Abstract
Background:
There is concern about increasing utilization of low-value health care services, including preoperative testing for low-risk surgical procedures. We investigated temporal trends, explanatory factors, and institutional and regional variation in the utilization of testing before low-risk procedures.
Methods:
For this retrospective cohort study, we accessed linked population-based administrative databases from Ontario, Canada. A cohort of 1 546 223 patients 18 years or older underwent a total of 2 224 070 low-risk procedures, including endoscopy and ophthalmologic surgery, from Apr. 1, 2008, to Mar. 31, 2013, at 137 institutions in 14 health regions. We used hierarchical logistic regression models to assess patient- and institution-level factors associated with electrocardiography (ECG), transthoracic echocardiography, cardiac stress test or chest radiography within 60 days before the procedure.
Results:
Endoscopy, ophthalmologic surgery and other low-risk procedures accounted for 40.1%, 34.2% and 25.7% of procedures, respectively. ECG and chest radiography were conducted before 31.0% (95% confidence interval [CI] 30.9%–31.1%) and 10.8% (95% CI 10.8%–10.8%) of procedures, respectively, whereas the rates of preoperative echocardiography and stress testing were 2.9% (95% CI 2.9%–2.9%) and 2.1% (95% CI 2.1%–2.1%), respectively. Significant variation was present across institutions, with the frequency of preoperative ECG ranging from 3.4% to 88.8%. Receipt of preoperative ECG and radiography were associated with older age (among patients 66–75 years of age, for ECG, adjusted odds ratio [OR] 18.3, 95% CI 17.6–19.0; for radiography, adjusted OR 2.9, 95% CI 2.8–3.0), preoperative anesthesia consultation (for ECG, adjusted OR 8.7, 95% CI 8.5–8.8; for radiography, adjusted OR 2.2, 95% CI 2.1–2.2) and preoperative medical consultation (for ECG, adjusted OR 6.8, 95% CI 6.7–6.9; for radiography, adjusted OR 3.6, 95% CI 3.5–3.6). The median ORs for receipt of preoperative ECG and radiography were 2.3 and 1.6, respectively.
Interpretation:
Despite guideline recommendations to limit testing before low-risk surgical procedures, preoperative ECG and chest radiography were performed frequently. Significant variation across institutions remained after adjustment for patient- and institution-level factors.
In response to concerns about increasing utilization of low-value health care services, the American Board of Internal Medicine Foundation launched the Choosing Wisely campaign in the United States in 2012.1 The goal of the campaign is to encourage conversations between physicians and patients about low-value care by defining “top 5” lists of tests, treatments and procedures that may be unnecessary or unsupported by evidence.1 Subsequent Choosing Wisely campaigns have followed in other countries, including Canada starting in April 2014.2,3 Of interest for health policy-makers, payers and clinicians are current utilization rates for the procedures mentioned in these recommendations. Establishing baseline rates permits an understanding of the extent of the problem of low-value care, which in turn allows monitoring of the effect of initiatives such as Choosing Wisely on utilization rates over time.
One Choosing Wisely item included by many specialty societies is the recommendation to avoid routinely performing preoperative testing (including chest radiography, echocardiography and cardiac stress tests) for patients undergoing low-risk surgery.4–6 This recommendation was previously included in the 2007 American College of Cardiology/American Heart Association guidelines on perioperative cardiovascular evaluation for noncardiac surgery7 and was reconfirmed in a recent update.8 Avoiding preoperative investigations in this setting is supported by evidence that routine testing in patients undergoing low-risk surgery does not improve outcomes or change management and may lead to further unnecessary downstream testing, cancellation of surgery, and increases in patient anxiety and cost.7,9–12 To date, neither the rate of preoperative testing across a large and diverse jurisdiction nor the degree of variation at regional and institutional levels, where data may be “actionable,” is well understood.
The objectives of this study were to determine utilization rates of preoperative tests before hospital-based low-risk surgical procedures at the jurisdictional, regional and institutional level. In addition, we aimed to evaluate temporal trends of preoperative testing over a 5-year period. We hypothesized that there would be significant regional and institutional variation in preoperative cardiac testing before low-risk surgery and that patients with prior cardiac comorbidities would have a higher rate of preoperative testing than those without such comorbidities.
Methods
Study design and data sources
We conducted a retrospective cohort study in Ontario, Canada, using population-based administrative health care databases. The datasets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences. Using the Canadian Institute for Health Information’s Discharge Abstract Database and Same-Day Surgery database, we identified all Ontario adults (≥ 18 yr) with an elective hospital admission between Apr. 1, 2008, and Mar. 31, 2013, who underwent one of the following surgical procedures on the date of admission: endoscopy, ophthalmologic surgery or other low-risk surgery (e.g., knee arthroscopy, hernia repair). Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150174/-/DC1, lists the procedures included, all of which have a low cardiac risk (i.e., estimated risk of myocardial infarction or cardiac death < 1%) and generally do not require preoperative cardiac testing.7,8 We excluded patients with incomplete data for the index procedure or demographic characteristics and those who underwent the procedure during an existing inpatient admission or secondary to another higher-risk surgical procedure within the same hospital stay (e.g., endoscopy before colon surgery). Because we conducted a per-procedure analysis, we included all procedures for patients who underwent more than one eligible procedure during the study period.
We collected patient demographic characteristics from the Registered Persons Database and used neighbourhood income quintile to estimate patients’ socioeconomic status. We obtained the surgical setting (inpatient or outpatient) and the institution number from the Discharge Abstract Database and the Same-Day Surgery database. Using validated data algorithms, we identified patients with chronic obstructive pulmonary disease, asthma and the following cardiac risk factors: hypertension, diabetes mellitus and hyperlipidemia.13–16 We used codes from the International Statistical Classification of Diseases and Related Health Problems, 10th revision, for hospital admissions within 2 years before the index procedure to identify the following comorbidities: coronary artery disease, heart failure, atrial fibrillation, other cardiac arrhythmia, cardiac valvular disease, chronic renal disease, previous cerebrovascular disease, peripheral arterial disease and venous thromboembolism. Comorbidities identified from the index surgery included cardiac valvular disease, chronic renal disease and venous thromboembolism. We used the Discharge Abstract Database and the Ontario Health Insurance Plan (OHIP) claims database to identify the following cardiac procedures performed within 10 years before the index procedure: aortic valve replacement, mitral valve replacement, coronary artery revascularization and device implantation. We used OHIP claims to identify preoperative outpatient anesthesia consultation within 60 days before the index procedure.17 Using a validated algorithm, we identified preoperative medical consultations (cardiology, endocrinology, general internal medicine, geriatric medicine and nephrology) within 60 days of the index procedure from OHIP claims and the Institute for Clinical Evaluative Sciences Physician Database.18
Outcomes
We used OHIP claims to identify patients who underwent electrocardiography (ECG), transthoracic echocardiography, cardiac stress testing and chest radiography before their procedures. These tests encompass the range of cardiothoracic investigations advised against in the Choosing Wisely Canada recommendations of the Canadian Cardiovascular Society, the Canadian Association of General Surgeons and the Canadian Society of Internal Medicine,4–6 with ECG included for completeness. Tests occurring within 60 days before the index date were considered preoperative.17 Although institutional policies vary, tests conducted between 30 and 60 days before surgical procedures are generally considered current, and their results are accepted for preoperative evaluation.
Analyses
We compared patient characteristics across procedure categories (endoscopy, ophthalmologic surgery and low-risk surgeries) using analysis of variance and the χ2 test as appropriate. We assessed rates of preoperative ECG, transthoracic echocardiography, stress testing and chest radiography for the overall study cohort and by procedure category.
Initially, we determined regional and institutional variation with unadjusted rates of preoperative testing for all procedures combined. Regional variation was assessed across Ontario’s 14 Local Health Integration Networks, which are geographically organized administrative regions. Institutions were included in the analysis if they had 250 or more procedures in at least one category and 500 or more procedures overall. Subgroup analyses were conducted by procedure category for institutions with at least 250 procedures for that category. We calculated descriptive statistics for each procedure category and all procedures combined.
We developed hierarchical random-intercept multivariable logistic regression models to separately assess the adjusted associations of patient- and institution-level factors with preoperative ECG and chest radiography. Adjusted analyses were not performed for preoperative echocardiography and stress tests, as the event rates were too low for stable regression modelling. Institution was included as a random effect to account for random differences in rates of preoperative testing across hospitals. Patient-level factors were procedure category, age, sex, rural or urban residence, neighbourhood income quintile, comorbidities, cardiac risk factors, prior cardiac procedures, preoperative anesthesia consultation and preoperative medical consultation. Institution-level factors were hospital teaching status and total surgical volume tertile (low and high v. intermediate).
We assessed institutional variation in rates of preoperative ECG and chest radiography using the median odds ratio (OR).19 The median OR compares the adjusted odds of preoperative testing for 2 patients with the same covariates at 2 randomly selected institutions and can be interpreted as the median value of these ORs.19 The median OR always has a value greater than or equal to 1.0 because it involves comparing randomly selected pairs of higher-ranked versus lower-ranked institutions.19 It characterizes heterogeneity across institutions, is adjusted for patient-level covariates and may be directly compared with ORs of fixed-effect patient-level factors.19 For example, a value of 1.50 for ECG suggests 50% adjusted higher odds of undergoing preoperative ECG if the same patient has surgery at one randomly selected institution as opposed to another.
To estimate adjusted institutional testing rates for ECG and chest radiography, we predicted the probabilities of testing for each patient using logistic regression models that adjusted for patient- and procedure-level factors (see Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150174/-/DC1 for the model ORs). These probabilities were summed to estimate the expected event count for each institution. We indirectly standardized these institutional rates by multiplying the observed to expected event count ratio for each institution by the overall mean event rate for the whole cohort.
Results
Study cohort
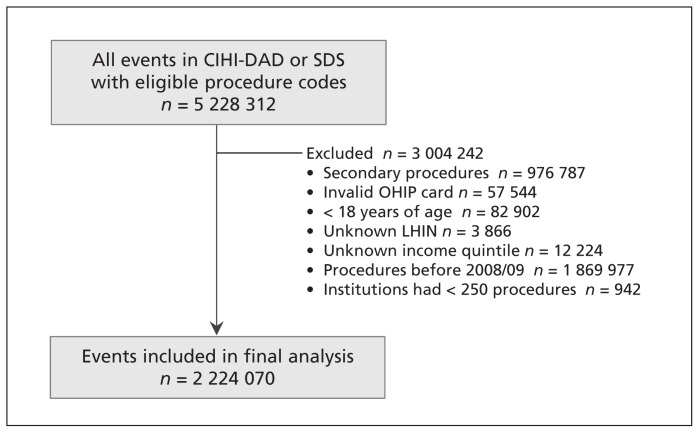
Assembly of the final cohort, which consisted of 1 546 223 patients who underwent 2 224 070 distinct procedures at 137 institutions between Apr. 1, 2008, and Mar. 31, 2013, is described in Figure 1. The annual volume of all procedures decreased from 474241 in 2008/09 to 404488 in 2012/13. Endoscopy accounted for 40.1% of procedures, whereas ophthalmologic and other low-risk surgery accounted for 34.2% and 25.7%, respectively.
Figure 1:
Study flow diagram. Data represent procedures included in the analysis (some patients underwent more than 1 procedure in the study period). CIHI = Canadian Institute for Health Information, DAD = Discharge Abstract Database, LHIN = Local Health Integration Network, OHIP = Ontario Health Insurance Plan, SDS = Same-Day Surgery database.
Patient demographic, clinical and surgical characteristics
Demographic, clinical and surgical characteristics of the cohort are presented in Table 1. The mean age was 61.6 years, with those undergoing ophthalmologic surgery being older than those undergoing endoscopy or low-risk surgery. The comorbidity burden was generally low, particularly for cardiac comorbidities. Most procedures (95.8%) were performed in an outpatient setting. The proportion of inpatient procedures was higher for low-risk surgeries than for endoscopy and ophthalmologic surgery (15.8% v. <0.1% v. 0.3%, p < 0.001).
Table 1:
Characteristics of the study cohort by procedure category
| Characteristic* | Procedure; no. (%) of patients† | |||
|---|---|---|---|---|
| Endoscopy n = 892 644 |
Ophthalmologic surgery n = 759 906 |
Low-risk surgery n = 571 520 |
Overall n = 2 224 070 |
|
| Proportion of procedures, % | 40.1 | 34.2 | 25.7 | 100.0 |
| Age, yr | ||||
| Mean ± SD | 58.0 ± 14.2 | 70.8 ± 11.4 | 54.9 ± 16.9 | 61.6 ± 15.6 |
| 18–25 | 19 792 (2.2) | 3 312 (0.4) | 30 616 (5.4) | 53 720 (2.4) |
| 26–35 | 42 279 (4.7) | 5 866 (0.8) | 48 371 (8.5) | 96 516 (4.3) |
| 36–45 | 94 468 (10.6) | 13 968 (1.8) | 88 178 (15.4) | 196 614 (8.8) |
| 46–55 | 219 006 (24.5) | 47 132 (6.2) | 126 071 (22.1) | 392 209 (17.6) |
| 56–65 | 216 192 (24.2) | 116 037 (15.3) | 103 851 (18.2) | 436 080 (19.6) |
| 66–75 | 185 624 (20.8) | 253 539 (33.4) | 94 371 (16.5) | 533 534 (24.0) |
| 76–85 | 98 143 (11.0) | 259 458 (34.1) | 63 169 (11.1) | 420 770 (18.9) |
| > 85 | 17 140 (1.9) | 60 594 (8.0) | 16 893 (3.0) | 94 627 (4.3) |
| Sex, female | 494 953 (55.4) | 432 103 (56.9) | 293 430 (51.3) | 1 220 486 (54.9) |
| Rural–urban status | ||||
| Urban | 542 194 (60.7) | 493 190 (64.9) | 365 993 (64.0) | 1 401 377 (63.0) |
| Suburban | 233 001 (26.1) | 184 372 (24.3) | 142 080 (24.9) | 559 453 (25.2) |
| Rural | 109 679 (12.3) | 76 751 (10.1) | 59 036 (10.3) | 245 466 (11.0) |
| Missing | 7 770 (0.9) | 5 593 (0.7) | 4 411 (0.8) | 17 774 (0.8) |
| Neighbourhood income quintile | ||||
| 1 (lowest) | 150 477 (16.9) | 148 162 (19.5) | 100 650 (17.6) | 399 289 (18.0) |
| 2 | 169 475 (19.0) | 158 352 (20.8) | 111 146 (19.4) | 438 973 (19.7) |
| 3 | 177 422 (19.9) | 152 269 (20.0) | 114 399 (20.0) | 444 090 (20.0) |
| 4 | 194 595 (21.8) | 152 948 (20.1) | 123 138 (21.5) | 470 681 (21.2) |
| 5 (highest) | 200 675 (22.5) | 148 175 (19.5) | 122 187 (21.4) | 471 037 (21.2) |
| Surgical site | ||||
| Inpatient procedure | 182 (<0.1) | 2 502 (0.3) | 90 188 (15.8) | 92 872 (4.2) |
| Outpatient procedure | 892 462 (>99.9) | 757 404 (99.7) | 481 332 (84.2) | 2 131 198 (95.8) |
| Comorbidities | ||||
| Coronary artery disease | 20 824 (2.3) | 30 949 (4.1) | 12 406 (2.2) | 64 179 (2.9) |
| Atrial fibrillation or flutter | 10 885 (1.2) | 18 773 (2.5) | 7 121 (1.2) | 36 779 (1.7) |
| Other cardiac arrhythmia | 4 227 (0.5) | 6 929 (0.9) | 2 716 (0.5) | 13 872 (0.6) |
| Cardiac valvular disease | 2 272 (0.3) | 4 174 (0.5) | 1 489 (0.3) | 7 935 (0.4) |
| Cerebrovascular disease | 3 465 (0.4) | 7 050 (0.9) | 2 387 (0.4) | 12 902 (0.6) |
| Peripheral arterial disease | 3 771 (0.4) | 6 106 (0.8) | 2 548 (0.4) | 12 425 (0.6) |
| Venous thromboembolism | 1 477 (0.2) | 1 385 (0.2) | 882 (0.2) | 3 744 (0.2) |
| Heart failure | 33 167 (3.7) | 68 129 (9.0) | 21 258 (3.7) | 122 554 (5.5) |
| Myocardial infarction | 6 388 (0.7) | 9 529 (1.3) | 3 539 (0.6) | 19 456 (0.9) |
| Chronic renal disease | 5 551 (0.6) | 10 410 (1.4) | 4 710 (0.8) | 20 671 (0.9) |
| Asthma | 133 034 (14.9) | 106 252 (14.0) | 88 580 (15.5) | 327 866 (14.7) |
| COPD | 127 959 (14.3) | 161 236 (21.2) | 77 121 (13.5) | 366 316 (16.5) |
| Cardiac risk factors | ||||
| Diabetes mellitus | 160 083 (17.9) | 239 743 (31.5) | 93 573 (16.4) | 493 399 (22.2) |
| Hypertension | 398 390 (44.6) | 516 992 (68.0) | 229 330 (40.1) | 1 144 712 (51.5) |
| Hyperlipidemia | 38 011 (4.3) | 57 009 (7.5) | 22 362 (3.9) | 117 382 (5.3) |
| Prior cardiac procedures | ||||
| Aortic valve replacement | 2 750 (0.3) | 4 995 (0.7) | 1 777 (0.3) | 9 522 (0.4) |
| Mitral valve replacement | 805 (0.1) | 1 490 (0.2) | 507 (0.1) | 2 802 (0.1) |
| Coronary revascularization | 32 346 (3.6) | 49 468 (6.5) | 19 020 (3.3) | 100 834 (4.5) |
| Device implantation | 7 015 (0.8) | 14 760 (1.9) | 4 777 (0.8) | 26 552 (1.2) |
| Preoperative consultations | ||||
| Outpatient anesthesia | 6 074 (0.7) | 45 455 (6.0) | 107 538 (18.8) | 159 067 (7.2) |
| Medical consult | 50 968 (5.7) | 40 124 (5.3) | 29 623 (5.2) | 120 715 (5.4) |
Note: COPD = chronic obstructive pulmonary disease, SD = standard deviation.
For all characteristics (except prior cardiac procedures), p < 0.001 across procedure categories.
Except where indicated otherwise.
The rates of outpatient preoperative anesthesia and medical consultation were 7.2% and 5.4%, respectively, with the highest rate of preoperative anesthesia occurring before low-risk surgeries and the highest rate of medical consultation before endoscopy.
Temporal trends
Table 2 and Appendix 3 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150174/-/DC1) describe annual and overall rates of preoperative testing from 2008/09 to 2012/13 for the whole cohort and by procedure category. The rate of ECG testing before all procedures was 31.0%, with the highest frequency among patients undergoing low-risk surgery, followed by ophthalmologic and endoscopic procedures (54.6% v. 32.0% v. 15.1%, p < 0.001). Chest radiography was performed before 10.8% of procedures, with the highest frequency among patients undergoing low-risk surgery, followed by endoscopic and ophthalmologic procedures (19.0% v. 9.0% v. 6.7%, p < 0.001). Provincial rates of preoperative transthoracic echocardiography and stress testing were 2.9% and 2.1%, respectively.
Table 2:
Temporal trends in rates of various types of preoperative testing from 2008/09 to 2012/13
| Type of testing and year | Procedure; % of cases with preoperative test (95% CI) | |||
|---|---|---|---|---|
| Endoscopy | Ophthalmologic surgery | Low-risk surgery | Overall | |
| Electrocardiography | ||||
| 2008/09 | 15.7 (15.6–15.9) | 42.4 (42.1–42.6) | 54.1 (53.8–54.3) | 34.5 (34.3–34.6) |
| 2009/10 | 16.0 (15.8–16.1) | 39.3 (39.1–39.6) | 54.9 (54.6–55.1) | 33.9 (33.8–34.1) |
| 2010/11 | 15.2 (15.0–15.4) | 30.5 (30.3–30.7) | 55.5 (55.2–55.8) | 30.7 (30.6–30.8) |
| 2011/12 | 14.6 (14.5–14.8) | 23.8 (23.6–24.0) | 55.7 (55.4–56.0) | 28.4 (28.2–28.5) |
| 2012/13 | 13.5 (13.3–13.7) | 21.9 (21.6–22.1) | 52.8 (52.5–53.1) | 26.9 (26.7–27.0) |
| All years | 15.1 (15.0–15.2) | 32.0 (31.9–32.1) | 54.6 (54.5–54.7) | 31.0 (30.9–31.1) |
| Echocardiography* | ||||
| 2008/09 | 2.4 (2.4–2.5) | 3.1 (3.0–3.2) | 2.5 (2.5–2.6) | 2.7 (2.7–2.7) |
| 2009/10 | 2.7 (2.6–2.8) | 3.4 (3.4–3.5) | 2.6 (2.5–2.7) | 2.9 (2.9–3.0) |
| 2010/11 | 2.7 (2.6–2.8) | 3.2 (3.1–3.3) | 2.9 (2.8–3.0) | 2.9 (2.9–3.0) |
| 2011/12 | 2.8 (2.7–2.9) | 3.3 (3.2–3.4) | 2.9 (2.8–3.0) | 3.0 (2.9–3.0) |
| 2012/13 | 2.8 (2.7–2.9) | 3.1 (3.0–3.2) | 2.8 (2.7–2.9) | 2.9 (2.8–3.0) |
| All years | 2.7 (2.7–2.7) | 3.2 (3.2–3.2) | 2.7 (2.7–2.7) | 2.9 (2.9–2.9) |
| Stress test | ||||
| 2008/09 | 2.1 (2.0–2.1) | 1.8 (1.7–1.9) | 1.9 (1.9–2.0) | 1.9 (1.9–2.0) |
| 2009/10 | 2.2 (2.2–2.3) | 1.8 (1.7–1.9) | 2.3 (2.2–2.4) | 2.1 (2.1–2.1) |
| 2010/11 | 2.3 (2.2–2.4) | 1.8 (1.7–1.8) | 2.6 (2.5–2.7) | 2.2 (2.2–2.2) |
| 2011/12 | 2.3 (2.2–2.3) | 1.8 (1.7–1.9) | 2.7 (2.6–2.8) | 2.2 (2.2–2.3) |
| 2012/13 | 2.2 (2.1–2.3) | 1.8 (1.7–1.8) | 2.8 (2.7–2.9) | 2.2 (2.2–2.3) |
| All years | 2.2 (2.2–2.2) | 1.8 (1.8–1.8) | 2.5 (2.5–2.5) | 2.1 (2.1–2.1) |
| Chest radiography | ||||
| 2008/09 | 8.9 (8.8–9.1) | 7.4 (7.2–7.5) | 19.9 (19.7–20.2) | 11.1 (11.0–11.2) |
| 2009/10 | 9.2 (9.1–9.4) | 7.2 (7.1–7.3) | 20.0 (19.8–20.2) | 11.3 (11.2–11.4) |
| 2010/11 | 9.0 (8.9–9.2) | 6.6 (6.5–6.7) | 19.4 (19.2–19.6) | 10.8 (10.7–10.9) |
| 2011/12 | 8.8 (8.7–9.0) | 6.0 (5.9–6.1) | 18.6 (18.4–18.8) | 10.4 (10.3–10.5) |
| 2012/13 | 8.8 (8.6–8.9) | 5.9 (5.8–6.1) | 17.1 (16.9–17.3) | 10.1 (10.0–10.2) |
| All years | 9.0 (8.9–9.1) | 6.7 (6.6–6.8) | 19.0 (18.9–19.1) | 10.8 (10.8–10.8) |
Note: CI = confidence interval.
Transthoracic echocardiography.
Regional and institutional variation in preoperative testing
Across Local Health Integration Networks, the proportion of patients who underwent preoperative ECG ranged from 21.0% (95% CI 20.7%–21.2%) to 38.7% (95% CI 38.5%–38.9%). For preoperative chest radiography, the range was 7.5% (95% CI 7.4%–7.7%) to 15.1% (95% CI 14.8%–15.4%). Ordering of preoperative transthoracic echocardiography ranged from 1.6% (95% CI 1.6%–1.7%) to 4.2% (95% CI 4.1%–4.3%) and of preoperative stress tests, from 1.4% (95% CI 1.3–1.5) to 2.9% (95% CI 2.8%–3.0%).
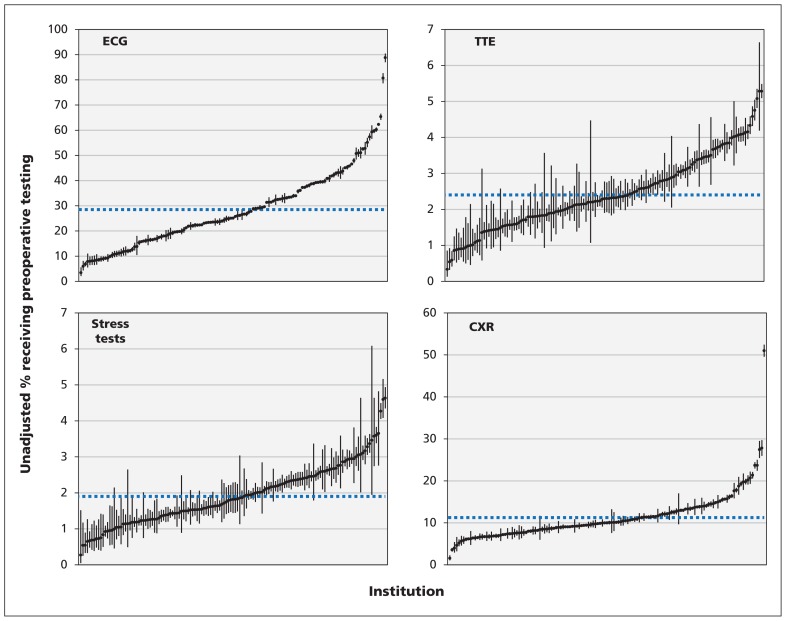
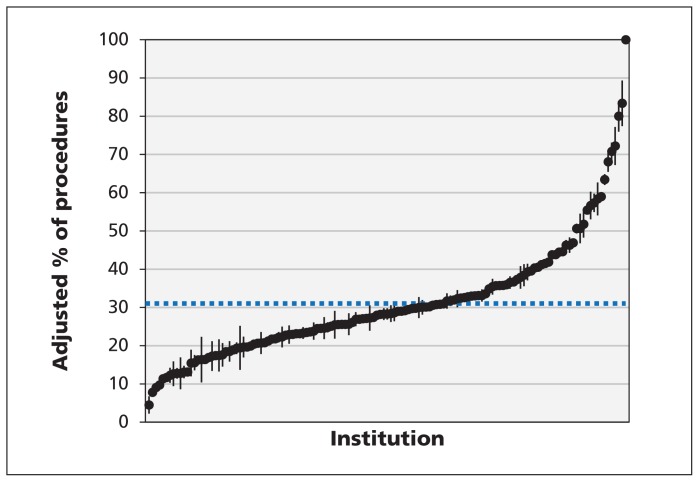
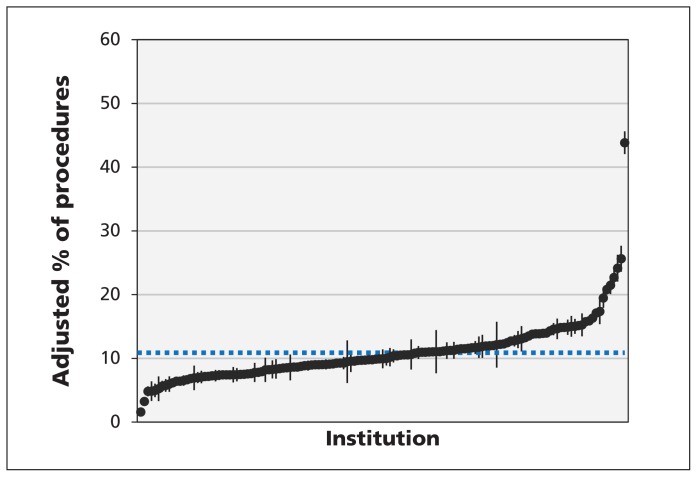
The unadjusted rates of preoperative ECG and chest radiography for all procedures ranged from 3.4% to 88.8% and from 1.6% to 51.0%, respectively (Figure 2). The unadjusted rates of preoperative transthoracic echocardiography and stress tests were 0.3% to 5.3% and 0.3% to 4.6%, respectively (Figure 2). Figures 3 and 4 show the indirect standardized rates of preoperative ECG and chest radiography, respectively, across the 137 institutions over the study period.
Figure 2:
Institutional variation in preoperative testing for the 4 preoperative procedures. Each point represents the unadjusted rate of testing for a single institution, with vertical lines representing 95% confidence intervals. The dashed horizontal lines denote mean rates of testing across all institutions. CXR = chest radiography, ECG = electrocardiography, TTE = transthoracic echocardiography.
Figure 3:
Indirect standardized rates of preoperative electrocardiography (ECG). Each point represents the indirect standardized rate of preoperative ECG for a single institution, and the associated vertical line represents the institution’s 95% confidence interval for the testing rate. The dashed horizontal line denotes the mean rate of testing across all institutions.
Figure 4:
Indirect standardized rates of preoperative chest radiography. Each point represents the indirect standardized rate of preoperative chest radiography for a single institution, and the associated vertical line represents the 95% confidence interval for the testing rate. The dashed horizontal line denotes the mean rate of testing across all institutions.
Adjusted analyses
Table 3 shows the adjusted associations of patient- and institution-level factors with receipt of preoperative ECG and chest radiography. Preoperative testing was associated with older age, and the adjusted odds of preoperative ECG among patients aged 66–75 years was 18.3 (95% CI 17.6–19.0) relative to patients aged 18–25 years. Several cardiac comorbidities were associated with preoperative ECG, but the effect sizes were small. There were strong associations between preoperative ECG and preoperative anesthesia consultation (adjusted OR 8.7, 95% CI 8.5–8.8) and preoperative medical consultation (adjusted OR 6.8, 95% CI 6.7–6.9). Preoperative chest radiography was also significantly associated with preoperative anesthesia consultation (adjusted OR 2.2, 95% CI 2.1–2.2) and preoperative medical consultation (adjusted OR 3.6, 95% CI 3.5–3.6).
Table 3:
Association of preoperative testing with characteristics at patient and institutional levels
| Characteristic | Preoperative test; adjusted OR* (95% CI) | |
|---|---|---|
| Electrocardiography | Chest radiography | |
| Age, yr | ||
| 18–25 (reference) | 1.0 | 1.0 |
| 26–35 | 1.8 (1.7–1.9) | 0.1 (0.1–0.1) |
| 36–45 | 5.0 (4.8–5.2) | 1.3 (1.3–1.4) |
| 46–55 | 13.3 (12.8–13.9) | 1.8 (1.8–1.9) |
| 56–65 | 17.2 (16.6–17.9) | 2.3 (2.2–2.4) |
| 66–75 | 18.3 (17.6–19.0) | 2.6 (2.5–2.7) |
| 76–85 | 17.7 (17.0–18.4) | 2.9 (2.8–3.0) |
| > 85 | 15.8 (15.1–16.4) | 3.1 (3.0–3.3) |
| Sex, female | 0.9 (0.9–1.0) | 3.3 (3.2–3.5) |
| Rural–urban status | ||
| Urban (reference) | 1.0 | 1.0 |
| Suburban | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| Rural | 1.0 (1.0–1.0) | 1.0 (1.0–1.1) |
| Missing | 1.0 (0.9–1.0) | 1.1 (1.0–1.2) |
| Neighbourhood income quintile | ||
| 1 (lowest) (reference) | 1.0 | 1.0 |
| 2 | 1.0 (1.0–1.0) | 0.9 (0.9–1.0) |
| 3 | 1.0 (1.0–1.0) | 0.9 (0.9–0.9) |
| 4 | 1.0 (0.9–1.0) | 0.9 (0.9–0.9) |
| 5 (highest) | 0.9 (0.9–0.9) | 0.8 (0.8–0.9) |
| Procedure type | ||
| Low-risk surgery (reference) | 1.0 | 1.0 |
| Endoscopy | 0.1 (0.1–0.1) | 0.4 (0.4–0.4) |
| Ophthalmologic surgery | 0.2 (0.2–0.2) | 0.2 (0.2–0.2) |
| Comorbidities | ||
| Coronary artery disease | 1.2 (1.2–1.3) | 1.4 (1.4–1.4) |
| Atrial fibrillation or flutter | 1.3 (1.3–1.4) | 1.4 (1.3–1.4) |
| Other cardiac arrhythmia | 1.3 (1.2–1.3) | 1.2 (1.2–1.3) |
| Cardiac valvular disease | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) |
| Cerebrovascular disease | 1.1 (1.1–1.1) | 1.2 (1.2–1.3) |
| Peripheral arterial disease | 1.1 (1.0–1.1) | 1.3 (1.2–1.4) |
| Venous thromboembolism | 1.0 (1.0–1.1) | 1.6 (1.5–1.8) |
| Heart failure | 1.2 (1.2–1.2) | 1.3 (1.3–1.3) |
| Myocardial infarction | 1.1 (1.0–1.1) | 1.0 (1.0–1.1) |
| Chronic renal disease | 1.1 (1.1–1.2) | 1.7 (1.6–1.7) |
| Asthma | 1.0 (1.0–1.0) | 1.2 (1.2–1.2) |
| COPD | 1.0 (1.0–1.0) | 1.5 (1.5–1.5) |
| Cardiac risk factors | ||
| Diabetes mellitus | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| Hypertension | 1.2 (1.2–1.2) | 1.0 (1.0–1.1) |
| Hyperlipidemia | 1.1 (1.0–1.1) | 1.1 (1.1–1.1) |
| Prior cardiac procedures | ||
| Aortic valve replacement | 0.9 (0.9–1.0) | 0.9 (0.8–0.9) |
| Mitral valve replacement | 1.1 (1.0–1.2) | 0.9 (0.8–1.0) |
| Coronary revascularization | 1.1 (1.1–1.1) | 0.9 (0.9–0.9) |
| Device implantation | 1.2 (1.1–1.2) | 1.1 (1.1–1.2) |
| Preoperative consultations | ||
| Outpatient anesthesia consult | 8.7 (8.5–8.8) | 2.2 (2.1–2.2) |
| Medical consult | 6.8 (6.7–6.9) | 3.6 (3.5–3.6) |
| Teaching hospital | 1.1 (0.9–1.2) | 1.0 (1.0–1.0) |
| Procedure volume | ||
| Low (reference) | 1.0 | 1.0 |
| Intermediate | 1.2 (1.0–1.4) | 1.0 (1.0–1.1) |
| High | 1.4 (1.1–1.7) | 1.1 (1.0–1.1) |
| Median OR | 2.3 | 1.6 |
Note: CI = confidence interval, COPD = chronic obstructive pulmonary disease, OR = odds ratio.
ORs from logistic regression with mutual adjustment for all other variables listed in the table.
For preoperative ECG, the median OR was 2.3, which means that the odds of a patient receiving preoperative ECG at one randomly selected institution were 2.3 times those of receiving this form of testing at another randomly selected institution. The median OR for preoperative chest radiography was 1.6.
Interpretation
Cardiac investigations (including ECG, stress tests and transthoracic echocardiography) and chest radiography are not routinely indicated before low-risk surgical procedures, according to published guidelines and multiple Choosing Wisely specialty lists.4–8 In this large retrospective cohort study, we found — despite existing recommendations — that testing before low-risk procedures was common. Although some tests occurred infrequently, ECG was performed before one-third of procedures. In addition, significant regional and institution-level variation was present, with a 30-fold difference between institutions with the lowest and highest rates of ordering tests. Institutional variation persisted despite modelling for patient-, procedure- and hospital-related factors, including institution type and surgical volume. After correction for such confounders, the median ORs related to procedure location (inpatient v. outpatient) exceeded the ORs for many clinically sensible patient characteristics. Our results suggest that the major drivers of preoperative testing are older age, procedure type, concurrent preoperative consultation and the institution at which the procedure was conducted. Interestingly, patient comorbidities, particularly cardiac comorbidities, were not major drivers of preoperative testing.
These findings support results from earlier investigations showing high rates of preoperative testing. Thanh and colleagues20 found that rates of testing before elective noncardiac surgery were 13.4% for ECG and 23.2% for chest radiography. Similarly, in a study of the Medicare population in the United States, Sheffield and associates21 found that the rate of stress testing before low- and intermediate-risk surgery was 6.4%. More recently, in an examination of the prevalence of practices targeted by the Choosing Wisely recommendations, also in the Medicare population, Schwartz and coworkers22 found that the rate of preoperative stress testing before low- and intermediate-risk surgery was between 0.3% and 0.7%, that of transthoracic echocardiography between 0.3% and 0.8% and that of preoperative chest radiography between 1.6% and 5.5%. These authors also observed significant regional variation.22
Our results add a number of important contributions to the literature. First, unlike prior investigations, our study was restricted to low-risk, predominantly outpatient procedures. The decision to include only low-risk procedures was in line with current Choosing Wisely recommendations on preoperative testing and was intended to increase the specificity of the measure to detect low-value care. Second, prior studies of the US Medicare population included only patients over age 65, whereas our study examined all adults, thereby providing a more complete picture of test-ordering behaviour for this population.22,23 Finally, whereas prior studies have shown regional variation in rates of preoperative testing, we assessed both regional and institution-specific rates and found substantial institution-level variation that persisted after adjustment for patient comorbidities. This latter point is critical, as interventions to modify test ordering are “actionable” predominantly at the institution and provider levels. Our finding emphasizes the need for re-evaluation of ordering decisions and clinical pathways for patients preparing for low-risk procedures. In particular, preoperative anesthesia and medical consultations have been shown to increase preoperative testing rates.18 In a previous study, Wijeysundera and colleagues24 showed substantial variation in preoperative medical consultation rates across institutions, albeit for major elective noncardiac surgery. This variation in preoperative consultation practices may underlie some of the variation we observed in institutional preoperative testing rates.
Although the magnitude was small, reductions in the rates of ECG and chest radiography over time suggest increased penetrance of guideline recommendations and a response to other policy changes. In particular, the government of Ontario adjusted fee codes in 2010 to eliminate reimbursement for routine preoperative chest radiography and ECG before cataract surgery.25 This change likely has resulted in reductions in the rates of ECG and chest radiography ordered before ophthalmologic surgery.
The results of our study have substantial importance for public policy. Choosing Wisely, a grass-roots, physician-led movement, aims to reduce unnecessary low-value practices by changing the attitudes of both physicians and patients. This approach may avoid the need for top–down mechanisms, such as delisting of services, which can restrict the shared decision-making between patients and physicians that allows for individualized care. Although administrative data do not provide the clinical granularity needed to determine the reason for each test ordered, it is clear that provincial-level consistency of preoperative ordering practices could be substantially improved. Although the optimal rate of preoperative testing cannot be defined from these administrative data, institutional rates of 88.8% for ECG and 51.0% for chest radiography would seem difficult to justify. The dramatic institution-level variation that we observed offers an opportunity for providers, administrators and policy-makers to not only explore underlying reasons for ordering tests but also improve ordering behaviour, particularly in those institutions that are significant outliers, where the impact of change may be greatest. Feeding institution-level data back to administrators and providers as a quality improvement measure can help raise provider awareness of the frequency of low-value care decisions and enable high-ordering institutions to examine and improve on local processes.
Limitations
This study had several limitations. First, administrative data do not encompass the clinical information, such as presence of symptoms or abnormal findings of a physical examination, that would be needed to determine the appropriateness of preoperative tests. Not all preoperative testing is of low value, and there are patients for whom these tests provide valuable clinical data. However, our selection of low-risk procedures and the low-risk clinical characteristics of the cohort make it unlikely that the majority of tests were ordered to evaluate new clinical symptoms or abnormal physical findings.
Second, there is no comprehensive, validated list of “low-risk” surgical procedures to guide such an investigation. We investigated very low risk procedures, including endoscopy, ophthalmologic surgery and the minimally invasive procedures listed in Appendix 1. The choice of procedures included is in line with the broad definition of “low-risk procedures” outlined in existing guidelines on perioperative cardiac evaluation.7,8 Procedure groups were analyzed separately because of the heterogeneity of procedures. We made an effort to exclude more invasive procedures, and the high rate of outpatient surgery (95.8%) indicates our success in this regard.
Third, we included in our analysis all tests conducted within 60 days before the index procedure, and it is possible that these investigations were ordered for indications other than preoperative testing. Although testing during this 60-day period may have been due to unrelated indications, many institutions within the jurisdiction accept preprocedure testing within this period to meet local institutional guidelines for preoperative investigations. As such, the results of these tests would often be considered by perioperative physicians and would influence whether additional investigations were conducted.
Finally, we do not know what effect the results of testing had on surgical decisions or patient outcomes.
Despite these limitations, this study adds substantially to the literature on health care overuse and suggests a need for improved alignment with guidelines in the use of preoperative testing before low-risk surgical procedures.
Conclusion
Rates of preoperative testing before low-risk procedures were higher than expected, given current guidelines and recommendations, with a significant degree of regional and institution-level variation across hospitals in a large, diverse jurisdiction with a single-payer health system. More study is needed to determine the underlying causes of this variation and to develop care pathways to reduce low-value preoperative testing.
Footnotes
CMAJ Podcasts: author interview at soundcloud.com/cmajpodcasts/150174-res
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Sacha Bhatia had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Sacha Bhatia, Kyle Kirkham and Duminda Wijeysundera were responsible for study concept and design. Sacha Bhatia and Ryan Ng were responsible for acquisition of data. Ryan Ng performed the statistical analysis. All authors analyzed and interpreted the data. Sacha Bhatia, Kyle Kirkham and Ciara Pendrith drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final version to be published. Sacha Bhatia obtained funding. Sacha Bhatia, Kyle Kirkham and Duminda Wijeysundera supervised the study. All authors agree to serve as guarantors for the work.
Funding: This study was supported by the Ontario Ministry of Health and Long-Term Care (MOHLTC) and by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the MOHLTC. The funders had no role in the design and conduct of the study; the collection, management, analysis or interpretation of the data; the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Duminda Wijeysundera is supported in part by a New Investigator Award from the Canadian Institutes of Health Research and a Merit Award from the Department of Anesthesia, University of Toronto. Jack Tu is supported by a Canada Research Chair in Health Services Research and a Career Investigator Award from the Heart and Stroke Foundation, Ontario Provincial Office.
References
- 1.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 2012;307: 1801–2. [DOI] [PubMed] [Google Scholar]
- 2.Levinson W, Kallewaard M, Bhatia RS, et al. ‘Choosing Wisely’: a growing international campaign. BMJ Qual Saf 2015; 24:167–74. [DOI] [PubMed] [Google Scholar]
- 3.Levinson W, Huynh T. Engaging physicians and patients in conversations about unnecessary tests and procedures: Choosing Wisely Canada. CMAJ 2014;186:325–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Cardiovascular Society. Cardiology: five things physicians and patients should question. Choosing Wisely Canada; 2014. Available: www.choosingwiselycanada.org/recommendations/cardiology/ (accessed 2015 May 14). [Google Scholar]
- 5.Canadian Association of General Surgeons. Six things physicians and patients should question. Choosing Wisely Canada; 2014. Available: www.choosingwiselycanada.org/recommendations/general-surgery/ (accessed 2015 May 14). [Google Scholar]
- 6.Canadian Society of Internal Medicine. Five things physicians and patients should question. Choosing Wisely Canada; 2014. Available: www.choosingwiselycanada.org/recommendations/internal-medicine/ (accessed 2015 May 13). [Google Scholar]
- 7.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery [published erratum in J Am Coll Cardiol 2008;52:793–4]. J Am Coll Cardiol 2007; 50:e159–241. [DOI] [PubMed] [Google Scholar]
- 8.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130: e278–333. [DOI] [PubMed] [Google Scholar]
- 9.Chung F, Yuan H, Yin L, et al. Elimination of preoperative testing in ambulatory surgery. Anesth Analg 2009;108:467–75. [DOI] [PubMed] [Google Scholar]
- 10.Fritsch G, Flamm M, Hepner DL, et al. Abnormal pre-operative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand 2012;56:339–50. [DOI] [PubMed] [Google Scholar]
- 11.Routine preoperative tests — are they necessary? Edmonton: Institute of Health Economics; 2007. Available: www.ihe.ca/documents/IHE_Report_Routine_Preoperative_Tests_May_2007.pdf (accessed 2015 Jan. 7). [Google Scholar]
- 12.Schein OD, Katz J, Bass EB, et al. ; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med 2000;342:168–75. [DOI] [PubMed] [Google Scholar]
- 13.Tu K, Campbell NR, Chen ZL, et al. Accuracy of administrative databases in identifying patients with hypertension. Open Med 2007;1:e18–26. [PMC free article] [PubMed] [Google Scholar]
- 14.Gershon AS, Wang C, Guan J, et al. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD 2009;6:388–94. [DOI] [PubMed] [Google Scholar]
- 15.Gershon AS, Wang C, Guan J, et al. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J 2009;16:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–6. [DOI] [PubMed] [Google Scholar]
- 17.Bugar JM, Ghali WA, Lemaire JB, et al. ; Canadian Perioperative Research Network. Utilization of a preoperative assessment clinic in a tertiary care centre. Clin Invest Med 2002;25:11–8. [PubMed] [Google Scholar]
- 18.Wijeysundera DN, Austin PC, Beattie WS, et al. A population-based study of anesthesia consultation before major noncardiac surgery. Arch Intern Med 2009;169:595–602. [DOI] [PubMed] [Google Scholar]
- 19.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol 2005;161:81–8. [DOI] [PubMed] [Google Scholar]
- 20.Thanh NX, Rashiq S, Jonsson E. Routine preoperative electrocardiogram and chest x-ray prior to elective surgery in Alberta, Canada. Can J Anaesth 2010;57:127–33. [DOI] [PubMed] [Google Scholar]
- 21.Sheffield KM, McAdams PS, Benarroch-Gampel J, et al. Overuse of preoperative cardiac stress testing in Medicare patients undergoing elective noncardiac surgery. Ann Surg 2013;257:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz AL, Landon BE, Elshaug AG, et al. Measuring low-value care in Medicare. JAMA Intern Med 2014;174:1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colla CH, Sequist TD, Rosenthal MB, et al. Use of non-indicated cardiac testing in low-risk patients: Choosing Wisely. BMJ Qual Saf 2015;24:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijeysundera DN, Austin PC, Beattie WS, et al. Variation in the practice of preoperative medical consultation for major elective noncardiac surgery: a population-based study. Anesthesiology 2012;116:25–34. [DOI] [PubMed] [Google Scholar]
- 25.Changes to OHIP based on best evidence. Toronto: Ministry of Health and Long-Term Care; 2011. Available: www.health.gov.on.ca/en/news/bulletin/2011/docs/bg_20110401_2.pdf (accessed 2015 Jan. 9). [Google Scholar]