Abstract
Dietary intervention of freeze-dried black raspberries (BRBs) in a group of human colorectal cancer patients has demonstrated beneficial effects, including pro-apoptosis, anti-proliferation and anti-angiogenesis. The aim of this study was to investigate BRB-mediated metabolite changes from this same cohort of patients. 28 colorectal cancer patients were given 60 g BRB powder daily for 1–9 weeks. Urine and plasma specimens were collected before and after BRB intervention. A mass spectrometry-based nontargeted metabolomic analysis was conducted on each specimen. A total of more than 400 metabolites were annotated in each specimen. Of these 34 and 6 metabolites were significantly changed by BRBs in urine and plasma, respectively. Increased levels of 4-methylcatechol sulfate in both post-BRB urine and post-BRB plasma were significantly correlated with a higher level of apoptotic marker (TUNEL) in post-BRB tumors. One tricarboxylic acid (TCA) cycle metabolites, Cis-aconitate, was increased in post-BRB urine. Furthermore, BRB-derived polyphenols were absorbed and metabolized to various benzoate species, which were significantly increased in post-BRB specimens. Increased benzoate levels were positively correlated with enhanced levels of amino acid metabolites. These results suggest that BRBs induce significant metabolic changes and affect energy generating pathways. This study supports the hypothesis that BRBs might be beneficial to colorectal cancer patients through the regulation of multiple metabolites.
Keywords: Colorectal cancer, Black raspberries, Metabolomics, cis-aconitate, 4-methylcatechol sulfate
Introduction
Colorectal cancer is preventable with removal of polyps during colonoscopy (1). Nevertheless, it is the third most common cancer in the world with 3.5 million diagnosed patients in 2012 (2). In the United States, an estimated 132,700 new cases and 49,700 death were attributed to colorectal cancer, accounting for approximately 8.4% of all cancer deaths in 2015 (3). Many risk factors have been shown to contribute to colorectal cancer incidence, including aging, obesity, diabetes, smoking, heavy alcohol use and family history (4). Tumor promoters, such as inflammation, carcinogens, growth factors and hormones, increase proliferation and progression of initiated cells and pre-cancerous lesions. Various dietary sources have been intensively studied as chemopreventive agents against colorectal cancer (5). Diets rich in fruits and vegetables have been reported to be inversely associated with colorectal cancer risk (6). In addition, consumption of a dry bean diet has been shown to reduce the recurrence of advanced colorectal adenomas (7).
Given the complex nature of both dietary components themselves and the interactions they have with pre-cancer cells, a comprehensive metabolomic approach has been used to investigate the effects of disease and therapeutic prevention/intervention (8, 9). With the advantages of being sensitive, precise, consistent and quantitative, metabolomics measures the dynamic status of small-molecular-weight metabolites. Thus, metabolomics characterizes the end-products of transcription and translation and is a closer representation of the phenotype (10). Tumor-specific phenotypes have been reported in colorectal cancer using metabolomics, including changes in amino acid metabolism, polyamine metabolism, glycolysis, tricarboxylic acid (TCA) cycle, nucleic acid metabolism, and methylation (8, 11–15). More importantly, metabolomics can identify bioactive dietary components and provide insights to the mechanisms of action these dietary components have on tumor-specific metabolic pathways (16). For instance, black raspberries (BRBs)-derived anthocyanin compounds, cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside, which have showed anti-proliferative effects on colon cancer cells, were the predominant bioactive components identified using metabolomics (17). In addition, the concept of “personalized health” has been proposed to characterize the specific profile for each individual (18) which could predict the patient’s risk factors, stage of cancer and response to chemotherapy or dietary interventions. Ideally, personalized therapy based on an individual’s unique metabolic profile could be combined with genomic and proteomic profiles to provide the greatest efficacy against diseases.
We previously reported that an average of 4 weeks of dietary intervention with freeze-dried BRBs resulted in demethylation of tumor suppressor genes and modulation of several biomarkers of tumor development in the human colon and rectum tissues (19). The current study was conducted to determine if metabolic pathways are altered by BRBs in these patients. We performed a metabolomic study to identify metabolites whose levels in urine and plasma are changed by BRBs and to assess the biochemical pathways which may be impacted by BRBs. Dietary BRB intervention led to significant alterations of benzoates derived from BRB components both in urine and plasma. An increased level of 4-methylcatechol sulfate was significantly correlated with a higher level of apoptotic marker (TUNEL) in post-BRB tumors. Our data suggest that several metabolic pathways, e.g., amino acid metabolism, energy and lipid metabolism, are altered by BRB intervention, which might be beneficial to colorectal cancer patients.
Materials and Methods
Human clinical trials
The present trial was approved by the Institutional Review Boards of the Ohio State University Comprehensive Cancer Center and the University of Texas, San Antonio. Inclusion criteria and exclusion criteria were described in our previous publication (19). 20 g freeze-dried berry powder was mixed with 100 mL of water and orally administered three times a day (a total of 60 g/day), six hours apart, for 1–9 weeks (19). Plasma and urine were collected at baseline and after berry treatment for metabolomic profiling.
Metabolomic profiling
The mass spectrometer platforms, sample extraction and preparation, instrument settings, conditions, and data handling were performed at Metabolon Inc. (Research Triangle Park, NC, USA), and have been previously described in detail (20). Briefly, the major components of the process can be summarized as follows. Osmolality of each urine sample was determined prior to processing. A cocktail of recovery standards was added to the urine and plasma samples and 100 uL aliquots were extracted in 500 uL methanol. The resulting urine and plasma extracts were divided into three fractions for untargeted metabolic profiling and samples were randomized for analysis. Each sample was dried under vacuum to remove organic solvent. Samples were characterized using three independent platforms: ultrahigh-performance liquid chromatography/tandem mass spectrometry (UHPLC-MS/MS) in the negative ion mode, UHPLC-MS/MS in the positive ion mode and gas chromatography-mass spectrometry (GC-MS) after siaylation. The reproducibility of the extraction protocol was assessed by the recovery of the xenobiotic compounds spiked in every urine sample prior to extraction. Urine and plasma extracts were analyzed using a platform consisting of a Waters ACQUITY UHPLC (Waters Corporation, Milford, MA, USA) and a Thermo-Finnigan LTQ mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA. USA). The derivatized samples for GC/MS were analyzed on a Thermo-Finnigan Trace DSQ single –quanrupole MS (Thermo Finnegan, San Jose, CA, USA). Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Identification of known chemical entities was based on comparison to metabolomic library entries of purified standards based on chromatographic properties and mass spectra.
A selection of QC compounds is added to every sample, including the experimental test samples. The internal standard compounds are carefully chosen so as not to interfere with the measurement of the endogenous compounds. These internal standards are used to assess instrument variability. In addition, a matrix pool is assembled from aliquots of all or most of the test samples and endogenous biochemicals in the matrix pool are measured in six replicates per set of 32 test samples. Each test sample is analyzed once with instrument variability determined by calculating the median relative standard deviation (RSD) for the internal standards that were added to each sample prior to injection into the mass spectrometers. Overall process variability was determined by calculating the median RSD for all endogenous metabolites (i.e., non-instrument standards) present in 100% of the Client Matrix samples, which are technical replicates of pooled client samples. Plasma internal standards and endogenous biochemical median RSDs were 6% and 13% respectively, while urine internal standards and endogenous biochemical median RSDs were 6% and 15% respectively.
Statistical analysis
Paired t-tests were performed in R version 2.14.2 (21) to determine the statistical significance of metabolite mean differences between pre- and post-BRB supplementation. The False Discovery Rate (FDR) analysis was performed and q-value was calculated for each metabolite. For all analyses, missing values (if any) were imputed with the observed minimum for that particular compound (imputed values were added after block-normalization). The statistical analyses were performed on natural log-transformed data to reduce the effect of any potential outliers in the data. In addition, urine data was normalized to sample osmolality to compensate for differences in urine concentration. Pearson correlation and linear regression tests were performed using SigmaStat3.5 to determine the association between metabolites in post-BRB urine/plasma, and between metabolites and TUNEL in post-BRB tumors. Metabolites with both a p value <0.05 and a q value <0.05 were considered statistically significant.
Results
To characterize the metabolic profiles induced by BRBs in colorectal cancer patients, we collected urine and plasma specimens before and after BRB treatment and performed MS-based nontargeted metabolomic analysis. A total of more than 400 metabolites were annotated in each sample. When data from all 28 patients were combined, 34 urinary metabolites (Table 1) were significantly changed by BRBs, including 31 increased metabolites and 3 decreased metabolites. In addition, BRBs significantly increased 6 plasma metabolites (Table 2). Significantly changed metabolites were referenced with the Koto Encyclopedia of Genes and Genomes (KEGG) database to identify specific metabolic pathways, i.e. amino acid metabolism, energy, carbohydrate metabolism, lipid metabolism, and xenobiotics. BRBs significantly increased and decreased multiple urinary (Figure 1 A) and plasma (Figure 1 B) metabolites in various metabolic pathways.
Table 1.
List of metabolites significantly changed in post-BRB urine
| Class | Metabolic Pathway | Metabolite | Fold Control | p-Value | q-Value |
|---|---|---|---|---|---|
| Amino acid | Histidine Metabolism | 3-methylhistidine | 3.57 | 0.0072 | 0.0458 |
| N-acetyl-3-methylhistidine | 2.58 | 0.0062 | 0.0419 | ||
| Phenylalanine and Tyrosine Metabolism | 3-hydroxyphenylacetate | 9.43 | 7.64E-05 | 0.001 | |
| gentisate | 4.92 | 0.0011 | 0.0098 | ||
| phenylpropionylglycine | 2.42 | 0.0023 | 0.0178 | ||
| 3-[3-(sulfooxy)phenyl]propanoic acid | 32.25 | 1.40E-05 | 0.0004 | ||
| 5-hydroxymethyl-2-furoic acid | 8.56 | 0.0071 | 0.0458 | ||
| Tryptophan Metabolism | anthranilate | 5.81 | 3.21E-10 | 3.48E-08 | |
| Urea cycle; Arginine and Proline Metabolism | N-acetylproline | 10.39 | 4.00E-08 | 2.89E-06 | |
| Carbohydrate | Pentose Metabolism | xylose | 2.19 | 4.10E-05 | 0.0008 |
| xylitol | 0.82 | 0.0043 | 0.0304 | ||
| Fructose, Mannose and Galactose Metabolism | methyl-beta-glucopyranoside | 3.65 | 0.0016 | 0.0142 | |
| Energy | TCA Cycle | cis-aconitate | 1.36 | 0.0001 | 0.0017 |
| Lipid | Inositol Metabolism | chiro-inositol | 5.63 | 0.0035 | 0.0256 |
| Glycerolipid Metabolism | glycerol 3-phosphate (G3P) | 0.77 | 0.0018 | 0.0143 | |
| Steroid | cortisol | 0.76 | 0.0018 | 0.0143 | |
| cortisone | 1.63 | 0.0026 | 0.0194 | ||
| Xenobiotics | Benzoate Metabolism | hippurate | 3.71 | 9.02E-05 | 0.0012 |
| 3-hydroxyhippurate | 36.59 | 3.06E-05 | 0.0007 | ||
| 4-hydroxyhippurate | 2.45 | 0.0002 | 0.0026 | ||
| 2,4,6-trihydroxybenzoate | 21.27 | 4.92E-11 | 1.07E-08 | ||
| catechol | 3.1 | 7.05E-05 | 0.001 | ||
| catechol sulfate | 5.97 | 2.77E-07 | 1.50E-05 | ||
| 4-methylcatechol sulfate | 4.25 | 0.0006 | 0.0064 | ||
| Food Component/Plant | 2,5-furandicarboxylic acid | 6.45 | 2.23E-05 | 0.0005 | |
| vanillate | 5.48 | 6.79E-07 | 2.94E-05 | ||
| 2,3-dihydroxyisovalerate | 5.22 | 0.0008 | 0.008 | ||
| 2-isopropylmalate | 6.28 | 1.71E-05 | 0.0005 | ||
| 2-oxindole-3-acetate | 5.25 | 0.0001 | 0.0017 | ||
| abscisate | 5.23 | 4.98E-05 | 0.0008 | ||
| cinnamoylglycine | 4.15 | 5.96E-05 | 0.0009 | ||
| N-(2-furoyl)glycine | 5.73 | 0.001 | 0.0094 | ||
| Drug | 4-acetylphenol sulfate | 4.71 | 3.18E-06 | 0.0001 | |
| Chemical | succinimide | 1.9 | 4.94E-05 | 0.0008 |
Fold control is calculated as the ratio of post-BRB urine over pre-BRB urine.
Table 2.
List of metabolites significantly changed in post-BRB plasma
| Class | Metabolic Pathway | Metabolite | Fold Control | p-Value | q-Value |
|---|---|---|---|---|---|
| Amino acid | Phenylalanine & tyrosine metabolism | 3-phenylpropionate (hydrocinnamate) | 2.25 | 0.0004 | 0.0367 |
| 5-hydroxymethyl-2-furoic acid | 3.44 | 0.0006 | 0.0413 | ||
| Carbohydrate | Fructose, mannose, galactose, starch, and sucrose metabolism | methyl-beta-glucopyranoside | 6.92 | 1.00E-15 | 3.15E-13 |
| Nucleotide sugars, pentose metabolism | xylose | 2.67 | 5.77E-11 | 1.22E-08 | |
| Xenobiotics | Benzoate metabolism | 4-methylcatechol sulfate | 2.72 | 0.0004 | 0.0367 |
| catechol sulfate | 3.78 | 6.05E-08 | 8.49E-06 |
Fold control is calculated as the ratio of post-BRB plasma over pre-BRB plasma.
Figure 1. Metabolic pathway classes for metabolites detected in urine and plasma.
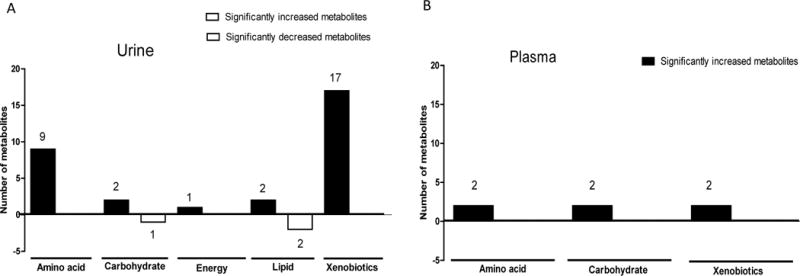
Historical graphs represent the number of urinary (A) and plasma (B) metabolites significantly increased (black) and decreased (white) in each metabolic pathway class.
BRBs significantly increased amino acid metabolism
BRBs significantly increased 9 urinary (Table 1) and 2 plasma (Table 2) metabolites in colorectal cancer patients. These increased metabolites were associated with histidine, phenylalanine, tyrosine, tryptophan, and proline metabolism. Particularly, there was a marked increase in several metabolites in post-BRB urine (Table 1), such as 3-[3-(sulfooxy) phenyl] propanoic acid (32.25 fold), N-acetylproline (10.39 fold), 3-hydroxyphenylacetate (9.43 fold), 5-hydroxymethyl-2-furoic acid (8.56 fold) and anthranilate (5.81 fold) (Figure 2 A).
Figure 2. BRBs significantly increased amino acid metabolism and TCA intermediate.
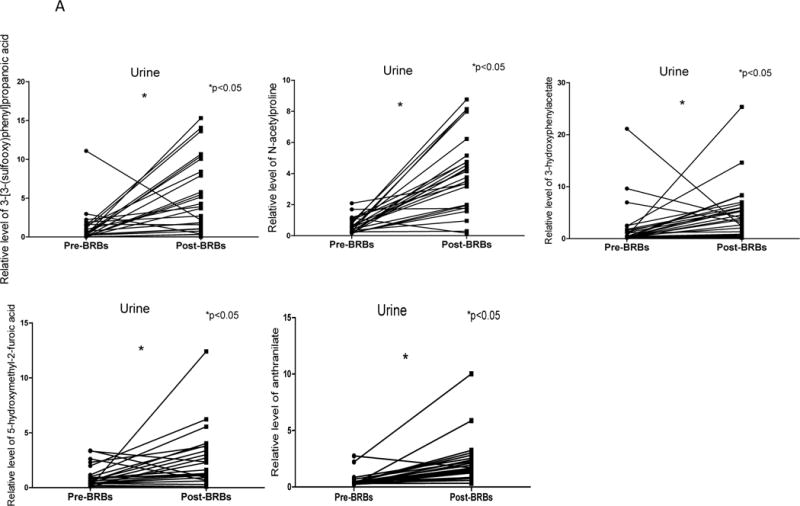
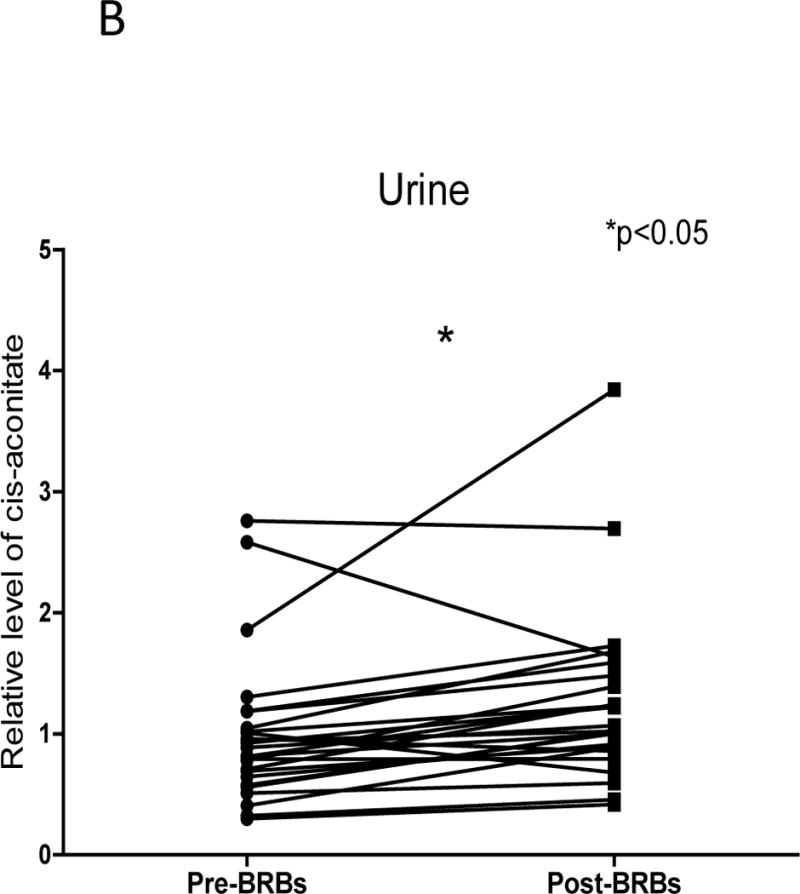
(A) Significantly increased levels of 3-[3-(sulfooxy) phenyl] propanoic acid, N-acetylproline, 3-hydroxyphenylacetate, 5-hydroxymethyl-2-furoic acid and anthranilate in post-BRB urine. (B) Significantly increased levels of cis-aconitate in post-BRB urine.
BRBs significantly increased TCA cycle intermediate
Regarding tumor cell energy generation, lactate accumulation is frequently associated with an increased dependence on aerobic glycolysis and depletion of TCA cycle intermediates has been reported in nearly every study of colorectal cancer patients at various pathologic stages (11–13, 15, 22–24). Intriguingly, BRBs significantly increased one TCA cycle intermediate, cis-aconitate in urine (Figure 2 B). However, no significant changes in glycolytic metabolites were detected.
BRBs significantly increased benzoate metabolites
One distinct feature of post-BRB metabolic profiles was the substantially increased levels of benzoate metabolites in urine (Table 1), such as 3-hydroxyhippurate (36.59 fold), 2,4,6-trihydroxybenzoate (21.27 fold), and catechol sulfate (5.97 fold) (Figure 3 A). The extent of the increase in benzoates was less in plasma (Table 2). Increased levels of 4-methylcatechol sulfate in post-BRB urine and plasma were correlated with a higher level of apoptotic marker (TUNEL) in post-BRB tumors (Figure 3 B). These benzoate metabolites were most likely produced from the metabolism of BRB polyphenols and anthocyanins and were absorbed in the large intestine (25).
Figure 3. BRBs significantly increased benzoate metabolites.
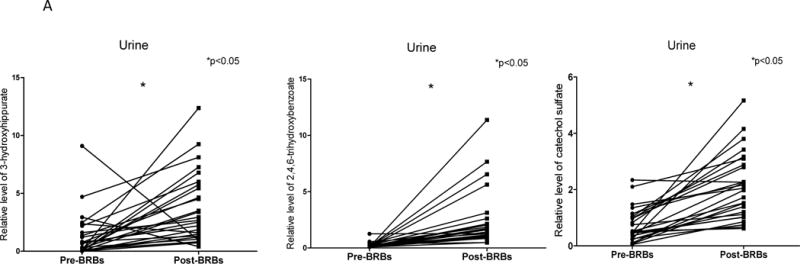
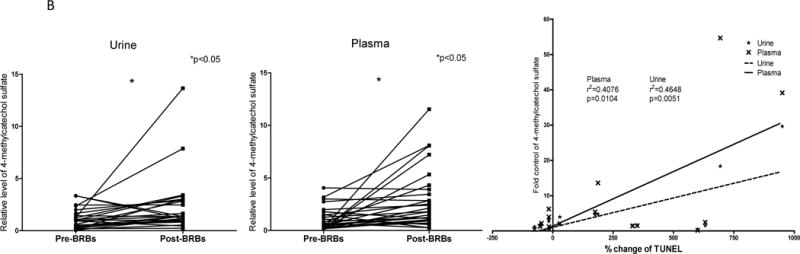
(A) Significantly increased levels of 3-hydroxyhippurate, 2,4,6-trihydroxybenzoate, and catechol sulfate in post-BRB urine. (B) Increased levels of 4-methylcatechol sulfate in post-BRB urine (dash line) and post-BRB plasma (solid line) were significantly correlated with TUNEL staining in post-BRB tumors. *p<0.05
Significant correlations between benzoates and amino acid metabolites
Positive correlations were observed between various benzoate metabolites and amino acid metabolites. For example, in urine, increased levels of hippurate were positively correlated with three increased amino acid metabolites, 3-hydroxyphenylacetate, gentisate and phenylpropionylglycine (Figure 4 A). In plasma, increased levels of catechol sulfate and 4-methylcatechol sulfate were positively correlated with two increased amino acid metabolites, 3-phenylpropionate and 5-hydroxymethyl-2-furoic acid (Figure 4 B).
Figure 4. Positive correlations between benzoates and amino acid metabolites in urine (A) and plasma (B).
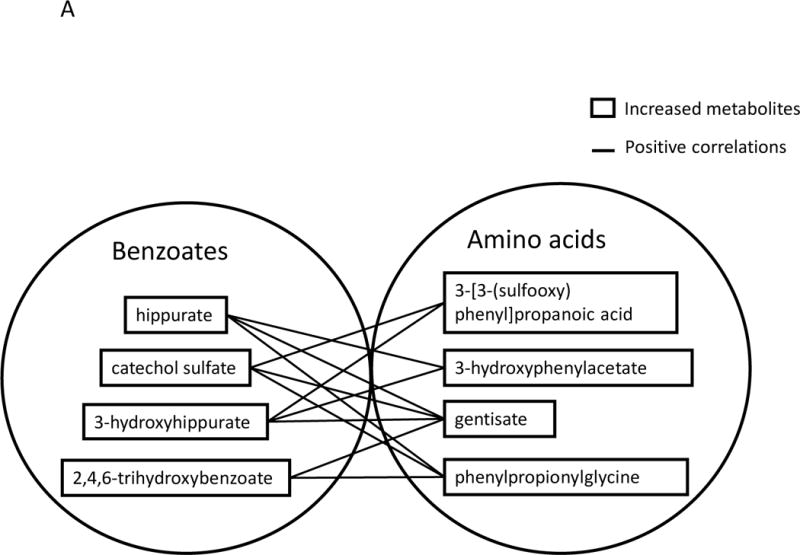
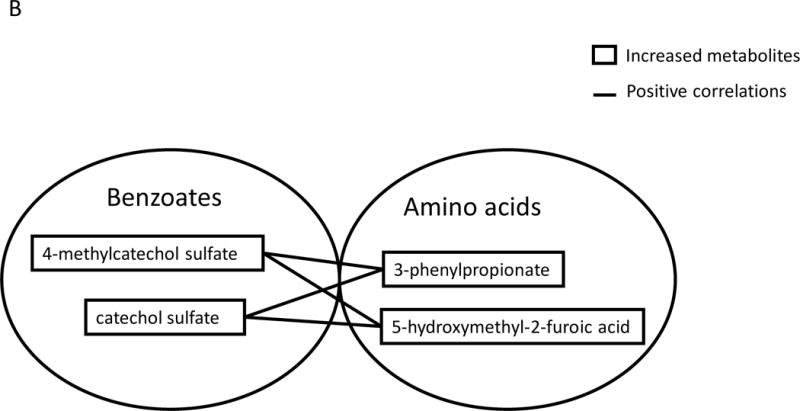
Metabolites labeled in solid line boxes were up-regulated in post-BRB urine (A) and plasma (B). Solid lines indicate significant positive (p<0.05) correlations between benzoates and amino acid metabolites.
Discussion
Our prior clinical trial treated 28 colorectal cancer patients with 60 g freeze-dried BRB powder daily for 1–9 weeks (19) and presented BRB-induced beneficial effects against colorectal tumors, including anti-proliferation, pro-apoptosis and anti-angiogenesis. Our current study performed a comprehensive analysis of metabolic profiles in urine and plasma specimens collected from the same cohort of patients before and after BRB intervention. We detected more than 400 metabolites in each specimen. Of these 34 urinary (Table 1) and 6 plasma (Table 2) metabolites were significantly changed by BRBs. These metabolites are associated with amino acid, energy, carbohydrate, lipid metabolism, and xenobiotics (Figure 1). Diverse distribution of metabolites suggests that BRBs could induce a systemic impact on colorectal cancer patients. Interestingly, our current study showed that increased levels of 4-methylcatechol sulfate in post-BRB urine and plasma were linearly correlated with a higher level of apoptosis (TUNEL) in post-BRB tumors (Figure 3 B) (19). The correlations between metabolites in urine/plasma and tumor markers suggest that BRB-mediated metabolite changes in urine/plasma may be reflective of modulation of tumor metabolism affecting colorectal tumors.
Significant changes in amino acid metabolites in colorectal cancer patients have been reported, such as a decrease in histidine (15, 26), lysine (15, 22, 26), tryptophan (15, 22, 27) and valine metabolism (22, 26) in serum, as well as decreased tryptophan, tyrosine (14) and valine metabolism (28) in urine. However, observed alterations varied significantly between different groups. For instance, Alexander et al. (26) detected a decrease in urinary tyrosine, leucine, isoleucine and methionine metabolites, whereas Nishiumi et al. (27) reported enhanced concentrations of these amino acids. This inconsistency might be attributed to sample preparation, diet and patient classification, such as racial population. Alexander et al. conducted a study in German with European patients, while Nishiumi et al. recruited Asian patients in Japan. In contrast, others have shown that colon tumor tissues presented a highly consistent pattern of changes in amino acid metabolism. Most of the essential amino acids have been reported to be elevated in colon tumors, including alanine, threonine, leucine, isoleucine, valine, lysing, glycine, methionine and tyrosine (23, 24, 29). It is possible that these tumors increase demand for specific amino acids to support rapid cell proliferation (8). Our current study demonstrated numerous amino acid metabolites changed in post-BRB urine and plasma in colorectal cancer patients (Tables 1 and 2). The increase in amino acid metabolites might be attributed directly to BRBs or the metabolism of BRBs.
Numerous studies have consistently confirmed accumulation of lactate and reduced levels of TCA cycle intermediates in many cancer types, including colorectal cancer (11–13, 15, 22–24, 30, 31). This change is commonly referred to as the “Warburg effect” (32). Consistent with our previous studies which demonstrated anti-proliferative effects of BRBs in colorectal cancer patients (19), current metabolomics identified one TCA cycle intermediates, cis-aconitate, that was up-regulated by BRBs in urine (Figure 2 B). No significant changes were observed in lactate levels or lactate/pyruvate ratio in plasma specimens. However, nucleotide sugars, i.e. xylose and xylitol, were increased in post-BRB plasma/urine, which might suggest more glucose was processed through pentose phosphate pathways. Accumulation of TCA cycle intermediates suggests a higher TCA cycle turnover rate and further leads to an enhanced rate of mitochondrial oxidative respiration.
Our current study detected several considerably increased polyphenol-derived benzoate metabolites in post-BRB urine specimens, such as 3-hydroxyhippurate (36.59 fold), 2,4,6-trihydroxybenzoate (21.27 fold) and catechol sulfate (5.97 fold) (Figure 3 A). Importantly, we observed significant positive correlations between polyphenol-derived benzoate metabolites and amino acid metabolites (Figure 4 A and B). In addition, some amino acid metabolites have been shown to derive from other dietary sources using in vitro anaerobic fecal fermentation mode. For example, 3-hydroxyphenylacetate has been reported from rutin (33); 5-hydroxymethyl-2-furoic acid was detected in urine from subjects that consumed dried plum juice (34).
All of the tumor samples from these patients were used to validate the effects of BRB on inhibition of cell proliferation by Ki67 immunohistochemistry, on apoptosis by TUNEL assays and to determine the levels of gene methylation on tumor suppressor genes (19). In the current study, the urinary and plasma specimens of the colorectal patients before BRB intervention served as their own control in comparison with the specimens collected after BRB intervention; therefore observed differences could be associated with the BRB intervention, dietary changes during the BRB intervention, or other time-associated differences. We used published information from many groups that have conducted comprehensive metabolomic studies to determine the tumor-specific metabolic profiles in colorectal cancer patients (8, 11–15). Thus, large scale studies which include metabolic analysis of tumors, of the microenvironment and of the microbiome are needed to accurately measure the effects of BRBs on colorectal cancer patients in comparison with healthy controls.
Together, our results suggest that BRBs significantly induce metabolic changes in colorectal cancer patients. These BRB-derived metabolites may contribute to an overall beneficial regulation against colorectal tumors.
Acknowledgments
We thank Dr. Mark Arnold, Dr. Edward Martin and Dr. Christine Sardo, as well as Comprehensive Cancer Center Tissue Procurement Shared Resource at Ohio State University for managing the trial and handling the specimens in this trial at the Ohio State University. We also thank Dr. John Winston III and Sara Olivarri for their help in the trial at the University of Texas, San Antonio. Finally, we thank all patients for their participation in this trial.
Funding: This work was supported by NIH grant 5 R01 CA148818 04 and American Cancer Society, RSG-13-138-01—CNE to L.-S. Wang.
Footnotes
Conflict of interest: No potential conflicts of interest were disclosed.
References
- 1.http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-treating-by-stage-colon.
- 2.http://publications.cancerresearchuk.org/downloads/product/CS_REPORT_WORLD.pdf.
- 3.http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf.
- 4.http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-risk-factors.
- 5.Miller AB, Linseisen J. Achievements and future of nutritional cancer epidemiology. International journal of cancer Journal international du cancer. 2010;126:1531–7. doi: 10.1002/ijc.25006. [DOI] [PubMed] [Google Scholar]
- 6.van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. The American journal of clinical nutrition. 2009;89:1441–52. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 7.Bobe G, Sansbury LB, Albert PS, Cross AJ, Kahle L, Ashby J, et al. Dietary flavonoids and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:1344–53. doi: 10.1158/1055-9965.EPI-07-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Tso VK, Slupsky CM, Fedorak RN. Metabolomics and detection of colorectal cancer in humans: a systematic review. Future oncology. 2010;6:1395–406. doi: 10.2217/fon.10.107. [DOI] [PubMed] [Google Scholar]
- 9.Mamas M, Dunn WB, Neyses L, Goodacre R. The role of metabolites and metabolomics in clinically applicable biomarkers of disease. Archives of toxicology. 2011;85:5–17. doi: 10.1007/s00204-010-0609-6. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica; the fate of foreign compounds in biological systems. 1999;29:1181–9. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 11.Tan B, Qiu Y, Zou X, Chen T, Xie G, Cheng Y, et al. Metabonomics identifies serum metabolite markers of colorectal cancer. J Proteome Res. 2013;12:3000–9. doi: 10.1021/pr400337b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manna SK, Tanaka N, Krausz KW, Haznadar M, Xue X, Matsubara T, et al. Biomarkers of coordinate metabolic reprogramming in colorectal tumors in mice and humans. Gastroenterology. 2014;146:1313–24. doi: 10.1053/j.gastro.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Y, Cai G, Su M, Chen T, Liu Y, Xu Y, et al. Urinary metabonomic study on colorectal cancer. J Proteome Res. 2010;9:1627–34. doi: 10.1021/pr901081y. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y, Xie G, Chen T, Qiu Y, Zou X, Zheng M, et al. Distinct urinary metabolic profile of human colorectal cancer. J Proteome Res. 2012;11:1354–63. doi: 10.1021/pr201001a. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Djukovic D, Deng L, Gu H, Himmati F, Chiorean EG, et al. Colorectal cancer detection using targeted serum metabolic profiling. J Proteome Res. 2014;13:4120–30. doi: 10.1021/pr500494u. [DOI] [PubMed] [Google Scholar]
- 16.Kim YS, Milner JA. Bioactive food components and cancer-specific metabonomic profiles. Journal of biomedicine & biotechnology. 2011;2011:721213. doi: 10.1155/2011/721213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paudel L, Wyzgoski FJ, Giusti MM, Johnson JL, Rinaldi PL, Scheerens JC, et al. NMR-based metabolomic investigation of bioactivity of chemical constituents in black raspberry (Rubus occidentalis L.) fruit extracts. Journal of agricultural and food chemistry. 2014;62:1989–98. doi: 10.1021/jf404998k. [DOI] [PubMed] [Google Scholar]
- 18.McNiven EM, German JB, Slupsky CM. Analytical metabolomics: nutritional opportunities for personalized health. The Journal of nutritional biochemistry. 2011;22:995–1002. doi: 10.1016/j.jnutbio.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Wang LS, Arnold M, Huang YW, Sardo C, Seguin C, Martin E, et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase I pilot study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:598–610. doi: 10.1158/1078-0432.CCR-10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical chemistry. 2009;81:6656–67. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 21.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna Austria: 2013. [Google Scholar]
- 22.Qiu Y, Cai G, Su M, Chen T, Zheng X, Xu Y, et al. Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS. J Proteome Res. 2009;8:4844–50. doi: 10.1021/pr9004162. [DOI] [PubMed] [Google Scholar]
- 23.Chan EC, Koh PK, Mal M, Cheah PY, Eu KW, Backshall A, et al. Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and gas chromatography mass spectrometry (GC/MS) J Proteome Res. 2009;8:352–61. doi: 10.1021/pr8006232. [DOI] [PubMed] [Google Scholar]
- 24.Denkert C, Budczies J, Weichert W, Wohlgemuth G, Scholz M, Kind T, et al. Metabolite profiling of human colon carcinoma–deregulation of TCA cycle and amino acid turnover. Molecular cancer. 2008;7:72. doi: 10.1186/1476-4598-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacological research : the official journal of the Italian Pharmacological Society. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Leichtle AB, Nuoffer JM, Ceglarek U, Kase J, Conrad T, Witzigmann H, et al. Serum amino acid profiles and their alterations in colorectal cancer. Metabolomics: Official journal of the Metabolomic Society. 2012;8:643–53. doi: 10.1007/s11306-011-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiumi S, Kobayashi T, Ikeda A, Yoshie T, Kibi M, Izumi Y, et al. A novel serum metabolomics-based diagnostic approach for colorectal cancer. PloS one. 2012;7:e40459. doi: 10.1371/journal.pone.0040459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma YL, Qin HL, Liu WJ, Peng JY, Huang L, Zhao XP, et al. Ultra-high performance liquid chromatography-mass spectrometry for the metabolomic analysis of urine in colorectal cancer. Digestive diseases and sciences. 2009;54:2655–62. doi: 10.1007/s10620-008-0665-4. [DOI] [PubMed] [Google Scholar]
- 29.Ong ES, Zou L, Li S, Cheah PY, Eu KW, Ong CN. Metabolic profiling in colorectal cancer reveals signature metabolic shifts during tumorigenesis. Molecular & cellular proteomics : MCP. 2010 doi: 10.1074/mcp.M900551-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Ni Y, Xie G, Jia W. Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. J Proteome Res. 2014;13:3857–70. doi: 10.1021/pr500443c. [DOI] [PubMed] [Google Scholar]
- 31.Qiu Y, Cai G, Zhou B, Li D, Zhao A, Xie G, et al. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:2136–46. doi: 10.1158/1078-0432.CCR-13-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. The Journal of general physiology. 1927;8:519–30. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aura AM, O’Leary KA, Williamson G, Ojala M, Bailey M, Puupponen-Pimia R, et al. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. Journal of agricultural and food chemistry. 2002;50:1725–30. doi: 10.1021/jf0108056. [DOI] [PubMed] [Google Scholar]
- 34.Prior RL, Wu X, Gu L. Identification and urinary excretion of metabolites of 5-(hydroxymethyl)-2-furfural in human subjects following consumption of dried plums or dried plum juice. Journal of agricultural and food chemistry. 2006;54:3744–9. doi: 10.1021/jf0601113. [DOI] [PubMed] [Google Scholar]


