Abstract
A combined experimental and mathematical model of intermittent hypoxia (IH) conditioned engineered tissue was used to characterize the effects of IH on the formation of in vitro vascular networks. Results showed that the frequency of hypoxic oscillations has pronounced influence on the vascular response of endothelial cells and fibroblasts.
Keywords: endothelial cells, vasculogenesis, oxygen, intermittent hypoxia, 3D culture, vascular endothelial growth factor
Oxygen tension is a key regulator in the angiogenic process required for sustained tumor growth [1]. While universal to all solid tumors, this process is inefficient, leading to the presence of both chronic hypoxia (CH; sustained low levels of oxygen) and intermittent hypoxia (IH; the periodic cycling between hypoxia and normoxia). The effects of IH on vascular development are poorly understood, due in large part to the difficulties of controlling dynamic oxygen conditions [2]. Furthermore, the kinetics of IH measured in vivo varies significantly, spanning frequencies on the order of minutes to days per cycle [3], and fluctuating across tumors as well as within the same tumor [4]. Of particular interest is the response of the endothelial lining of tumor blood vasculature, considering the critical role of angiogenesis in tumor progression, and the fact that endothelial cells in normal (non-cancerous) tissue rarely experience hypoxia. Relatively little is known about how IH modulates endothelial cell function, although some reports suggest that IH may protect endothelial cells against apoptosis [5, 6], or even render them radioresistant [7]. In this note, we investigate the capacity of endothelial cells to form new vasculature structures during IH. We describe a combined experimental and mathematical model of thick, engineered tissue exposed to IH, and use this model to characterize the effects of IH on the formation of vascular networks in vitro.
A multi-chambered oxygen control system (Fig. 1A) was fabricated out of polymethyl methacrylate (PMMA) and housed in a 37°C incubator. The LabVIEW controlled system cycles premixed gases containing 1% O2, 5% CO2, 94% N2 (CO2, low) and 20% O2, 5% CO2, 75% N2 (CO2, high). A dynamic fluorescence quenching-based oxygen sensor system (PreSens, Germany) was used to monitor real-time oxygen levels within each chamber.
Figure 1.
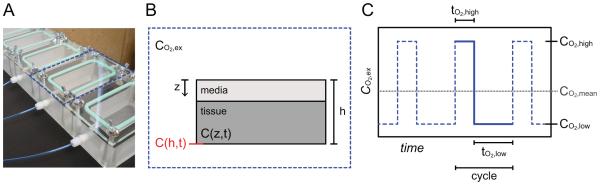
A) The fabricated temporal oxygen control system facilitates in vivo-like IH dynamics. The blue dotted line outlines a single incubation chamber. B) Each chamber may be subjected to an external oxygen profile, CO2,ex, which is applied to the z = 0 boundary of the media/tissue assembly that is housed inside. C) A representative IH profile shown by the blue dotted line is characterized by the cycling between CO2,high for tO2,high and CO2,low for tO2,low, with a mean oxygen level of CO2,mean. The blue solid line segment represents a single cycle.
Endothelial colony forming cell-derived endothelial cells (ECFC-EC) were isolated from human umbilical cord blood as previously described [8], and fed with EGM-2 (Lonza, Wakersfield, MD, USA). Normal human lung fibroblasts (NHLF; Lonza) were cultured in FGM-2 (Lonza). 1χ106 ECFC-EC/mL and 2χ106 NHLF/mL were suspended in a fibrinogen solution (10 mg/mL; Sigma-Aldrich, St. Louis, MO, USA), mixed with thrombin (50 units/mL; Sigma-Aldrich), and pipetted onto a circular glass cover slip with an affixed polydimethylsiloxane (PDMS) retaining ring as previously described [9]. Tissues were first maintained at 20% O2 for 24 hours, and then transferred to the desired oxygen condition for an additional 7 days. Supernatant was collected from the tissues on Days 1, 3, 5, and 7 and analyzed for human VEGF-A protein production using ELISA (R&D Systems, Minneapolis, MN, USA).
Tissues were fixed in 10% formalin, blocked with a 2% BSA (Sigma-Aldrich) and 0.1% Tween 20 (Sigma-Aldrich) solution, and incubated with 1:200 mouse anti-human CD31 antibody (Dako, Carpinteria, CA, USA) followed by 1:500 Alexa Fluor 555-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA). Vessels were analyzed using AngioTool [10]. 5 random locations were analyzed in each of 9 tissues (n = 45) per experimental condition. Statistical comparisons were performed using one-way analysis of variance (ANOVA).
Fick’s second law was used to describe the mass balance of oxygen within the media and tissue domains (Fig. 1B), assuming net diffusion in the z-direction only:
where Cmedia(z,t) and C(z,t) (mol O2/m3, or % O2) are the concentrations of oxygen in the media and tissue domains, respectively, and Dmedia and Dtissue (m2/s) are the molecular diffusion coefficients of oxygen in the media and tissue domains. The cellular oxygen consumption rate (mol O2·m−3·s−1) in the tissue domain was assumed to follow Michaelis-Menten kinetics where ρcell (cells/m3) is the volumetric cell density, Vmax (mol·cell−1·s−1) is the maximum rate of oxygen consumption, and Km (mol/m3) is the concentration at which the oxygen consumption rate is half that of Vmax. Values used for the parameters Dmedia, Dtissue, Vmax and Km were 3.0χ10−9 m2/s, 1.7χ10−9 m2/s, 3.6χ10−5 mol/m3/s and 8.0χ10−3 mol/m3, respectively [11].
No flux boundary conditions were used except for the z = 0 boundary, which was exposed to the externally applied time-varying oxygen profile CO2,ex. 20% O2 was used for initial conditions. The model was solved using the COMSOL Multiphysics time-dependent solver for non-steady state analysis within the built-in module for diffusion-reaction equations. An interpolation function was defined for each time varying boundary condition.
Our fabricated oxygen control system has a 90% response time to a 20% to 1% O2 step change of < 30 seconds, which contrasts sharply with commercially available incubators (e.g. Eppendorf Galaxy 48R) that approach 30 minutes (data not shown). Thus, our system may facilitate rapid and precise fluctuations in oxygen, which enables comprehensive evaluation of IH with temporal dynamics (Fig. 1C) at the scale of minutes.
Most cells in vivo experience normoxia levels of ~5% O2, and hypoxia as levels approach ~2% O2 [12], but are adapted to in vitro culture under hyperoxic conditions (~20% O2). Thus, our strategy for IH was to oscillate between 20% and 1% O2, representing a high and low (CO2,high and CO2,low, respectively) value, yet maintain a mean oxygen level (CO2,mean) of 5% O2. This strategy would ensure a periodic decrease below 2% O2, but maintain a CO2,mean consistent with normoxia. We explored a range of IH frequencies consistent with those observed in vivo (Table 1). Here, we describe “frequency” as the number of cycles per hour, where a cycle is defined as the sum of tO2,high and tO2,low (the time at CO2,high and CO2,low, respectively), or the solid blue line segment in Figure 1C.
Table 1.
Experimental oxygenation conditions of varying frequencies.
| Condition | Frequency (hr−1) |
Total number of cycles |
CO2,high
(%) |
tO2,high
(min) |
CO2,low
(%) |
tO2,low
(min) |
|---|---|---|---|---|---|---|
| IH 1 | 1.8 | 300 | 20 | 7 | 1 | 27 |
| IH 2 | 0.6 | 100 | 20 | 21 | 1 | 80 |
| IH 3 | 0.12 | 20 | 20 | 106 | 1 | 398 |
| IH 4 | 0.06 | 10 | 20 | 212 | 1 | 796 |
| IH 5 | 0.03 | 5 | 20 | 424 | 1 | 1591 |
| IH 6 | 0.012 | 2 | 20 | 1061 | 1 | 3978 |
| 5% | - | 0 | 5 | constant | - | - |
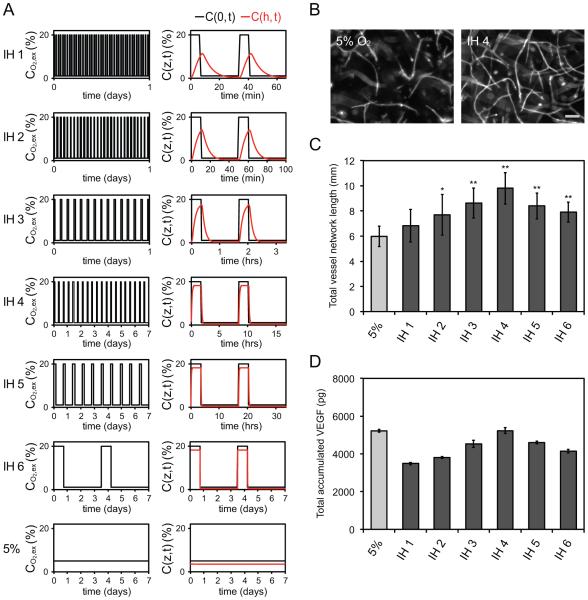
To our knowledge, there are currently no published mathematical models that characterize the response of 3D cellularized tissues subjected to dynamic oxygen conditions. We therefore created a non-steady state diffusion-reaction model with time-varying boundary conditions, and used finite element simulations to predict the resulting oxygen distribution (Fig. 2A). The notable difference between the oxygen profile imposed at the z = 0 boundary (C(0,t); black line in Fig. 2A), and the oxygen profile at the base of the tissue (C(h,t); red line in Fig. 2A) demonstrates the attenuation in transport due to diffusion and cellular consumption. Furthermore, it confirms that for each IH condition, oxygen levels decreased enough during the tO2,low intervals to be considered hypoxic. Finally, we used our model to predict CO2,mean and found that all IH conditions were within 14% of the 5% O2 control condition (data not shown).
Figure 2.
A) Experimental oxygenation conditions imposed at the z = 0 boundary are shown as a function of time (black line). Finite element simulations were used to predict the oxygen profile at the base of the tissue (red line). The time lag and dispersion effects are induced by limitations in oxygen diffusion and reaction. B) Fluorescent images of CD31 labeled endothelial cells show that IH may promote greater vessel network development than the constant 5% O2 control. Scale bar represents 100 μm. C) Quantification of total vessel network length demonstrates frequency dependence of IH conditioned tissues. *p < 0.05 and **p < 0.001 compared to 5% O2 control. D) Total accumulated VEGF in the conditioned tissue samples reveal parallel frequency dependence.
We next used our system to examine the effects of IH on the potential of endothelial cells to form vessel networks in vitro. Interestingly, we found that most IH conditions demonstrated greater vessel development compared to the constant 5% O2 control, despite having similar CO2,mean. Thus, endothelial cells are sensitive to the temporal frequency at which oxygen is delivered. Specifically, we observed a bell-shaped response, with IH 4 (frequency equal to 0.06 hr−1) demonstrating the most pronounced vascular enhancement (Fig. 2B,C).
Blood vessel formation is mediated by hypoxia inducible factor-1 (HIF-1) regulated expression of pro-angiogenic proteins such as vascular endothelial growth factor (VEGF). To investigate if the observed vascular response was related to VEGF production, we measured the total (accumulated) concentration of VEGF over the 7-day assay. We similarly found that VEGF production is sensitive to the temporal frequency at which oxygen is delivered (Fig. 2D), and further observed an analogous bell-shaped response with IH 4 producing the highest amounts of VEGF. Well accepted as the most potent angiogenesis promoting cytokine, it is clear that the frequency-dependent VEGF production is strongly correlated to the frequency-dependent vessel development. However, considering that no IH condition surpassed the VEGF level produced by the 5% O2 control, it is evident that other frequency-induced angiogenic factors are at play.
Our data reflects the time-dependent cellular response to hypoxia, in which the balance between pro- and anti-angiogenic mediators is modulated by the severity and duration of hypoxia. Short-term exposure (on the order of minutes to hours) may instigate the post-translational modification of proteins or the activation of pre-existing proteins, most notably the HIF-1 transcription factor [13], leading to VEGF production. Hence, it is likely that the hypoxic exposure (tO2,low) in the higher frequency IH profiles in our study (i.e. approximating IH 1) are sufficient to instigate a corresponding transcriptional response. In contrast, prolonged hypoxic exposure has been shown to lead to endothelial cell death [14]; thus, as frequency decreases (i.e. approximating IH 6), the cells may respond in a fashion more similar to chronic hypoxia, which is characterized by cellular stress and damage. Future studies should focus on understanding the expression of angiogenic proteins other than VEGF in response to IH to more fully understand the impact of IH on angiogenesis.
In conclusion, we presented an experimental platform, validated with a computational model, capable of efficiently conferring temporal oxygen control of 3D tissues. We used this system to demonstrate that IH stimulates vessel network formation and VEGF production in a highly correlated fashion that is frequency-dependent. This work may inform how the vascular tumor microenvironment responds to or modulates IH, and thus provides insight into developing strategies for therapeutic intervention.
Acknowledgments
This work was supported by the National Institute of Health (grants R01 CA170879 and UH2 TR000481).
References
- 1.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature Reviews Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 2.Baumgardner JE, Otto CM. In vitro intermittent hypoxia: challenges for creating hypoxia in cell culture. Respiratory Physiology and Neurobiology. 2003;136:131–139. doi: 10.1016/s1569-9048(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 3.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nature Reviews Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto S, Yasui H, Mitchell JB, Krishna MC. Imaging Cycling Tumor Hypoxia. Cancer Research. 2010;70:10019–10023. doi: 10.1158/0008-5472.CAN-10-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartel FV, Holl M, Arshad M, Aslam M, Gunduz D, Weyand M, Micoogullari M, Abdallah Y, Piper HM, Noll T. Transient hypoxia induces ERK-dependent anti-apoptotic cell survival in endothelial cells. American Journal of Physiology-Cell Physiology. 2010;298:C1501–C1509. doi: 10.1152/ajpcell.00333.2009. [DOI] [PubMed] [Google Scholar]
- 6.Feron O, Martinive P, Defresne F, Quaghebeur E, Daneau G, Crokart N, Gregoire V, Gallez B, Dessy C. Impact of cyclic hypoxia on HIF-1 alpha regulation in endothelial cells - new insights for anti-tumor treatments. FEBS Journal. 2009;276:509–518. doi: 10.1111/j.1742-4658.2008.06798.x. [DOI] [PubMed] [Google Scholar]
- 7.Martinive P, Defresne F, Bouzin C, Saliez J, Lair F, Gregoire V, Michiels C, Dessy C, Feron O. Preconditioning of the Tumor Vasculature and Tumor Cells by Intermittent Hypoxia: Implications for Anticancer Therapies. Cancer Research. 2006;66:11736–11744. doi: 10.1158/0008-5472.CAN-06-2056. [DOI] [PubMed] [Google Scholar]
- 8.Chen XF, Aledia AS, Popson SA, Him L, Hughes CCW, George SC. Rapid Anastomosis of Endothelial Progenitor Cell-Derived Vessels with Host Vasculature Is Promoted by a High Density of Cotransplanted Fibroblasts. Tissue Engineering Part A. 2010;16:585–594. doi: 10.1089/ten.tea.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CC, George SC. A three-dimensional in vitro model of tumor cell intravasation. Integr Biol (Camb) 2014;6:603–10. doi: 10.1039/c3ib40170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zudaire E, Gambardella L, Kurcz C, Vermeren S. A Computational Tool for Quantitative Analysis of Vascular Networks. Plos One. 2011;6 doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehsan SM, George SC. Nonsteady State Oxygen Transport in Engineered Tissue: Implications for Design. Tissue Engineering Part A. 2013;19:1433–1442. doi: 10.1089/ten.tea.2012.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL. Perspectives on oxygen sensing. Cell. 1999;98:281–284. doi: 10.1016/s0092-8674(00)81957-1. [DOI] [PubMed] [Google Scholar]
- 14.Stempien-Otero A, Karsan A, Cornejo CJ, Xiang H, Eunson T, Morrison RS, Kay M, Winn R, Harlan J. Mechanisms of hypoxia-induced endothelial cell death - Role of p53 in apoptosis. Journal of Biological Chemistry. 1999;274:8039–8045. doi: 10.1074/jbc.274.12.8039. [DOI] [PubMed] [Google Scholar]