Abstract
Dehydroalanine (Dha) is a nonproteinogenic electrophilic amino acid that is a synthetic intermediate or product in the biosynthesis of several bioactive cyclic peptides such as lantibiotics, thiopeptides, and microcystins. Dha also enables labeling of proteins and synthesis of post-translationally modified proteins and their analogues. However, current chemical approaches to introducing Dha into peptides have substantial limitations. Using in vitro selection, here we show that DNA can catalyze Zn2+ or Zn2+/Mn2+-dependent formation of Dha from phosphoserine (pSer), i.e., exhibit pSer lyase activity, a fundamentally new DNA-catalyzed reaction. Two new pSer lyase deoxyribozymes, named Dha-forming deoxyribozymes 1 and 2 (DhaDz1 and DhaDz2), each function with multiple turnover on the model hexapeptide substrate that was used during selection. Using DhaDz1, we generated Dha from pSer within an unrelated linear 13-mer peptide. Subsequent base-promoted intramolecular cyclization of homocysteine into Dha formed a stable cystathionine (thioether) analogue of the complement inhibitor compstatin. These findings establish the fundamental catalytic ability of DNA to eliminate phosphate from pSer to form Dha and suggest that with further development, pSer lyase deoxyribozymes will have broad practical utility for site-specific enzymatic synthesis of Dha from pSer in peptide substrates.
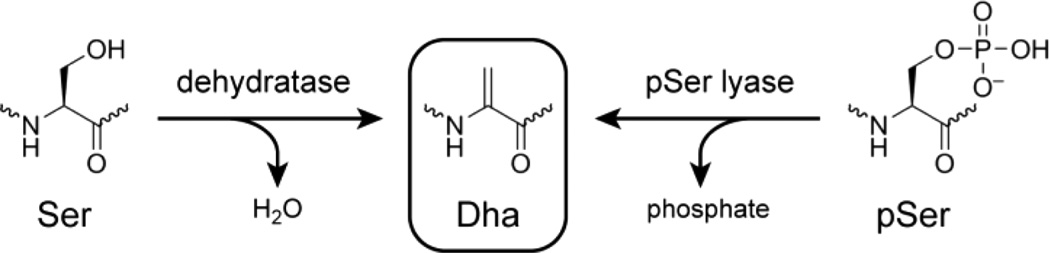
Nature rarely forms electrophilic sites on peptides and proteins.1 A notable exception is dehydroalanine (Dha),2 formally created from serine (Ser) by net elimination of water (Figure 1).3 Dha and related functional groups are intermediates or products in the biosynthesis of a wide range of cyclic peptides, including lantibiotics,4 thiopeptides,5 microcystins,6 and other natural products.6 When introduced synthetically into a peptide or protein, Dha enables preparation of many valuable natural and artificial modifications, usually via thiol addition.7 Dha-containing peptides and proteins can be formed via base-catalyzed elimination of phosphate from pSer-containing substrates, e.g., by treatment with strong base (~1 M hydroxide) for phosphoproteomics analysis.8 However, such relatively harsh conditions lead to numerous side reactions and are not typically used to prepare Dha-containing products. Davis et al. have surveyed the use of various more sophisticated reagents for conversion of cysteine (Cys) to Dha, concluding that a bis-alkylation-elimination strategy is optimal.7,9 However, any exposed Cys will react with such reagents, which therefore cannot readily be used when the newly created Dha is intended for subsequent cyclization with a second Cys residue. Without a gentle and general route available, one must resort to difficult or lengthy synthetic approaches to install Dha or to obviate its need.7,10 This is especially true considering that the Dha monomer for solid-phase peptide synthesis (SPPS) is, after nitrogen deprotection, a simple enamine that presents substantial difficulties for traditional SPPS, necessitating use of alternative Dha-related monomers.11
Figure 1.
Dha and its formation by elimination from either Ser or phosphoserine (pSer, SP).
We envisioned enzymatic catalysis rather than reagent-based synthetic approaches to form Dha from a readily introduced precursor. The natural protein enzymes that create Dha residues proceed via an activated Ser intermediate, such as pSer3 or glutamylated serine.12 Lanthipeptides contain lanthionine or methyllanthionine rings, characterized by addition of Cys into either Dha or the analogous dehydrobutyrine (Dhb) that is obtained by dehydration of threonine (Thr). Biosynthesis of lanthipeptides involves enzyme-catalyzed elimination reactions.13 However, these enzymes often require subsequently removed leader peptides on their substrates, and in general lanthipeptide dehydratases are not preparatively useful beyond their narrow natural sequence contexts.14 We therefore targeted de novo identification of entirely new enzymes for formation of Dha residues. We chose pSer as the Dha precursor (Figure 1), noting that the standard Fmoc-pSer monomer for SPPS is commercially available. Any resulting deoxyribozymes will have pSer lyase activity, in analogy to natural formation of Dhb from pThr by phosphothreonine lyases.15
Deoxyribozymes (DNA enzymes) are particular sequences of DNA that have catalytic activity,16 analogous to protein enzymes as catalytic amino acid sequences. We and others identify deoxyribozymes using in vitro selection,17 an approach first developed with ribozymes (RNA enzymes).18 Deoxyribozymes have been identified to have many kinds of catalytic activity, with substrates that include other oligonucleotides, small molecules, and peptides.19 Here, we sought to identify pSer lyase deoxyribozymes, i.e., DNA catalysts that specifically eliminate phosphate from pSer, forming Dha (Figure 1). We identified two new deoxyribozymes and found them to perform multiple turnovers with the model hexapeptide that was used during selection. We subsequently used DhaDz1 to prepare a stable cystathionine (thioether) analogue of the disulfide-cyclized compstatin. This compound is a 13-mer peptide inhibitor of complement activation that was previously made only after multistep organic synthesis of a thioether dipeptide precursor.20
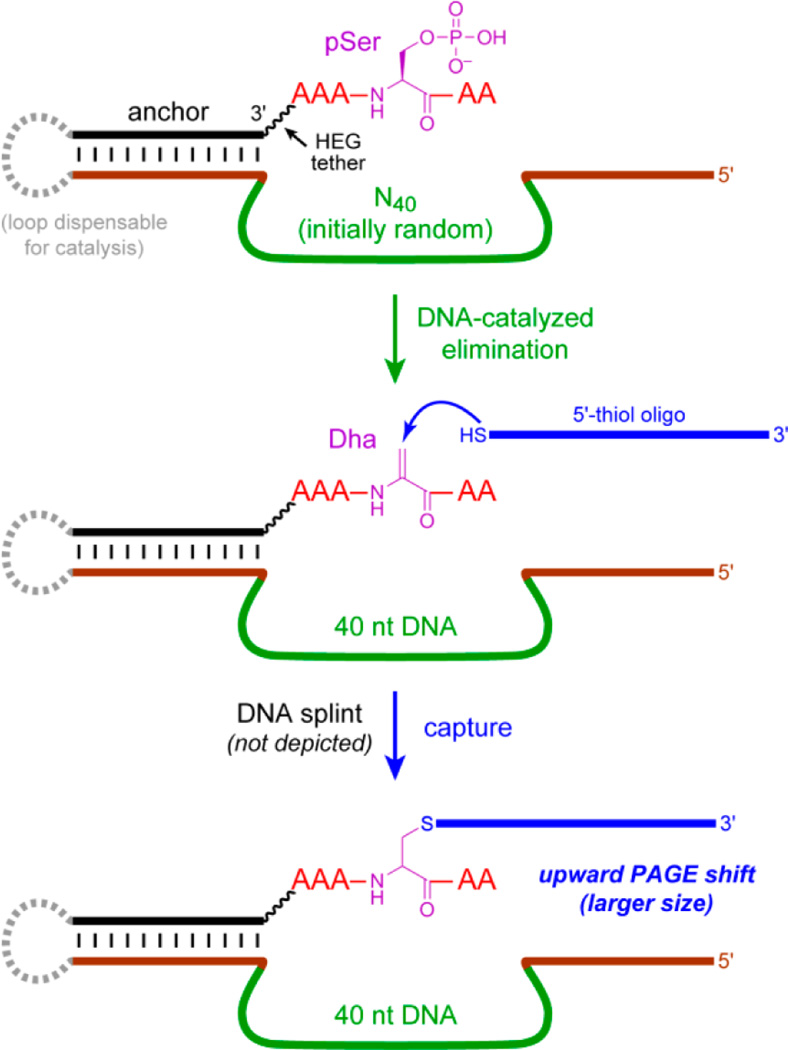
To perform in vitro selection for pSer lyase deoxyribozymes, we needed to develop a reliable “capture” reaction,19f by which specific DNA sequences that convert pSer to Dha are physically separable from the vast majority of catalytically inactive sequences. This capture was achieved with a simple 5′-thiol oligonucleotide that reacts via Michael-type addition with Dha, increasing the mass of only the catalytic DNA sequences and enabling their separation (Figure 2). The capture yield was ~40%, which was readily sufficient to enable the selection process. Using this strategy, in vitro selection was implemented using a DNA-anchored AAASPAA model peptide (SP = pSer) that was attached via the DNA anchor’s remote 5′-end to the deoxyribozyme pool, which included an N40 region (40 random nucleotides). During each round’s key selection step, incubation conditions were 70 mM HEPES, pH 7.5, 1 mM ZnCl2, 20 mM MnCl2, 40 mM MgCl2, and 150 mM NaCl at 37 °C for 14 h. In many of our previous selection experiments, the ions Zn2+, Mn2+, and Mg2+ were each effective deoxyribozyme cofactors, sometimes alone and sometimes in combination. Following the selection and capture steps, PAGE-separated sequences were amplified by PCR21 and ligated to the DNA-anchored pSer peptide substrate for the next selection round.
Figure 2.
In vitro selection of pSer lyase deoxyribozymes. DNA-catalyzed elimination of phosphate from the pSer residue in the DNA-anchored peptide, forming Dha, is followed by DNA-splinted capture of Dha using a 5′-thiol oligonucleotide, which results in an upward PAGE shift for only the active DNA sequences. See Figure S1 for details, including depiction of the hexa(ethylene glycol) [HEG] tether connecting the DNA anchor to the peptide and validation of the capture reaction. The dashed loop on the left enables the selection process but is dispensable for catalysis.
DNA-catalyzed pSer lyase activity of the pool was 15% at round 9, which is relative to the maximum (control) capture yield of ~40% (Figure S2), and individual deoxyribozymes were cloned. In parallel, selection in 50 mM CHES, pH 9.0, 40 mM MgCl2, and 150 mM NaCl at 37 °C showed no activity through round 10, and this second selection experiment was discontinued. This experiment was performed because the higher pH could have fostered more efficient phosphate elimination, but catalysis was not observed.
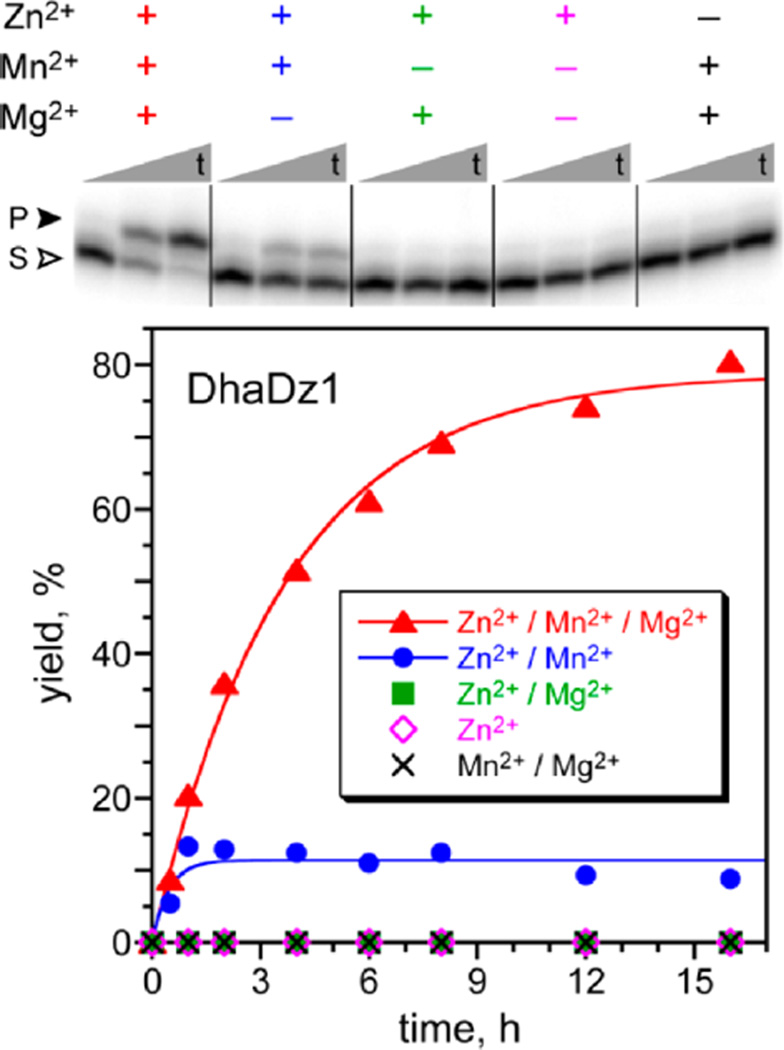
From the round 9 pool, two unrelated 76 nt deoxyribozymes were identified (see Supporting Information for sequences) and named DhaDz1 and DhaDz2, for Dha-forming deoxyribozymes 1 and 2. DhaDz1 catalyzed single-turnover Zn2+/Mn2+-dependent elimination of phosphate from DNA-anchored AAASPAA in high yield when this substrate was not attached to the deoxyribozyme (Figure 3). Although catalysis strictly required only Zn2+ and Mn2+, optimal activity was observed only in the additional presence of Mg2+. DhaDz2 catalyzed the same elimination reaction, strictly requiring only Zn2+ but with greater activity in the additional presence of Mn2+, Mg2+, or both of these ions (Figure S3). For DhaDz1 and each of 1.5 mM Zn2+, 20 mM Mn2+, and 40 mM Mg2+, kobs = 0.28 ± 0.01 h−1 (n = 3) with 80% yield. For DhaDz2 and 2.0 mM Zn2+, 20 mM Mn2+, and 40 mM Mg2+, kobs = 0.43 ± 0.07 h−1 (n = 3) with 68% yield. MALDI mass spectrometry validated the pSer lyase activities of DhaDz1 and DhaDz2.22 For both DhaDz1 and DhaDz2, omitting the deoxyribozyme or using a random-sequence oligonucleotide led to no activity. Removal of the 18 nt fixed-sequence 5′-segment of DhaDz1 or DhaDz2 led to only ~10% yield in 16 h in both cases. This finding suggests that during the selection process, some portion of the fixed-sequence 5′-segment was recruited for folding or catalysis. DhaDz1 and DhaDz2 do not function with a pThr substrate. Selections using pThr are underway.
Figure 3.
Single-turnover pSer lyase activity of the DhaDz1 deoxyribozyme with DNA-anchored model peptide AAASPAA. S = pSer substrate; P = Dha product. Incubation conditions: 70 mM HEPES, pH 7.5, combinations of 1.5 mM ZnCl2, 20 mM MnCl2, and 40 mM MgCl2 as appropriate, and 150 mM NaCl at 37 °C (10 nM 5′-32P-radiolabeled DNA-anchored peptide, 1 µM DhaDz1). The PAGE image shows time points at t = 30 s, 4 h, and 16 h. The Zn2+ concentration of 1.5 mM was optimal at 20 mM Mn2+ and 40 mM Mg2+. At 1.5 mM Zn2+ in the absence of Mg2+, a higher yield of 55% was observed at 10 mM Mn2+. Data for the DhaDz2 deoxyribozyme is in Figure S3A.
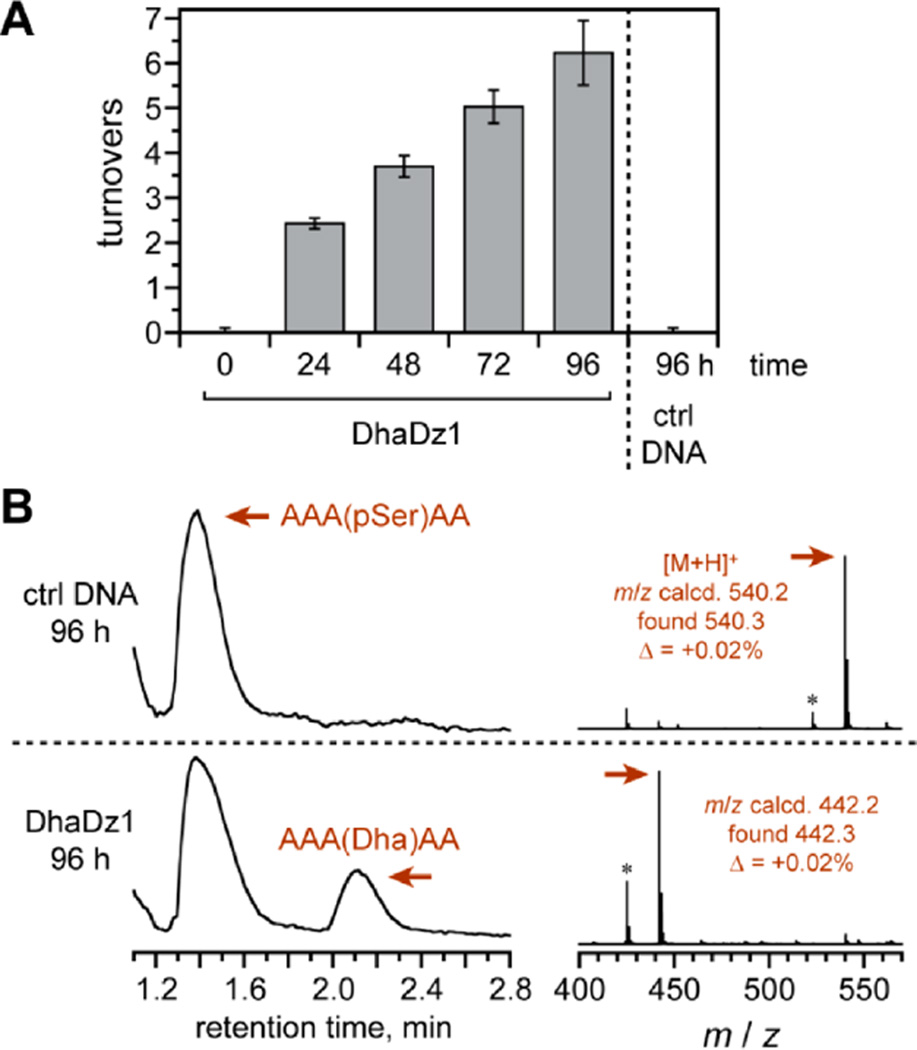
DhaDz1 and DhaDz2 were assessed for multiple-turnover activity with the free (discrete; not DNA-anchored) model AAASPAA peptide substrate. Each deoxyribozyme was assayed both by colorimetric detection of the released inorganic phosphate using malachite green dye (Figure 4A) and by LC-MS of the peptide mixture (Figure 4B). DhaDz1 achieved up to 6–7 turnovers in 96 h as determined by both assays. pSer lyase activity was observed only when the 19 nt fixed-sequence 3′-segment of DhaDz1 was part of a Watson–Crick duplex with the separate anchor oligonucleotide; either deletion of the 3′-segment or omission of the anchor led to no reaction (dye assay, 40:1 peptide:DhaDz1, <0.1 turnovers). DhaDz2 also showed multiple-turnover activity (4 turnovers in 96 h; Figure S3B).
Figure 4.
Multiple-turnover pSer lyase activity of the DhaDz1 deoxyribozyme with free model peptide AAASPAA. The incubation conditions were as in Figure 3, except with 6 mM Zn2+ as optimized to accommodate the high DNA and peptide concentrations (100 µM DhaDz1, 4 mM peptide). (A) Malachite green assay for released inorganic phosphate. At each time point, n = 3 (± sd); “ctrl DNA” refers to an inactive negative control of arbitrary sequence. (B) LC-MS assay (TIC; ESI-MS of indicated peaks). The observed 18% yield is equivalent to 7.2 turnovers. The asterisks in the MS denote loss of ammonia from the peptide N-terminus.23
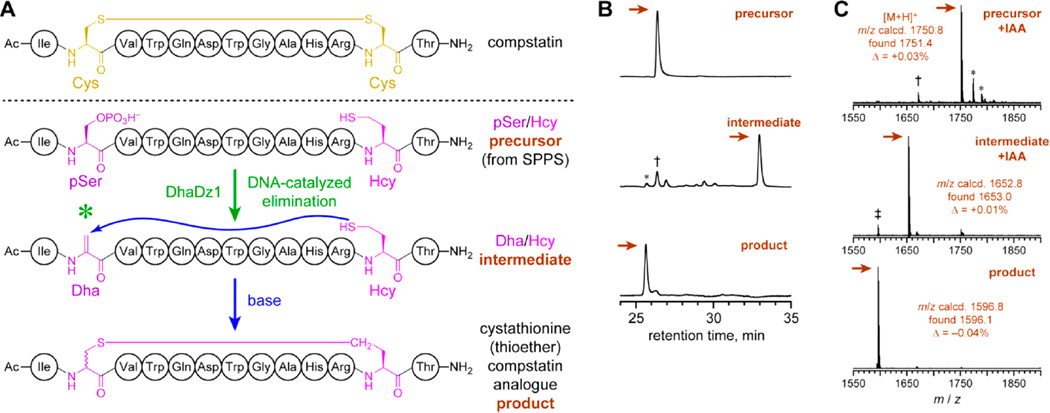
For practical utility, a pSer lyase deoxyribozyme must function with a realistic peptide substrate sequence, rather than the model peptide used during selection. Compstatin is a 13 amino acid cyclic peptide, identified by phage display, that inhibits the proteolytic activation of human complement component C3 and may have therapeutic value.20,24 Disulfide-containing cyclic peptides such as compstatin are stabilized by replacing the disulfide bond with a cystathionine bridge, i.e., –S–CH2– in place of –S–S–. This replacement is typically accompanied by minimal loss of bioactivity,25 including for compstatin.20 Here we used DhaDz1 to enable synthesis of a more stable cystathionine analogue of compstatin (Figure 5A). DhaDz1 was found to catalyze preparative-scale (nanomole) single-turnover formation of Dha within a compstatin-derived linear peptide precursor that includes pSer and homocysteine (Hcy) in place of the two Cys in the native sequence (Figure 5B). The linear Dha/Hcy intermediate was then efficiently cyclized by simple base treatment to the compstatin analogue in 70% two-step yield. Analysis of HPLC-purified intermediate and cyclic product after iodoacetamide treatment (to alkylate free cysteine thiol groups) by MALDI mass spectrometry confirmed their structures (Figure 5C). Because the cyclization step is not enzyme-catalyzed, both (R,S) and (S,S) diastereomers (i.e., epimers at the initially pSer residue) of the compstatin analogue are presumably formed. Formation of diastereomers upon thiol addition into Dha is well-recognized and not an inherent impediment to a wide range of experiments that exploit the products of such a reaction.7,26
Figure 5.
Application of the DhaDz1 pSer lyase deoxyribozyme for synthesis of a more stable compstatin analogue. (A) Compstatin, and its stable cystathionine (thioether) analogue as prepared using DhaDz1. One of the two possible pSer/Hcy combinations is shown. (B) HPLC analysis of the reaction process. The three traces show (top to bottom) the HPLC-purified linear precursor pSer/Hcy peptide, the crude linear intermediate Dha/Hcy peptide after treatment of the precursor with DhaDz1, and the crude cyclic product (compstatin thioether analogue) after subsequent base treatment of the HPLC-purified intermediate Dha/Hcy peptide. Single-turnover conditions for Dha formation: 100 µM DhaDz1, 125 µM untethered DNA anchor oligo, 50 µM precursor peptide, 70 mM HEPES, pH 7.5, 4 mM ZnCl2 (optimized), 20 mM MnCl2, 40 mM MgCl2, 150 mM NaCl, 37 °C, 96 h. Conditions for cyclization: 100 mM Tris, pH 8.0, 25 °C, 4 h (shorter times not attempted). The two-step yield was 70%. For the intermediate, the asterisk denotes autocyclization product, the dagger denotes unreacted precursor, and unmarked peaks are side products not specifically identified. (C) MALDI mass spectrometry of HPLC-purified precursor, intermediate, and product, each after iodoacetamide (IAA) treatment to modify any free thiol groups (+57; one free thiol in each of precursor and intermediate; no free thiol in product).28 For the precursor, the asterisks denote [M + Na]+ and [M + K]+, and the dagger denotes hydrolysis of pSer. For the intermediate, the double dagger denotes autocyclization product.
In summary, we used in vitro selection to identify DhaDz1 and DhaDz2, pSer lyase deoxyribozymes that eliminate phosphate from pSer in a peptide substrate to form Dha. This elimination is a fundamentally new DNA-catalyzed reaction, which expands the repertoire of reactions known to be catalyzed by DNA. A mechanistic understanding of how the DNA catalyzes the elimination process requires more detailed experiments, likely preceded by high-resolution structural information that is currently unavailable for any deoxyribozyme.27 DhaDz1 was used to synthesize a Dha-containing linear peptide intermediate to a cystathionine analogue of compstatin, a cyclic inhibitor of complement activation. With further development of pSer and pThr lyase deoxyribozymes to improve the elimination rate and yield and to establish peptide sequence generality beyond compstatin, we anticipate that such DNA catalysts will be useful for enzymatically converting pSer/pThr residues in peptide substrates into the corresponding Dha/Dhb residues. This ability will enable a broad range of experiments in biological chemistry, both via study of natural products and their analogues as well as by site-specific labeling of peptides and proteins at specifically engineered electrophilic sites. pSer/pThr lyase deoxyribozymes may also find utility in phosphoproteomics experiments.8
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grant R01GM065966 from the National Institutes of Health. We thank Manuel Ortega and Subha Mukherjee for assistance with the LC-MS experiments.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.5b06308.
Experimental details and additional data (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Gersch M, Kreuzer J, Sieber SA. Nat. Prod. Rep. 2012;29:659. doi: 10.1039/c2np20012k. [DOI] [PubMed] [Google Scholar]; (b) Wang L, Zhang Z, Brock A, Schultz PG. Proc. Natl. Acad. Sci. U. S. A. 2003;100:56. doi: 10.1073/pnas.0234824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siodłak D. Amino Acids. 2015;47:1. doi: 10.1007/s00726-014-1846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Chatterjee C, Miller LM, Leung YL, Xie L, Yi M, Kelleher NL, van der Donk WA. J. Am. Chem. Soc. 2005;127:15332. doi: 10.1021/ja0543043. [DOI] [PubMed] [Google Scholar]; (b) Knerr PJ, van der Donk WA. Annu. Rev. Biochem. 2012;81:479. doi: 10.1146/annurev-biochem-060110-113521. [DOI] [PubMed] [Google Scholar]
- 4.(a) Willey JM, van der Donk WA. Annu. Rev. Microbiol. 2007;61:477. doi: 10.1146/annurev.micro.61.080706.093501. [DOI] [PubMed] [Google Scholar]; (b) Ross AC, Vederas JC. J. Antibiot. 2011;64:27. doi: 10.1038/ja.2010.136. [DOI] [PubMed] [Google Scholar]
- 5.(a) Zhang Q, Liu W. Nat. Prod. Rep. 2013;30:218. doi: 10.1039/c2np20107k. [DOI] [PubMed] [Google Scholar]; (b) Just-Baringo X, Albericio F, Álvarez M. Mar. Drugs. 2014;12:317. doi: 10.3390/md12010317. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang S, Zhou S, Liu W. Curr. Opin. Chem. Biol. 2013;17:626. doi: 10.1016/j.cbpa.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 6.(a) Drahl C, Cravatt BF, Sorensen EJ. Angew. Chem. Int. Ed. 2005;44:5788. doi: 10.1002/anie.200500900. [DOI] [PubMed] [Google Scholar]; (b) Dittmann E, Fewer DP, Neilan BA. FEMS Microbiol. Rev. 2013;37:23. doi: 10.1111/j.1574-6976.2012.12000.x. [DOI] [PubMed] [Google Scholar]
- 7.(a) Chalker JM, Gunnoo SB, Boutureira O, Gerstberger SC, Fernández-González M, Bernardes GJL, Griffin L, Hailu H, Schofield CJ, Davis BG. Chem. Sci. 2011;2:1666. [Google Scholar]; (b) Spicer CD, Davis BG. Nat. Commun. 2014;5:4740. doi: 10.1038/ncomms5740. [DOI] [PubMed] [Google Scholar]
- 8.(a) Oda Y, Nagasu T, Chait BT. Nat. Biotechnol. 2001;19:379. doi: 10.1038/86783. [DOI] [PubMed] [Google Scholar]; (b) Rusnak F, Zhou J, Hathaway GM. J. Biomol. Technol. 2002;13:228. [PMC free article] [PubMed] [Google Scholar]; (c) Knight ZA, Schilling B, Row RH, Kenski DM, Gibson BW, Shokat KM. Nat. Biotechnol. 2003;21:1047. doi: 10.1038/nbt863. [DOI] [PubMed] [Google Scholar]
- 9.Chalker JM, Lercher L, Rose NR, Schofield CJ, Davis BG. Angew. Chem. Int. Ed. 2012;51:1835. doi: 10.1002/anie.201106432. [DOI] [PubMed] [Google Scholar]
- 10.Tabor AB. Org. Biomol. Chem. 2011;9:7606. doi: 10.1039/c1ob05946g. [DOI] [PubMed] [Google Scholar]
- 11.(a) Burrage SA, Rayhham T, Bradley M. Tetrahedron Lett. 1998;39:2831. [Google Scholar]; (b) Okeley NM, Zhu Y, van Der Donk WA. Org. Lett. 2000;2:3603. doi: 10.1021/ol006485d. [DOI] [PubMed] [Google Scholar]; (c) Pattabiraman VR, Stymiest JL, Derksen DJ, Martin NI, Vederas JC. Org. Lett. 2007;9:699. doi: 10.1021/ol063133j. [DOI] [PubMed] [Google Scholar]
- 12.Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK. Nature. 2014;517:509. doi: 10.1038/nature13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Zhang Q, van der Donk WA. Protein. Sci. 2013;22:1478. doi: 10.1002/pro.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, van der Donk WA. Chem. Eur. J. 2013;19:7662. doi: 10.1002/chem.201300401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Xu H, Zhou Y, Zhang J, Long C, Li S, Chen S, Zhou J-M, Shao F. Science. 2007;315:1000. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- 16.(a) Schlosser K, Li Y. Chem. Biol. 2009;16:311. doi: 10.1016/j.chembiol.2009.01.008. [DOI] [PubMed] [Google Scholar]; (b) Silverman SK. Angew. Chem. Int. Ed. 2010;49:7180. doi: 10.1002/anie.200906345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyce GF. Annu. Rev. Biochem. 2004;73:791. doi: 10.1146/annurev.biochem.73.011303.073717. [DOI] [PubMed] [Google Scholar]
- 18.Joyce GF. Angew. Chem. Int. Ed. 2007;46:6420. doi: 10.1002/anie.200701369. [DOI] [PubMed] [Google Scholar]
- 19.(a) Silverman SK. Chem. Commun. 2008:3467. doi: 10.1039/b807292m. [DOI] [PubMed] [Google Scholar]; (b) Silverman SK. Acc. Chem. Res. 2009;42:1521. doi: 10.1021/ar900052y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu J, Cao Z, Lu Y. Chem. Rev. 2009;109:1948. doi: 10.1021/cr030183i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhang XB, Kong RM, Lu Y. Annu. Rev. Anal. Chem. 2011;4:105. doi: 10.1146/annurev.anchem.111808.073617. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gong L, Zhao Z, Lv YF, Huan SY, Fu T, Zhang XB, Shen GL, Yu RQ. Chem. Commun. 2015;51:979. doi: 10.1039/c4cc06855f. [DOI] [PubMed] [Google Scholar]; (f) Silverman SK. Acc. Chem. Res. 2015;48:1369. doi: 10.1021/acs.accounts.5b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knerr PJ, Tzekou A, Ricklin D, Qu H, Chen H, van der Donk WA, Lambris JD. ACS Chem. Biol. 2011;6:753. doi: 10.1021/cb2000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The forward and reverse single-stranded PCR products were separable because formation of the reverse product was initiated using a primer containing a nonamplifiable spacer that stops Taq polymerase (see Supporting Information.
- 22.All substrates were DNA-anchored. pSer substrate m/z calcd, 6963.8; found, 6964.0, Δ = +0.003%; DhaDz1 Dha product m/z calcd, 6865.8; found, 6867.7, Δ = +0.03%. DhaDz2 Dha product m/z calcd, 6865.8; found, 6860.6, Δ = −0.08%.
- 23.Wysocki VH, Resing KA, Zhang Q, Cheng G. Methods. 2005;35:211. doi: 10.1016/j.ymeth.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 24.(a) Sahu A, Kay BK, Lambris JD. J. Immunol. 1996;157:884. [PubMed] [Google Scholar]; (b) Janssen BJ, Halff EF, Lambris JD, Gros P. J. Biol. Chem. 2007;282:29241. doi: 10.1074/jbc.M704587200. [DOI] [PubMed] [Google Scholar]; (c) Mastellos DC, Yancopoulou D, Kokkinos P, Huber-Lang M, Hajishengallis G, Biglarnia AR, Lupu F, Nilsson B, Risitano AM, Ricklin D, Lambris JD. Eur. J. Clin. Invest. 2015;45:423. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Dekan Z, Vetter I, Daly NL, Craik DJ, Lewis RJ, Alewood PF. J. Am. Chem. Soc. 2011;133:15866. doi: 10.1021/ja206408q. [DOI] [PubMed] [Google Scholar]; (b) Muttenthaler M, Andersson A, de Araujo AD, Dekan Z, Lewis RJ, Alewood PF. J. Med. Chem. 2010;53:8585. doi: 10.1021/jm100989w. [DOI] [PubMed] [Google Scholar]
- 26.(a) Hofmann FT, Szostak JW, Seebeck FP. J. Am. Chem. Soc. 2012;134:8038. doi: 10.1021/ja302082d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tang W, Jimenez-Oses G, Houk KN, van der Donk WA. Nat. Chem. 2014;7:57. doi: 10.1038/nchem.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowakowski J, Shim PJ, Prasad GS, Stout CD, Joyce GF. Nat. Struct. Biol. 1999;6:151. doi: 10.1038/5839. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S, van der Donk WA. J. Am. Chem. Soc. 2014;136:10450. doi: 10.1021/ja504692v. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.