Abstract
Polyethylene glycol (PEG) and related polymers are often used in the functionalization of carbon nanomaterials in procedures that involve sonication. However, PEG is very sensitive to sonolytic degradation and PEG degradation products can be toxic to mammalian cells. Thus, it is imperative to assess potential PEG degradation to ensure that the final material does not contain undocumented contaminants that can introduce artifacts into experimental results. Described here is a simple and inexpensive polyacrylamide gel electrophoresis method to detect the sonolytic degradation of PEG. The method was used to monitor the integrity of PEG phospholipid constructs and branched chain PEGs after different sonication times. This approach not only helps detect degraded PEG, but should also facilitate rapid screening of sonication parameters to find optimal conditions that minimize PEG damage.
Keywords: Polyethylene glycol, sonolysis, carbon nanotubes, graphene oxide, nanotoxicity
Introduction
A common practice in the preparation of carbon nanotubes, graphene oxide, and other nanomaterials for use in aqueous environments is their covalent or non-covalent functionalization with polyethylene glycol (PEG) to improve biocompatibility. Functionalization often involves sonication in the presence of a reagent that contains PEG, a phospholipid-PEG (PL-PEG), or a similar hydrophilic polymer.1–5 It is well known that the formation and collapse of cavitation bubbles during sonication creates reactive oxygen species, as well as shear forces, that can degrade polymers.6,7 PEG itself is fragmented upon sonication in aqueous solution as are polymers that contain PEG.8–11 Sonication not only degrades PEG and PEG-containing block co-polymers such as poloxamers (trade name Pluronic®), but the degradation products are toxic to mammalian cells.12 Further, mass spectrometry studies have demonstrated the sonolytic degradation of a widely used PL-PEG construct, and that the altered structure remaining on the nanotubes affects the interaction of the nanotubes with cells.13 The contamination of nanomaterials with undocumented PEG degradation products resulting from sonication may introduce artifacts into experimental results that impact conclusions.
Due to differences in commercially available sonicator types (probe or bath), and variables such as ultrasonic frequencies, delivered power, and temperature, it is typically difficult to replicate sonication conditions from one laboratory to another.14 This makes it challenging to predict the extent to which a polymer may be degraded or otherwise structurally altered by sonication based on experimental conditions described in the literature. To avoid potential artifacts that may arise in the event that a polymer solution or polymer component of a carbon nanomaterial suspension has been chemically changed by sonication, it is crucial to monitor polymer integrity after sonication. Herein is described a simple and inexpensive approach to rapidly detect sonolytic damage to PL-PEG and branched chain PEG polymers. Moreover, if polymer damage is detected in a particular experimental design, the approach described here should facilitate surveying sonication parameters to find conditions that induce minimal polymer damage.
Materials and methods
Chemicals and solutions
Carboxylated single-walled carbon nanotube (SWNT) powder (product no. P3, batch no. 03-A010) was purchased from Carbon Solutions, Inc. (Riverside, CA). Caution: a particulate respirator should be worn when handling dry SWNT powders. Distearoyl phosphatidylethanolamine-PEG-amine (DSPE-PEG-NH2) (product no. 880128P) and distearoyl phosphatidylethanolamine-PEG-carboxyl (DSPE-PEG-COOH) (product no. 880125P) were purchased from Avanti Polar Lipids (Alabaster, AL) and stored at –20℃. Four arm 2 kDa PEG-NH2 (product no. PSB430), four arm 10 kDa PEG-NH2 (product no. PSB431), and eight arm 10 kDa PEG-NH2 (product no. PSB811) were purchased from Creative PEGWorks (Winston Salem, NC) and stored at –20℃. Sodium phosphate buffer stock (0.1 M, pH 7.4) was sterilized by autoclaving at 121℃ for 45 min. All other chemicals were purchased form Sigma-Aldrich and were used as received.
Sonication and preparation of SWNT suspensions
All sonication was done using an Elmasonic P30H Ultrasonic Cleaner bath sonicator (Singen, Germany) in a 4℃ cold room. Typically, a glass vial containing 10 mL of aqueous sample was placed in a central location in the sonication tank filled with 1400 mL of DI water and sonicated at 37 kHz and 120 W for times as indicated in the figures. The temperature in the bath was controlled with a cooling coil that circulated water at 2℃ through the bath. During sonication, the temperature never exceeded 18℃. DSPE-PEG-COOH and DSPE-PEG-NH2 solutions were prepared by dissolving the powder in 10 mM phosphate buffer solution to a concentration of 0.35 mM. When SWNTs were present during sonication, 5 mg of SWNT powder was placed in a vial and 10 mL of the desired DSPE-PEG solution was added, followed by sonication. All three of the branched PEG samples, the four arm 2 kDa, the four arm 10 kDa, and the eight arm 10 kDa PEG, were dissolved in water to a concentration of 1 mg/mL. Ten milliliters each of these solutions was subjected to sonication as described earlier.
For purposes of comparison, the power delivered to the sonicator bath was determined by measuring the change in bath temperature (starting at 22℃) as a function of sonication time, at room temperature without a cooling coil, as described by Taurozzi et al.14 This method uses the equation P = (dT/dt) × M × Cp where P is the acoustic power (W), T is the temperature (K), t is time (s), M is the mass of water (1400 g for this bath sonicator), and Cp is the specific heat capacity of water at 298 K (4.18 J/g K). The power measured by this approach was ∼ 80 W for our sonication system. Even though this method does not measure the power delivered to a sample directly, it does provide a way to compare the power delivered to different bath sonication systems when measured under similar conditions.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) method to analyze the degradation of PEG-containing materials
Ten microliters of PEG-containing material was loaded on a 4% stacking and 15% resolving SDS polyacrylamide gel. The samples were electrophoresed at 100 V for 90 min and fixed in the gel with a solution of 50% methanol and 10% acetic acid for 5 min at room temperature. The gel was soaked in 5% BaCl2 for 10 min and the excess BaCl2 was removed. The gel was then soaked in 0.1 N iodine for 5 min and further destained with water to remove the background staining. Gels were scanned and digitized with a HP Scanjet G3110 flatbed scanner and the intensity of the bands was quantified using ImageJ software. The intensities were then plotted as percent of non-sonicated material that was set to 100%. The data presented are the mean ± SD of at least three different gels for each experiment.
Dynamic light scattering (DLS) particle size analysis
The particle size distribution of DSPE-PEG-COOH (140 µM in 10 mM phosphate buffer) was measured using a Malvern Zetasizer ZS 3600. Three independent DLS measurements of a sample, each consisting of 10 runs at 30 s/run, were averaged and the particle size distribution was plotted as hydrodynamic diameter against relative intensity.
Results
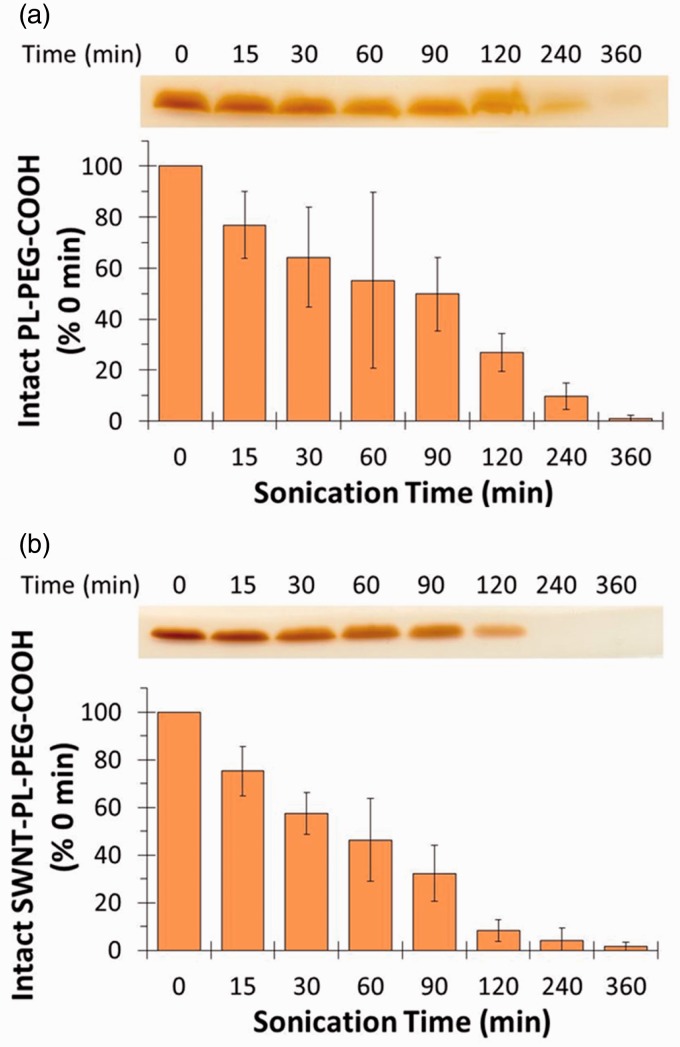
PEG covalently attached to proteins can be detected after SDS-PAGE based on the interaction of BaI2 with PEG.15 We reasoned that PL-PEG would bind SDS, acquire a negative charge, and migrate toward the anode during SDS-PAGE and that the PEG component could also be stained with BaI2. Degradation of PEG could then be detected by a decline in the intensity of BaI2 staining. To test the idea, a frequently used PL-PEG construct, DSPE-PEG-COOH, was sonicated in a bath sonicator for different times followed by SDS-PAGE and staining with BaI2. Figure 1(a) shows the BaI2-stained bands in a section of a representative gel. The relative intensities of bands in the digitized gel image were quantified and are shown as a bar graph below the gel image. In the absence of sonication, a single intense band was evident near the dye front marking the position of DSPE-PEG-COOH. Within 15 min of sonication the intensity of the band had visibly declined and continued to decline until it was almost absent by 360 min. Given that the degradation of PL-PEG into smaller fragments during sonication has been documented by mass spectrometry, these results suggest that BaI2 staining after SDS-PAGE readily detects the sonolysis of DSPE-PEG-COOH.13
Figure 1.
Sonolytic degradation of DSPE-PEG-COOH in the presence or absence of SWNTs. DSPE-PEG-COOH alone (a) or together with 0.5 mg/mL SWNTs (b) was sonicated for the indicated times as described in the “Materials and methods” section. The samples were analyzed by SDS-PAGE followed by BaI2 staining as shown by the bands in the gel image. The gels were then scanned and the band intensities quantitated using ImageJ software. (A color version of this figure is available in the online journal.)
Various PL-PEG constructs are widely used in the non-covalent functionalization of carbon nanotubes to disperse the nanotubes and for appending ligands to target the nanotubes to cells. To see if the presence of carbon nanotubes affected the apparent degradation of DSPE-PEG-COOH, SWNTs were sonicated for different times with DSPE-PEG-COOH and the resulting material was examined by SDS-PAGE followed by BaI2 staining. The intensity of the DSPE-PEG-COOH band (Figure 1(b)) decreased at a rate similar to material sonicated in the absence of SWNTs (Figure 1(a)). This suggests that the SWNTs have little influence on the sonolytic degradation of DSPE-PEG-COOH.
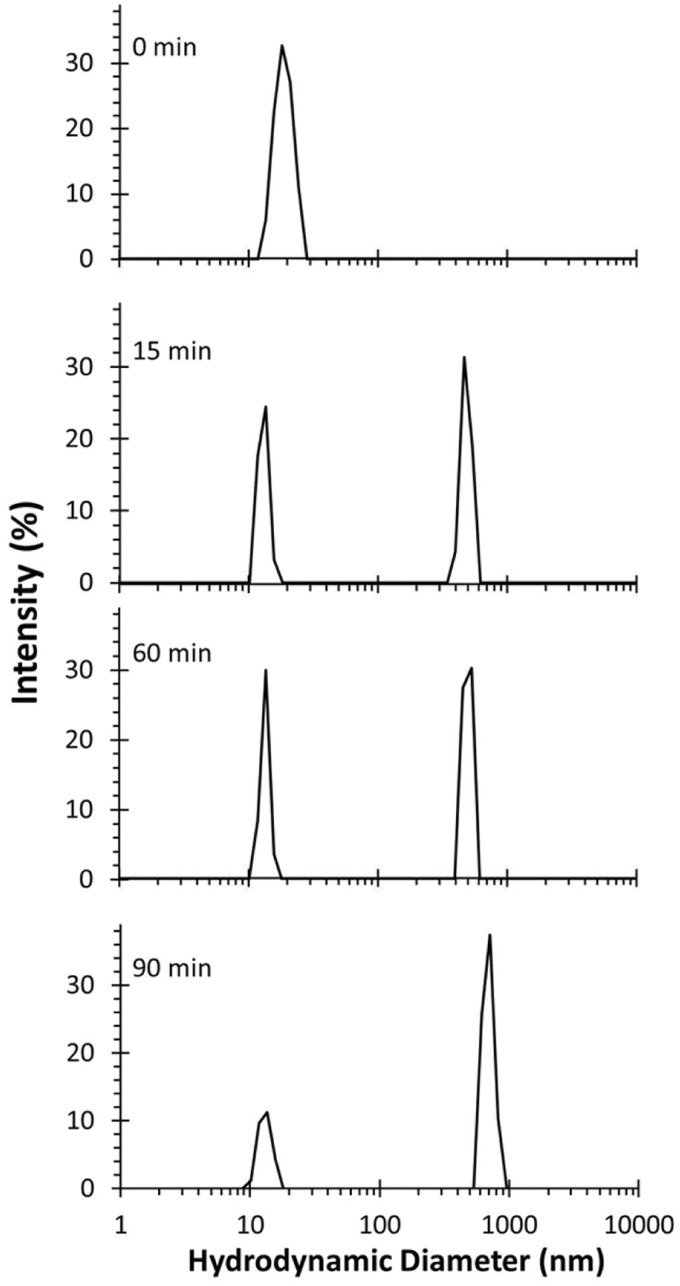
To independently verify that the structure of DSPE-PEG-COOH was altered by sonication, the particle size distribution of DSPE-PEG-COOH was monitored by DLS as a function of sonication time. Before sonication, DSPE-PEG-COOH was characterized as a single peak with a hydrodynamic diameter in the range of 10–20 nm (Figure 2). Within 15 min of sonication, the intensity of the peak declined and was reduced significantly at 90 min of sonication. Concomitant with sonication time, peaks of larger diameters appeared as a greater relative fraction of the total particle population. The nature of the larger particles is not known, but they may be fragments containing hydrophobic fatty acid tails that aggregate after the PEG has been degraded. These data are consistent with the results of SDS-PAGE suggesting that the PEG component of DSPE-PEG-COOH is lost during sonication.
Figure 2.
DLS particle size analysis of DSPE-PEG-COOH solution as a function of sonication time. An aqueous solution of DSPE-PEG-COOH was sonicated as described in the “Materials and methods” section and aliquots were collected at the indicated time points for analysis
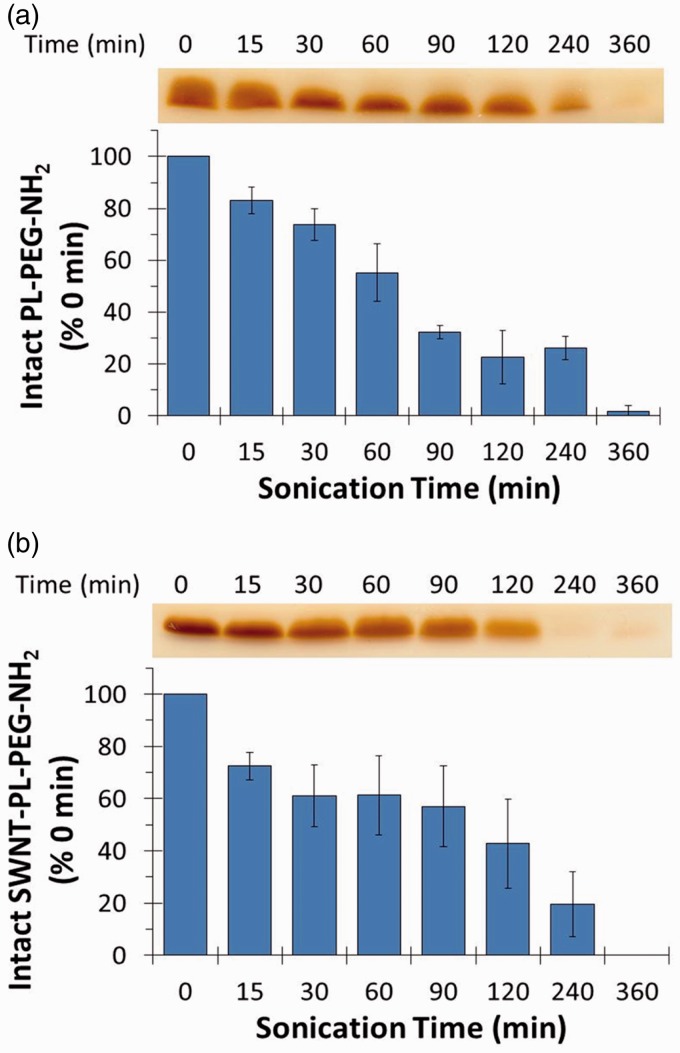
To determine whether the SDS-PAGE/BaI2 approach could be generalized to detect the sonolysis of other PL-PEG derivatives, the effect of sonication on DSPE-PEG-NH2 was assessed as a function of sonication time in the presence and absence of SWNTs. As with DSPE-PEG-COOH, a decrease in the intensity of the band stained with BaI2 was observed as a function of sonication time both in the presence and absence of SWNTs (Figure 3), confirming that the approach could be used with other PL-PEG compounds.
Figure 3.
Sonolytic degradation of DSPE-PEG-NH2 in the presence or absence of SWNTs. DSPE-PEG-NH2 was sonicated alone (a) or with 0.5 mg/mL of SWNTs (b) for the indicated times. Samples were analyzed by SDS-PAGE followed by BaI2 staining and quantification of band intensities as described in the “Materials and methods” section. (A color version of this figure is available in the online journal.)
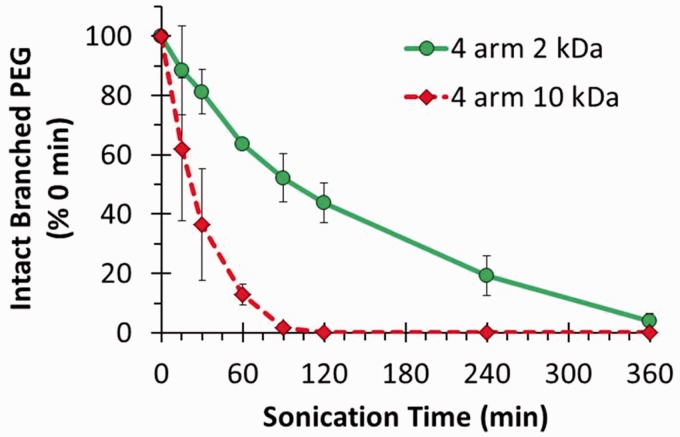
Branched chain PEG is often used in the covalent functionalization of graphene oxide for biomedical applications.2 We previously noted that a linear PEG polymer migrated toward the anode in SDS gels and could be detected with BaI2.12 To determine whether the SDS-PAGE/BaI2 approach could also apply to monitoring the integrity of branched chain PEG, four arm PEG-NH2 with molecular weights of 2 and 10 kDa were sonicated for different times and analyzed by SDS-PAGE with BaI2 staining (Figure 4). Partial degradation of the four arm 2 kDa PEG-NH2 was evident within 15 min and was complete within 360 min. The four arm 10 kDa PEG-NH2 degraded even faster with loss of about half of the polymer within 30 min, suggesting that higher molecular weight PEGs degrade faster than smaller PEGs. In a separate experiment, eight arm 10 kDa PEG-NH2 was also rapidly degraded using the exact same sonication conditions (data not shown).
Figure 4.
Sonolytic degradation of four arm 2 kDa and four arm 10 kDa PEG-NH2 was analyzed by SDS-PAGE followed by BaI2 staining as described in “Materials and methods” section. (A color version of this figure is available in the online journal.)
Discussion
The main objective of this paper was to determine whether SDS-PAGE followed by staining with BaI2 could be a rapid and inexpensive method to detect the potential sonolysis of PEG-containing compounds often used in the functionalization of carbon nanomaterials. For DSPE-PEG-COOH, a decline in the intensity of BaI2-stained bands was visible within 15 min of sonication and was at ∼ 50% loss within an hour. Similar results were found in the presence of SWNTs and with DSPE-PEG-NH2. The detection of PL-PEG degradation using the SDS-PAGE/BaI2 method was also consistent with changes in DSPE-PEG-COOH particle size distribution upon sonication as measured by DLS. Further, the method readily detected the sonolysis of branched chain PEG-NH2 species that are often used in the covalent functionalization of carboxylated carbon nanotubes and graphene oxide. Thus, SDS-PAGE/BaI2 approach appears to offer a rapid and inexpensive way to assess sonolytic damage to a variety of PEG-containing materials.
Despite the fact that sonolytic degradation of PEG has been well described in the literature, articles still appear in high profile journals where carbon nanomaterials are sonicated for extended times with agents that contain PEG and where the integrity of the PEG was not verified. For example, Sacchetti et al. sonicated amine functionalized PL-PEG for 360 min with SWNTs en route to preparing a variety of derivatives used in studies of protein binding to different constructs.16 Andersen et al. sonicated SWNTs with surfactant-PEG derivatives for an hour in a bath sonicator in studies of complement activation by the coated SWNTs.17 In both cases, the assumption appeared to be that the SWNTs were coated with intact polymer. In a recent Nature Protocols article, Yang et al. described the functionalization of various graphene oxides with branched chain PEG-NH2 that, depending on the construct, involved bath sonication for up to 90 min.2 We have no agenda in picking these specific examples other than to note the possibility that the sonication conditions could well have resulted in significant degradation of PEG-containing materials. Heretofore, detecting the sonolysis of PEG-containing materials usually involved mass spectrometry or other instrument-intensive approaches. The availability of the SDS-PAGE/BaI2 method should facilitate assessing whether PEG-containing materials are intact or fragmented upon sonication.
Acknowledgements
The authors thank the University of Arizona SEMATECH/Semiconductor Research Corporation Engineering Research Center for Environmentally Benign Semiconductor Manufacturing (Grant ERC425-042), the National Cancer Institute (Grant R15-CA152917), and the National Institute for Environmental Health Sciences (Grant R15-ES023666) for supporting this work.
Author contributions
Conceived and designed the experiments: VSM, RW, CAM, PP, and RD. Performed the experiments: VSM, and RW. Analyzed the data: VSM, RW, CAM, PP, and RD. Contributed reagents/materials/analysis tools: VSM, RW, CAM, PP, and RD. Wrote the manuscript: VSM, RW, CAM, PP, and RD.
References
- 1.Liu Z, Tabakman SM, Chen Z, Dai H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat Protoc 2009; 4: 1372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang K, Feng L, Hong H, Cai W, Liu Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat Protocols 2013; 8: 2392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottini M, Rosato N, Bottini N. PEG-modified carbon nanotubes in biomedicine: current status and challenges ahead. Biomacromolecules 2011; 12: 3381–93. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez VC, Jachak A, Hurt RH, Kane AB. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol 2012; 25: 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco A, Kostarelos K, Prato M. Making carbon nanotubes biocompatible and biodegradable. Chem Commun 2011; 47: 10182–8. [DOI] [PubMed] [Google Scholar]
- 6.Suslick KS, Flannigan DJ. Inside a collapsing bubble: sonoluminescence and the conditions during cavitation. Annu Rev Phys Chem 2008; 59: 659–83. [DOI] [PubMed] [Google Scholar]
- 7.Suslick KS. Sonochemistry. Science 1990; 247: 1439–45. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki H, Takeda Y, Arakawa R. Mass spectrometric analysis for high molecular weight synthetic polymers using ultrasonic degradation and the mechanism of degradation. Anal Chem 2007; 79: 4182–7. [DOI] [PubMed] [Google Scholar]
- 9.Singla R, Grieser F, Ashokkumar M. Kinetics and mechanism for the sonochemical degradation of a nonionic surfactant. J Phys Chem A 2009; 113: 2865–72. [DOI] [PubMed] [Google Scholar]
- 10.Vijayalakshmi SP, Madras G. Effect of temperature on the ultrasonic degradation of polyacrylamide and poly(ethylene oxide). Polym Degrad Stab 2004; 84: 341–4. [Google Scholar]
- 11.Vijayalakshmi SP, Madras G. Effect of initial molecular weight and solvents on the ultrasonic degradation of poly(ethylene oxide). Polym Degrad Stab 2005; 90: 116–22. [Google Scholar]
- 12.Wang R, Hughes T, Beck S, Vakil S, Li S, Pantano P, Draper RK. Generation of toxic degradation products by sonication of pluronic(R) dispersants: implications for nanotoxicity testing. Nanotoxicology 2013; 7: 1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeineldin R, Al-Haik M, Hudson LG. Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett 2009; 9: 751–7. [DOI] [PubMed] [Google Scholar]
- 14.Taurozzi JS, Hackley VA, Wiesner MR. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment – issues and recommendations. Nanotoxicology 2011; 5: 711–29. [DOI] [PubMed] [Google Scholar]
- 15.Kurfürst MM. Detection and molecular weight determination of polyethylene glycol-modified hirudin by staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem 1992; 200: 244–8. [DOI] [PubMed] [Google Scholar]
- 16.Sacchetti C, Motamedchaboki K, Magrini A, Palmieri G, Mattei M, Bernardini S, Rosato N, Bottini N, Bottini M. Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol-modified single-walled carbon nanotubes: potential implications on biological performance. ACS Nano 2013; 7: 1974–89. [DOI] [PubMed] [Google Scholar]
- 17.Andersen AJ, Robinson JT, Dai H, Hunter AC, Andresen TL, Moghimi SM. Single-walled carbon nanotube surface control of complement recognition and activation. ACS Nano 2013; 7: 1108–19. [DOI] [PubMed] [Google Scholar]