as degenerated (D).
For in vitro fertilization (IVF), mature motile sperm were collected from the cauda epididymis of proven CD-1 male breeders and capacitated in human tubal fluid (HTF) medium with 4 mg/ml BSA for 1.5 h (Kito et al. 2004). MII oocytes from follicles with different diameter ranges were collected for oocyte maturation, with follicle culture time periods ranging from 2 to 10 days. At least 20–40 oocytes were collected for each follicle size group. To eliminate the effect of ZP hardening on insemination, the zona pellucida (ZP) of the denuded MII oocytes was removed by incubating oocytes in Acidic Tyrodes solution (Sigma-Aldrich, St. Louis, MO, USA) for 10–15 sec. ZP-free oocytes were washed 3 times in HTF medium and combined with 50,000 sperm/ml for 4 h to increase fertilization rates and reduce the incidence of polyspermy (Evans et al. 1995, McAvey et al. 2002). Fertilized oocytes were identified by the presence of 2 pronuclei (2PN). Oocytes were then washed in fresh KSOM (EMD Millipore, Billerica, MA, USA) to remove bound sperm and cultured individually in 50 μl KSOM drops until the embryos developed to the blastocyst stage. Superovulated MII oocytes were used as an in vivo control. Superovulation was performed by 5 IU of pregnant mare’s serum gonadotropin (PMSG) (Sigma-Aldrich, St. Louis, MO, USA) intraperitoneal injection into the adult female mice, which was followed by 5 IU hCG injection 48 h after the PMSG injection. MII oocytes were collected from the ampulla 14 h after the hCG injection, and the ZP of MII oocytes was removed before performing IVF.
Histology
Follicles cultured in vitro were removed from the alginate beads and fixed for 3 h at 4°C in 3.8% paraformaldehyde in 1X PBS. Follicles were dehydrated in ascending concentrations of ethanol (50%–100%) and re-encapsulated in 1% alginate hydrogel before being embedded in paraffin using an automated tissue processor. Serial 5 μm sections were cut and stained with hematoxylin and eosin to identify the follicle diameter when antral cavity and cumulus-oocyte complex (COC) were formed during eIVFG.
Immunofluorescence
For spindle morphology and chromosome alignment analysis, gametes obtained following oocyte maturation were fixed in 3.8% paraformaldehyde containing 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 37°C. Oocytes were washed 3 times in blocking solution with 1x PBS containing 0.3% BSA and 0.01% Tween-20, incubated overnight in a 1:50 dilution of mouse anti-α-tubulin (Cell Signaling Technology, Danvers, MA, USA) in blocking solution. Then, oocytes were washed 3 times with blocking solution, mounted using Vectashield containing DAPI (Vector Laboratories, Burlingame, CA, USA), and analyzed using an EVOS FL AUTO microscope (Life Technology, Grand Island, NY, USA) in a blinded fashion. Oocytes with barrel-shaped bipolar spindles and well-organized microtubule fibers, along with tightly aligned chromosomes on the metaphase plate, were scored as normal. All other configurations were considered abnormal.
For chromosome cohesion analysis, gametes obtained following superovulation or in vitro oocyte maturation were cultured for 1 h in CZB containing 100 μM monastrol (Sigma, St. Louis, MO, USA), a kinesin-5 inhibitor, which causes the bipolar MII spindle to collapse into a monoplar spindle and results in the dispersion of chromosomes. Oocytes were then incubated with human CREST autoimmune serum (Immunovision, Springdale, AZ, USA), which was detected by an Alexa-Fluor 594-conjugated goat anti-human secondary antibody (Invitrogen, Carlsbad, CA, USA). Oocytes were washed in blocking solution, incubated in a 1: 5000 of Syto Green nucleic acid stain (Invitrogen, Carlsbad, CA, USA) for 10 min, mounted in Vectashield, and analyzed as previously described (Duncan et al. 2009).
Statistical analyses
Follicle size, oocyte size, and IVF data were analyzed from three to five independent cultures in which 16–24 follicles were cultured for each experimental group. Data were analyzed using one-way ANOVA, followed by Tukey range Test for significant difference. Categorized data were analyzed by the χ2 test; if significance was observed between groups, then we applied the Fisher’s exact test. The significance level was set at P < 0.05.
Results
Follicle size correlated with stage-specific follicle development during eIVFG
To better understand the relationship between follicle size and follicle development during eIVFG, we used the mouse model to define the specific follicle diameters associated with key stage-specific follicle developmental events and other reproductive outcomes. Alginate-based hydrogel encapsulation maintained follicle structure and phenocopied in vivo folliculogenesis from the multilayer secondary follicle to the early antral and late antral follicle stages (Fig. 1A). The average follicle diameter increased from 158 ± 15 μm on day 0 to 376 ± 31μm on day 8 and 390 ± 29 μm on day 10 (Fig. 1B). The average size of the oocyte increased from 60 ± 3 μm to 72 ± 4 μm from day 0 to day 8, and up to 74 ± 4 μm on day 10 (Fig. 1B). Our prior studies were limited to non-invasive light microscopy analysis of follicle morphology, which included the development of a central, cleared space described as the antrum and the movement of the oocyte to an acentric position. Here we extend these gross/superficial phenotypes to a more systematic histologic analysis of antral formation and cumulus-oocyte interactions. Using this terminal analysis required removal of follicles from culture on each day. The histology data showed that the antral cavity was present only after follicles reached 257 ± 19 μm, with this transition occurring in 8% of follicles cultured on day 3, 75% of follicles cultured on day 4 and 17% of follicles on day 5 (Fig. 1A and C). The oocytes became surrounded by cumulus cells and formed COCs when the follicle diameter reached 308 ± 26 μm, with this taking place in 12% of follicles cultured on day 6, 63% of follicles on day 7, and 25% of follicles on day 8 (Fig. 1A and D). After in vitro oocyte maturation, cumulus cell expansion was observed and meiotic resumption occurred, which resulted in the formation of MII oocytes as evidenced by the first polar body extrusion (Fig. 1A). These results demonstrate that follicle stage-specific development correlates with follicle size during eIVFG.
FIG. 1.
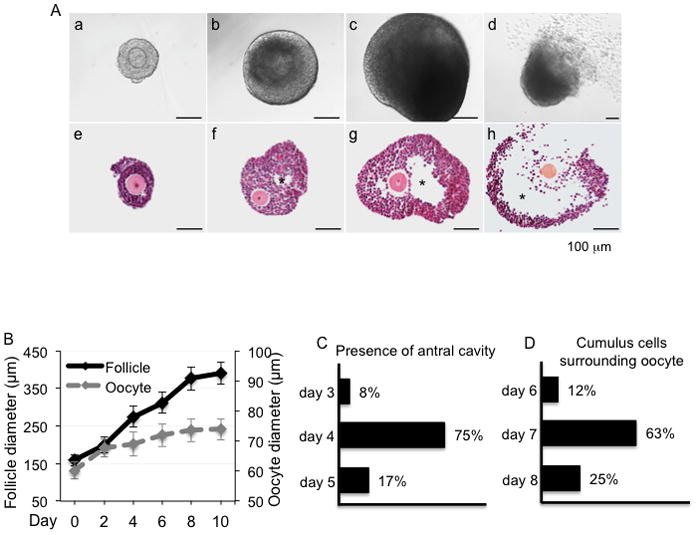
(A) Mouse follicle development in vitro in alginate-based hydrogels. a–d. Follicles developed from (a) multilayer secondary follicle to (b) early antral, and (c) antral stages during eIVFG. (d) The oocyte extruded the first polar body after oocyte maturation in vitro. Corresponding histology of follicles in different developmental stages during eIVFG: (e) pre-antral stage, (f) early antral stage, (g) antral stage, and (h) after oocyte maturation. (B) Follicle and oocyte growth during eIVFG from day 0 to day 10. Follicle distribution on different culture days (C) from preantral to antral stage with the presence of antral cativity and (D) cumulus-oocyte complex formation with cumulus cells surrounding the oocyte. Error bars: standard deviation; * Follicle antrum; scale bar: 100 μm. N=20–40 follicles for three replicates.
Follicle size correlates with oocyte chromatin configuration during eIVFG
During oocyte development and maturation, the oocyte chromatin configuration transits from NSN to SN state (De La Fuente & Eppig 2001). To determine the relationship between oocyte chromatin configuration changes and the follicle growth in our eIVFG system, we examined when the NSN to SN transition occurred. On day 0 and day 2 of eIVFG, all oocytes within follicles were in the NSN configuration with the chromatin dispersed throughout the nucleolus (Fig. 2A and B). There were oocytes with both the NSN and SN chromatin configurations on day 4 (Fig. 2A and B), and the oocytes predominantly had a SN chromatin configuration after day 6 (Fig. 2A and B) (75% on day 6, 96% on day 8, and 94% on day 10, respectively; Fig. 2B). Oocytes that had an NSN chromatin configuration were significantly smaller in diameter compared to oocytes with I and SN chromatin configruations (Fig. 2C). This data is consistent with previous studies demonstrating that the transition in oocyte chromatin configuration correlates with oocyte development (De La Fuente & Eppig 2001). The average follicle diameter was 181± 26 μm, 237 ± 31μm, and 330± 64 μm for follicles containing oocytes with NSN, intermediate, and SN chromatin configurations, respectively (Fig. 2D). These data indicate that oocyte chromatin configuration transitions from NSN to SN state when follicles reach the antral stage during eIVFG (Fig. 1), which correlates with oocyte chromatin redistribution in vivo (De La Fuente & Eppig 2001, Segers et al. 2010). These findings are significant because we are able to define for the first time the precise follicle diameter during eIVFG when follicle-enclosed oocytes achieve chromatin configuration in SN state. This determination is possible with the alginate hydrogel-based eIVFG system because the encapsulation maintains the follicle structure, so accurate diameter measurements can be obtained.
FIG. 2.
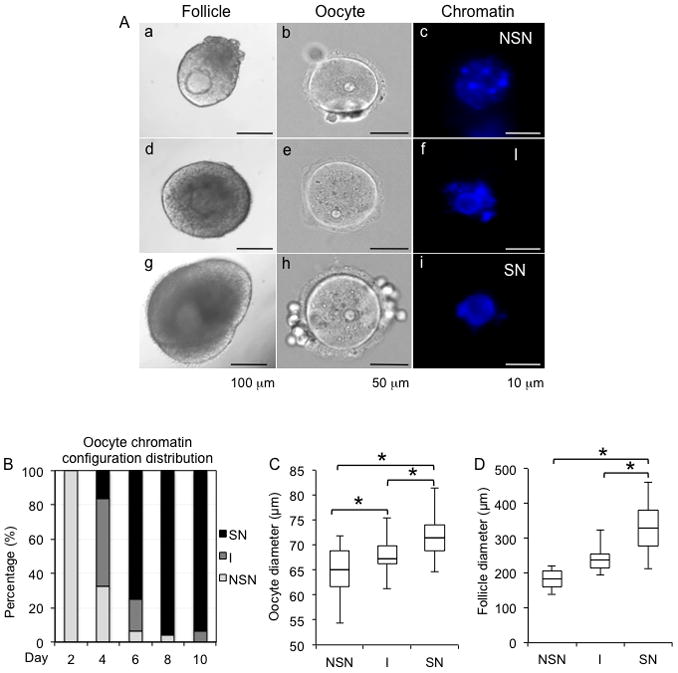
Representative images of follicles and the chromatin configuration within their enclosed oocytes during eIVFG (A). (a–c) follicle containing an oocyte with non-surrounded nucleus (NSN); (d–f) follicle containing an oocyte with an intermediate stage (I) chromatin configuration; (g–i) follicle containing an oocyte with surrounded nucleus (SN). (B) Chromatin configuration distribution from day 2 to day 10 during eIVFG. (C) Oocyte diameters associated with different oocyte chromatin configurations during eIVFG. (D) Follicle diameters associated with different oocyte chromatin configurations during eIVFG. Scale bars: 100 μm for a, d, and g; 50 μm for b, e, and h; 10 μm for c, f, and i. Error bars: standard deviation. *P < 0.05, compared to diameter of follicles with oocyte in I stage in C, and compared to the diameter of follicles with oocyte in SN stage in C and D. N=40–80 follicles for three replicates.
Oocyte maturation based on follicle size rather than absolute culture improves oocyte meiosis outcomes
Oocyte maturation was performed on follicles cultured for specific times or until they reached specific terminal diameter ranges to compare the oocyte meiotic outcomes of these two strategies. The MII percentage of oocytes from follicles cultured in vitro on day 2 was 25%, indicating that oocytes were not mature and could not support resumption of meiosis (Fig. 3A). The MII percentage on days 4, 6, 8, and 10, were all > 75%, with the MII percentage on day 6 being significantly higher than oocytes matured on days 4, 8, and 10 (85% vs. 75%, 75%, and 80%) and with follicle diameter at 289 ± 18 μm. When oocyte maturation was performed on follicles selected based on size, follicles that ranged from 300–350 μm showed the highest MII percentage, at 93% (Fig. 3B). This was significantly higher than the MII percentage for follicles cultured to day 6 (85%) and was also higher than the MII percentages for follicles <250 μm, 250–300 μm, and >350 μm in diameter, (Fig. 3A and B). Follicles that reached diameters of 300–350 μm developed over different culture times ranging from day 6 to day 9 (10% from day 6, 62% from day 7, 24% from day 8, and 4% from day 9, respectively). These results indicate that follicle size is a better predictor of oocyte meiotic competence compared to absolute culture time, and follicles with diameter of 300–350 μm showed the best oocyte meiotic competence. Therefore subsequent studies were focused on how follicle size correlated with oocyte reproductive outcomes.
FIG. 3.
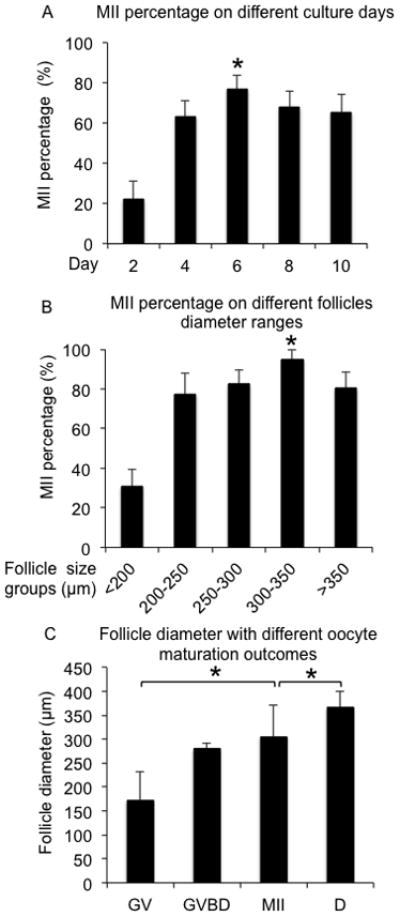
MII percentages after oocyte maturation for eIVFG cultured follicles. (A) MII percentages for follicles cultured for specific periods of time from day 2 to day 10. (B) MII percentages for follicles cultured to specific diameters. (C) Follicle diameter associated with different oocyte maturation outcomes. MII: metaphase II. GV: germinal vesicle. GVBD: germinal vesicle break down. D: degenerated oocyte. *P < 0.05, compared to the other groups in (A) and (B), and compared to the diameter of follicles with oocyte in MII stage after oocyte maturation in (C). N=100–160 follicles for five replicates.
To define the precise follicle diameters at which cultured follicles are fully meiotic competent, follicles were categorized by different meiotic outcomes after oocyte maturation. Oocytes that remained arrested at the germinal vesicle (GV) stage and did not resume meiosis came from follicles that had an average diameter at 173 ± 59 μm, while the oocytes that had undergone germinal vesicle breakdown (GVBD) were from follicles with a diameter of 280 ± 12 μm (Fig. 3C). Oocytes that were able to reach MII had an average follicle diameter of 305 ± 67 μm (Fig. 3C). The diameters of follicles that produced MII oocytes were significantly larger than those that produced oocytes that failed to resume meiosis (Fig. 3C). Interestingly, degenerated oocytes tended to be observed in follicles with the largest average diameter (367 ± 34 μm; Fig. 3C). Based on these data, we conclude that the oocyte maturation strategy based on follicle size is highly correlated to oocyte meiotic outcomes and could therefore have predictive value.
Follicle size correlates with gamete spindle morphology and chromosome alignment
To study the quality of the mature gametes obtained following eIVFG from follicles of different terminal diameters, oocytes that had progressed to MII after oocyte maturation were analyzed for spindle integrity and chromosome alignment, two critical markers of oocyte developmental competence (Moon et al. 2003, Li et al. 2014). Ninety percent of oocytes from follicles cultured to a diameter of 300–350 μm had barrel-shaped bipolar spindles and tightly aligned chromosomes on the metaphase plate. This morphology was comparable to what was observed in MII oocytes produced by superovulation in vivo (Fig. 4A–C and G). In contrast, a significantly higher percentage of MII oocytes from follicles cultured to diameters of < 250 μm or > 350 μm exhibited abnormal spindle morphology and/or chromosomal misalignment. These oocytes lacked barrel shaped spindles, and their chromosomes were dispersed instead of being tightly aligned on the metaphase plate (Fig. 4D–F and G; 35% and 41% of oocytes, respectively). Follicles with diameters between 250–300 μm produced MII oocytes that had an increased incidence of spindle abnormalities (15%), but there was no a significant difference compared to MII oocytes from 300–350 μm follicles (Fig. 4G).
FIG. 4.
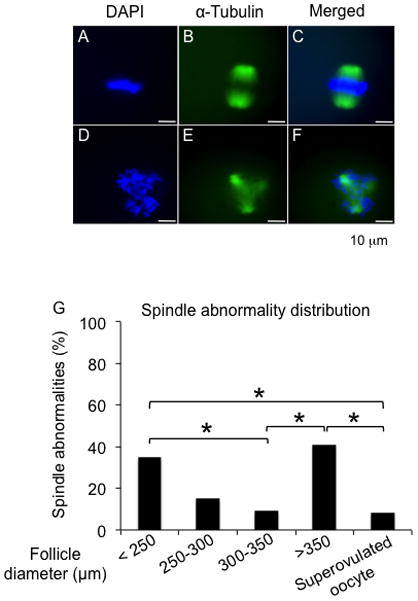
Spindle morphology and chromosome alignment of MII oocytes after oocyte maturation. (A–C) Representative images of meiotic spindles of MII oocytes with well-organized microtubule fibers (green) and tightly aligned chromosomes (blue). (D–F) Representative images of MII oocytes with abnormal spindle morphology and/or chromosomal alignment. (G) Incidence of spindle abnormality and chromosome misalignment in MII oocytes from follicles cultured to different sizes. MII: metaphase II. *P < 0.05. N=20–40 follicles for three replicates.
Follicle size is associated with IVF and embryo development outcomes
To determine the developmental competence of oocytes from different size follicles, MII oocytes from follicles cultured to various diameter ranges were selected for IVF and subsequent monitoring of fertilization and preimplantation embryo development. 85% of oocytes from follicles cultured to 300–350 μm reached the 2-PN stage after IVF, 81% developed to the 2-cell embryo stage, and 38% developed to the blastocyst stage. This developmental progression was significantly better than that observed with oocytes from follicles cultured to diameters of <250 μm, 250–300 μm, or >350 μm (Fig. 5). There was no significant difference in the fertilization percentages or the percentage of 2-cell embryos for MII oocytes from 300–350 μm cultured follicles compared to in vivo controls, in which superovulated MII oocytes were used for IVF (Fig. 5). However, the percentage of development to the blastocyst stage was significantly lower for MII oocytes from eIVFG compared to MII oocytes derived from in vivo superovulation (Fig. 5). Taken together these results indicate that, while eIVFG may not be able to produce gametes with as high quality as those produced in vivo, growing follicles to precise terminal diameters of 300–350 μm greatly improves the developmental competence of the resulting gametes.
FIG. 5.
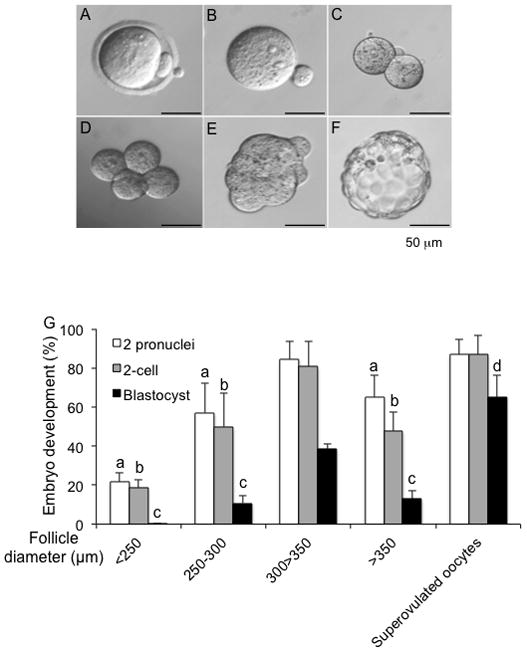
Follicle size is associated with IVF and preimplantation embryo development outcomes. Representative images of MII oocytes after IVF (A) showing 2 pronuclei (2-PN) (B). Fertilized oocyte developed to 2-cell (C), 4-cell (D), morula (E), and blastocyst stages (F). (G) Developmental outcomes for oocytes from follicles cultured up to specific follicle sizes or from in vivo control superovulated oocytes. a, b and c: P < 0.05 compared to the percentage of corresponding embryo development stage with follicle size group of 300–350 μm and the group of superovulated oocytes. d: P < 0.05 between follicle size group of 300–350 μm and superovulated oocytes. Scale bar: 50 μm. N=30–60 for three replicates.
Discussion
With the ultimate goal of translating eIVFG to young females with any fertility threatening condition, tremendous advances have been made in supporting follicle growth and oocyte development in large mammalian species such as domestic dogs, baboons, rhesus monkeys, and humans (Xu et al. 2009a, Xu et al. 2009b, Xu et al. 2010, Songsasen et al. 2011, Xu et al. 2011a, Xu et al. 2011b). Rhesus monkey secondary follicles have been cultured in vitro, produced MII oocytes, and cleaved to two-cell stage embryos and arrested with uneven cleavage after IVF (Xu et al. 2011a). Previous reports showed that in vivo, the size of isolated follicles correlated with oocyte quality in bovine and porcine models (Lucas et al. 2002, Raghu et al. 2002, Krisher 2004). The goal of this study was to definitively determine the in vitro size index that was most highly correlated to a high quality gamete, and to then adjust all future studies to size endpoints rather than days in culture, and monitoring follicle individually and choose follicle size as a non-invasive maker to predict follicle maturity.
Here, we demonstrate that oocytes obtain full meiotic and developmental competence when follicle diameters reach 300–350 μm (325 ± 27 μm). Although 85% of oocytes extruded the first polar body when oocyte maturation was performed on day 6 (Fig. 3A), the follicle diameter range on day 6 (270–310 μm) is smaller than the follicles that contained oocytes with the best meiotic and developmental outcomes (300–350 μm). The majority (>60%) of follicles with a diameter of 300–350 μm were obtained following 7 days of culture whereas only 19% were obtained after 8 days, which is the standard culture period for secondary follicles (Xu et al. 2006a). This reduced meiotic and developmental competence observed following a total of 8 days of culture is also consistent with our findings that these oocytes have a significant increase in unpaired sister chromatids (Fig. S1) and are slightly overgrown (Carabatsos et al. 1998, Hutt & Albertini 2007, Miao et al. 2009). These phenotypes are typically associated with gamete aging, so we propose that prolonged eIVFG results in a new form of aging, pre-ovulatory aging, which could account for compromised developmental competence (Tarin et al. 1999, Fissore et al. 2002, Mailhes 2008). These results highlight the need to shift towards size-specific follicle selection for oocyte maturation, which should increase the fraction of high-quality oocytes and improve reproductive outcomes.
A hallmark of oogenesis is the conversion of a highly transcriptionally active growing oocyte into a transcriptionally quiescent oocyte towards the end of its growth phase. This transition strongly correlates with a transition in the oocyte chromatin configuration transition from a NSN to SN state (Watson 2007). Oocytes synthesize and accumulate maternal RNAs to ensure the acquisition of oocyte nuclear and cytoplasmic maturation during oogenesis (Moore & Lintern-Moore 1978, Eichenlaub-Ritter & Peschke 2002). It has been reported that the transcriptome of oocytes from follicles cultured in alginate hydrogels is similar to the transcriptome of oocytes developing in vivo, suggesting that eIVFG supports and phenocopies the oocyte development in vitro (Mainigi et al. 2011). Pre-antral follicles, which contain oocytes in a transcriptionally active state, are not meiotically competent, and upon the follicle antral cavity formation and oocyte transcription repression, the oocytes obtain meiotic competence (Zuccotti et al. 1998, Bouniol-Baly et al. 1999). Consistent with previous results in vivo, our data suggest that the oocyte transcription transitions from an active to a quiescent state and oocytes become meiotic competent when their corresponding follicles develop to the antral stage during eIVFG (Fig. 1–2).
The maturation rate of follicles cultured for a total of 8 days (80%) in our study is higher than that in previous reports from our group (63%) (Xu et al. 2006c). Two factors could have resulted in this higher MII percentage independently of follicle size-based selection methods. First, we used 0.25% of alginate instead of the 1.5%, which is less rigid, thereby providing a more permissive environment as follicles increase in size to the antral stage. The decreased alginate concentration also promotes the transport and exchange of macromolecules from the culture media or secreted by the follicles, which may promote oocyte development (Xu et al. 2006b). Second, we used a combined growth medium of αMEM and F12 instead of αMEM medium alone. It has been reported that this combined growth medium improves oocyte meiotic competence (Tagler et al. 2013). F12 contains various inorganic salts and other components not present in the αMEM such as zinc sulfate and hypoxanthine, which are critical for the oocyte maturation (Eppig et al. 1985, Kim et al. 2010). However, even though our follicle culture strategies in this study are more advanced and result in higher follicle maturation rate, the size-specific follicle selection at certain follicle diameter range for oocyte maturation significantly improves the oocyte meiotic outcomes compared to absolute follicle culture time.
The percentage of oocytes with an extruded first polar body after oocyte maturation is typically used as a readout of oocyte meiotic competence. However, polar body extrusion is not a sufficient indicator of oocyte quality. In germ cells, the meiotic spindle is a unique subcellular structure composed of microtubules, which is essential for mediating proper chromosome segregation and producing a haploid gamete (Li et al. 2006). In our study, the majority of MII oocytes with normal spindle morphology and chromosome alignment came from follicles with diameters of 300–350 μm. However, follicles with diameters < 250 μm or > 350 μm not only had a lower MII percentage, but the MII oocytes also had a higher proportion of spindle abnormalities and the incidence of chromosome misalignment (Fig. 4), suggesting that both immature and prolonged cultured follicles have compromised oocyte quality.
Follicle survival rate and MII percentage have been used as endpoints in the development of eIVFG conditions (Tagler et al. 2012, Tagler et al. 2013); however, markers of developmental competence are equally critical to evaluate oocyte quality. Our data demonstrate that follicles with diameters of 300–350 μm have the highest MII percentage and the best oocyte developmental outcomes. MII oocytes retrieved from follicles ranging between 250–300 μm in diameter have similar spindle abnormalities as the oocytes from follicles with diameters of 300–350 μm and those obtained from superovulation in vivo. Therefore, these oocytes are meiotically competent. However, the fertilization capacity and embryo development outcomes of these oocytes are significantly compromised (Fig. 5), suggesting that the oocyte developmental competence has not yet been acquired, even though meiotic competence has. Repression of oocyte transcription and antral cavity formation correspond to oocyte meiotic competence acquisition (Segers et al. 2010). However, follicle development to this stage (250–300 μm) is not sufficient to support full oocyte developmental competence (Fig. 5), suggesting that post-transcriptional activities such as selective transcript degradation, mRNA polyadenylation, and translation are also required at later stages of oocyte development (de Moor & Richter 2001, Eichenlaub-Ritter & Peschke 2002, Chen et al. 2011). On the other hand, extending the culture time for follicles to reach diameters >350 μm also had detrimental outcomes. For example, the largest diameter follicles not only produced poor quality oocytes with lower MII percentages and increased oocyte degeneration (Fig. 3), but also contained oocytes with decreased developmental outcomes (Fig. 5), which could be attributed to increased spindle and chromosomal abnormalities (Fig. 4).
In conclusion, our study demonstrates that size-specific follicle selection, rather than absolute culture time, results in the identification of oocytes that will have the best meiotic and developmental outcomes. Oocytes from follicles that lie outside the 300–350 μm size window are immature or have undergone extended culture, both of which compromises gamete quality. Through individualized follicle monitoring, we hope to better identify the optimal endpoint for our eIVFG system that best recapitulates in vivo folliculogenesis and produces high-quality oocytes for maturation. The human follicle is one of the most difficult tissues to maintain in culture over extended periods of time. This is likely due to the source of the tissue (generally from an individual with a cancer diagnosis), age, previous treatments and tissue heterogeneity (Picton et al. 2003). The scarcity of the tissue for research (which is expected) and the fact that many follicles do not track from primordial to primary through antral and ovulated follicle. Thus, there are many complexities when growing follicles in culture. That said, we have had success with the mouse, goat, dog, bovine, baboon and rhesus monkey (Xu et al. 2006a, Xu et al. 2009a, Xu et al. 2009b, Xu et al. 2010, Songsasen et al. 2011, Xu et al. 2011a, Silva et al. 2014) and we, and others continue to develop in vivo and in vitro techniques to advance the field (De Vos et al. 2014). We believe the discoveries described in the present report will further refine the eIVFG system advancing the clinical application of human follicle culture for women who wish to preserve their fertility in the face of diseases or treatments that may compromise their reproductive health.
Supplementary Material
Figure S1. (A) Representative images of oocytes with (a) normal chromatid segregation and (b) unpaired sister chromatids. (B) Incidence of unpaired sister chromatids in MII oocytes from follicles cultured to 8 days. *P < 0.05 compared to the incidence of superovulated oocytes and MII oocytes from primed COCs. MII: metaphase II. COC: cumulus-oocyte complex. N=20–40 follicles for three replicates.
Acknowledgments
Funding: This work was supported by National Institutes of Health (NIH) (P50HD076188) to T. K. Woodruff.
We thank Megan M. Romero and Keisha M. Barreto (Northwestern University Ovarian Histology: P01HD021921) for sectioning our follicle samples, Rachel M. Smith for assistance on experiment design, and Stacey C. Tobin for editorial assistance on the manuscript.
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest.
References
- Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biology of Reproduction. 1999;60:580–587. doi: 10.1095/biolreprod60.3.580. [DOI] [PubMed] [Google Scholar]
- Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol. 1998;204:373–384. doi: 10.1006/dbio.1998.9087. [DOI] [PubMed] [Google Scholar]
- Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, Sette C, Blelloch R, Conti M. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25:755–766. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229:224–236. doi: 10.1006/dbio.2000.9947. [DOI] [PubMed] [Google Scholar]
- de Moor CH, Richter JD. Translational control in vertebrate development. Int Rev Cytol. 2001;203:567–608. doi: 10.1016/s0074-7696(01)03017-0. [DOI] [PubMed] [Google Scholar]
- De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Chiang T, Schultz RM, Lampson MA. Evidence That a Defective Spindle Assembly Checkpoint Is Not the Primary Cause of Maternal Age-Associated Aneuploidy in Mouse Eggs. Biol Reprod. 2009;81:768–776. doi: 10.1095/biolreprod.109.077909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Peschke M. Expression in in-vivo and in-vitro growing and maturing oocytes: focus on regulation of expression at the translational level. Hum Reprod Update. 2002;8:21–41. doi: 10.1093/humupd/8.1.21. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Ward-Bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biology of Reproduction. 1985;33:1041–1049. doi: 10.1095/biolreprod33.5.1041. [DOI] [PubMed] [Google Scholar]
- Evans JP, Schultz RM, Kopf GS. Mouse sperm-egg plasma membrane interactions: analysis of roles of egg integrins and the mouse sperm homologue of PH-30 (fertilin) beta. J Cell Sci. 1995;108 (Pt 10):3267–3278. doi: 10.1242/jcs.108.10.3267. [DOI] [PubMed] [Google Scholar]
- Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124:745–754. doi: 10.1530/rep.0.1240745. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Cytron J, Gracia C, Woodruff TK. Nonmalignant diseases and treatments associated with primary ovarian failure: an expanded role for fertility preservation. J Womens Health (Larchmt) 2011;20:1467–1477. doi: 10.1089/jwh.2010.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod Biomed Online. 2007;14:758–764. doi: 10.1016/s1472-6483(10)60679-7. [DOI] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito S, Hayao T, Noguchi-Kawasaki Y, Ohta Y, Uhara H, Tateno S. Improved in vitro fertilization and development by use of modified human tubal fluid and applicability of pronucleate embryos for cryopreservation by rapid freezing in inbred mice. Comparative Medicine. 2004;54:564–570. [PubMed] [Google Scholar]
- Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004;82(E-Suppl):E14–23. doi: 10.2527/2004.8213_supplE14x. [DOI] [PubMed] [Google Scholar]
- Li M, Zhao HC, Li R, Yu Y, Qiao J. Chromosomal aberrations in in-vitro matured oocytes influence implantation and ongoing pregnancy rates in a mouse model undergoing intracytoplasmic sperm injection. Plos One. 2014;9:e103347. doi: 10.1371/journal.pone.0103347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Feng HL, Cao YJ, Zheng GJ, Yang Y, Mullen S, Critser JK, Chen ZJ. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril. 2006;85:827–832. doi: 10.1016/j.fertnstert.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Lucas X, Martinez EA, Roca J, Vazquez JM, Gil MA, Pastor LM, Alabart JL. Relationship between antral follicle size, oocyte diameters and nuclear maturation of immature oocytes in pigs. Theriogenology. 2002;58:871–885. doi: 10.1016/s0093-691x(02)00699-4. [DOI] [PubMed] [Google Scholar]
- Mailhes JB. Faulty spindle checkpoint and cohesion protein activities predispose oocytes to premature chromosome separation and aneuploidy. Environ Mol Mutagen. 2008;49:642–658. doi: 10.1002/em.20412. [DOI] [PubMed] [Google Scholar]
- Mainigi MA, Ord T, Schultz RM. Meiotic and developmental competence in mice are compromised following follicle development in vitro using an alginate-based culture system. Biol Reprod. 2011;85:269–276. doi: 10.1095/biolreprod.111.091124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvey BA, Wortzman GB, Williams CJ, Evans JP. Involvement of calcium signaling and the actin cytoskeleton in the membrane block to polyspermy in mouse eggs. Biol Reprod. 2002;67:1342–1352. doi: 10.1095/biolreprod67.4.1342. [DOI] [PubMed] [Google Scholar]
- Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573–585. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- Moon JH, Hyun CS, Lee SW, Son WY, Yoon SH, Lim JH. Visualization of the metaphase II meiotic spindle in living human oocytes using the Polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod. 2003;18:817–820. doi: 10.1093/humrep/deg165. [DOI] [PubMed] [Google Scholar]
- Moore GP, Lintern-Moore S. Transcription of the mouse oocyte genome. Biol Reprod. 1978;18:865–870. doi: 10.1095/biolreprod18.5.865. [DOI] [PubMed] [Google Scholar]
- Picton HM, Danfour MA, Harris SE, Chambers EL, Huntriss J. Growth and maturation of oocytes in vitro. Reprod Suppl. 2003;61:445–462. [PubMed] [Google Scholar]
- Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28:517–524. doi: 10.1007/s10815-011-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu HM, Nandi S, Reddy SM. Follicle size and oocyte diameter in relation to developmental competence of buffalo oocytes in vitro. Reprod Fertil Dev. 2002;14:55–61. doi: 10.1071/rd01060. [DOI] [PubMed] [Google Scholar]
- Segers I, Adriaenssens T, Ozturk E, Smitz J. Acquisition and loss of oocyte meiotic and developmental competence during in vitro antral follicle growth in mouse. Fertil Steril. 2010;93:2695–2700. doi: 10.1016/j.fertnstert.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Silva GM, Rossetto R, Chaves RN, Duarte AB, Araujo VR, Feltrin C, Bernuci MP, Anselmo-Franci JA, Xu M, Woodruff TK, Campello CC, Figueiredo JR. In vitro development of secondary follicles from pre-pubertal and adult goats cultured in two-dimensional or three-dimensional systems. Zygote. 2014:1–10. doi: 10.1017/S0967199414000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songsasen N, Woodruff TK, Wildt DE. In vitro growth and steroidogenesis of dog follicles are influenced by the physical and hormonal microenvironment. Reproduction. 2011;142:113–122. doi: 10.1530/REP-10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagler D, Makanji Y, Anderson NR, Woodruff TK, Shea LD. Supplemented alphaMEM/F12-based medium enables the survival and growth of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng. 2013;110:3258–3268. doi: 10.1002/bit.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, Shea LD. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A. 2012;18:1229–1238. doi: 10.1089/ten.tea.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Aguilar A, Minarro J, Hermenegildo C, Cano A. Long-term effects of postovulatory aging of mouse oocytes on offspring: a two-generational study. Biology of Reproduction. 1999;61:1347–1355. doi: 10.1095/biolreprod61.5.1347. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M. Strategies to support human oocyte development in vitro. Int J Dev Biol. 2012;56:901–907. doi: 10.1387/ijdb.130001et. [DOI] [PubMed] [Google Scholar]
- Watson AJ. Oocyte cytoplasmic maturation: a key mediator of oocyte and embryo developmental competence. J Anim Sci. 2007;85:E1–3. doi: 10.2527/jas.2006-432. [DOI] [PubMed] [Google Scholar]
- Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Human Reproduction. 2011a;26:1061–1072. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Human Reproduction. 2009a;24:2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011b;84:689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006a;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006b;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biology of Reproduction. 2006c;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biology of Reproduction. 2009b;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotti M, Giorgi Rossi P, Martinez A, Garagna S, Forabosco A, Redi CA. Meiotic and developmental competence of mouse antral oocytes. Biol Reprod. 1998;58:700–704. doi: 10.1095/biolreprod58.3.700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A) Representative images of oocytes with (a) normal chromatid segregation and (b) unpaired sister chromatids. (B) Incidence of unpaired sister chromatids in MII oocytes from follicles cultured to 8 days. *P < 0.05 compared to the incidence of superovulated oocytes and MII oocytes from primed COCs. MII: metaphase II. COC: cumulus-oocyte complex. N=20–40 follicles for three replicates.


