Abstract
Persistent quadriceps muscle weakness is common after anterior cruciate ligament (ACL) reconstruction. The mechanisms underlying these chronic strength deficits are not clear. This study examined quadriceps strength in people two to fifteen years post ACL reconstruction and tested the hypothesis that chronic quadriceps weakness is related to levels of voluntary quadriceps muscle activation, antagonistic hamstrings moment, and peripheral changes in muscle. Knee extensor strength and activation were evaluated in fifteen ACL reconstructed and fifteen matched uninjured control subjects using an interpolated triplet technique. Electrically evoked contractile properties were used to evaluate peripheral adaptations in the quadriceps muscle. Antagonistic hamstrings moments were predicted using a practical mathematical model. Knee extensor strength and evoked torque at rest were significantly lower in the reconstructed legs (P < 0.05). Voluntary activation and antagonistic hamstrings activity were similar across legs and between groups (P > 0.05). Regression analyses indicated that side-to-side differences in evoked torque at rest explained 71% of the knee extensor strength differences by side (P < 0.001). Voluntary activation and antagonistic hamstrings moment did not contribute significantly (P > 0.05). Chronic quadriceps weakness in this sample was primarily related to peripheral changes in the quadriceps muscle, not to levels of voluntary activation or antagonistic hamstrings activity.
Keywords: Voluntary activation, Knee strength, Quadriceps, Electromyography, Coactivation
Introduction
Severe quadriceps weakness develops rapidly after ACL injury and surgery.1,2 The recovery of quadriceps strength is often incomplete even years after surgery. Quadriceps strength deficits from 2% to 20% have been reported in subjects more than two years post ACL surgery.3 While it is evident that quadriceps weakness is a common finding in people with ACL reconstruction, the underlying mechanisms for such weakness are currently unclear. Reports from people with acute ACL injury indicate that quadriceps atrophy and activation failure are the primary contributing factor for quadriceps weakness.1 Scientists have also suggested that an inability to completely activate the quadriceps muscle (i.e., voluntary activation failure) is a primary cause of chronic quadriceps weakness in people after ACL reconstruction.3-5 However, this premise has not been verified experimentally.
The net moment generated at a joint during maximal contractions is the algebraic sum of the moments produced by the agonist and antagonist muscle groups and is largely determined by the anatomical, physiological and biomechanical parameters of the muscle-tendon units spanning the joint.6 Accordingly, it is reasonable that neuromuscular adaptations subsequent to knee joint trauma such as quadriceps atrophy, activation failure, and altered levels of antagonist hamstrings activation contribute to the chronic quadriceps weakness often observed after ACL reconstruction. Identifying the mechanisms responsible for persistent knee extensor weakness is meaningful as this knowledge may help clinicians and scientists develop more effective intervention strategies for people who sustain intra-articular knee injuries or undergo knee joint surgery. Therefore, the purpose of this study was to assess the magnitude of quadriceps weakness in subjects two to fifteen years post ACL reconstruction and to test the hypothesis that chronic quadriceps weakness is a product of morphological and physiological changes of the quadriceps muscle (as determined by electrically evoked torque), incomplete voluntary quadriceps muscle activation, and increased antagonist hamstrings moment during knee extensor strength testing.
Materials and Methods
Subjects
Thirty (15 ACL reconstructed, 15 uninjured controls) active people (Tegner Activity Score ≥ 4) between 19 and 38 years of age volunteered to participate in this study. The ACL reconstructed and control subjects were matched by age (± 3 years), activity level (± 1 Tegner Activity score), height (± 5%), weight (± 10%), sex, and ethnicity. Exclusion criteria for ACL reconstructed subjects included ACL reconstruction less than two years or more than fifteen years previously, reports of failure to participate in physical rehabilitation after ACL surgery, history of injury or surgery to the contralateral knee, reports of injury or surgery to the reconstructed knee following ACL reconstruction, and history of posterior cruciate ligament injury. Exclusion criteria for both groups included an inability to perform resisted knee extension without significant pain (> 30 mm on a 100 mm visual analog scale), fracture of the pelvis, femur, tibia, fibula, or patella within the past two years, history of lower body nerve injury, lumbar radiculopathy, neurological disorder, and diabetes.
All subjects provided written consent to participation using an informed consent document approved by the University of Iowa Human Subjects Research Institutional Review Board. After obtaining consent to participation, a brief physical evaluation was performed to confirm that the subjects had no signs of conditions that would exclude them from participation. Subjects then completed the Knee Injury and Osteoarthritis Outcomes Survey (KOOS).
Testing Procedures
Knee Extensor Strength and Activation Testing
Subjects were asked to refrain from any strenuous physical activity for 24 hours prior to testing. Testing began by having subjects perform a five minute warm-up on a cycle ergometer. Subjects were then positioned on a HUMAC NORM Testing and Rehabilitation System (Computer Sports Medicine, Inc., Stoughton, MA, USA) with their hips and knees flexed at 90° of flexion.7-9 Knee extensor strength and voluntary activation of the quadriceps muscle were assessed using an interpolated triplet technique.10 Before testing, subjects were provided with several practice trials to familiarize them with test methods and to potentiate the quadriceps muscles.
Subjects performed three maximum voluntary isometric contractions (MVIC) of the knee extensors and flexors in an alternating fashion with three minutes of rest between each like trial. Loud verbal encouragement and visual feedback of the real-time torque were provided to facilitate maximal effort. A train of electrical pulses (three pulse, 100 Hz, 200 μs pulse duration, 400 V) at a predetermined subject-specific current intensity was superimposed on the subjects' maximal voluntary knee extension efforts using a constant current muscle and nerve stimulator (model DS7AH, Digitimer Ltd, Hertfordshire, England).11 A second stimulus was provided four seconds after the completion of the maximal contraction to obtain potentiated evoked knee extensor torque at rest. The electrical stimuli used during testing were delivered through two self-adhesive stimulating electrodes (2.75 × 5.00 inches, Dura-Stick II, Chattanooga Group, Hixon, TN, USA) applied over the vastus lateralis muscle proximally and largest part of the vastus medialis distally.12 The stimulator was triggered using an automated torque-based triggering approach.13 The trial that produced the highest voluntary torque was used in further analysis. The electromechanical delay between the stimulus delivery and the actual onset of evoked torque was taken into account when calculating voluntary activation values (Figure 1).14 Voluntary activation of the quadriceps muscle was determined for each leg using the following formula.10
Figure 1.
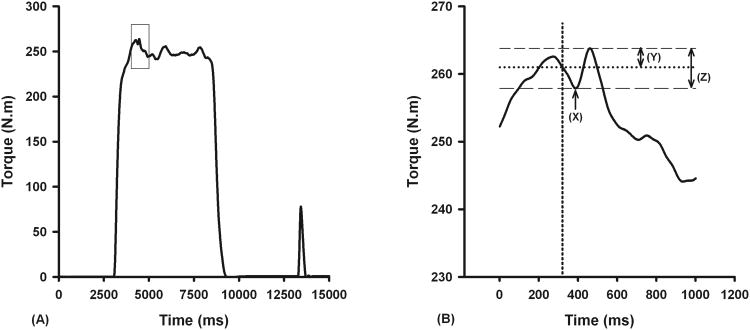
Figures illustrating the method used to determine the amplitude of evoked torque used when calculating percent voluntary activation. The right panel (B) depicts the close-up view of the rectangular region in the left panel (A). Voluntary activation was calculated by accounting for the electromechanical delay between the stimulus delivery (vertical dotted line) and the actual onset of the evoked torque (X). The amplitude of the evoked torque during MVIC would be underestimated if the electromechanical delay was not accounted for (Y vs. Z). In this subject, voluntary quadriceps muscle activation was 92.3% when electromechanical delay was accounted for and 96.4% when electromechanical delay was not accounted for.
| [1] |
Speed properties of the quadriceps muscle (rate of torque rise and fall) were assessed to determine if there were chronic peripheral adaptations in the quadriceps muscle associated with its metabolic and enzymatic function using the potentiated evoked torque at rest.15 The rate of torque rise and rate of torque fall were divided by the peak evoked torque at rest to obtain normalized rates in ms-1.
Muscle Specific EMG-Moment Relationships
After determining the subjects' isometric knee extensor and flexor strength, muscle specific EMG-moment relationships for the hamstrings muscles were obtained by having subjects match torque targets at four normalized moment magnitudes (10%, 20%, 30%, and 50% of peak voluntary flexor torque). Subjects viewed the moment targets and real-time feedback of their target matching efforts on a LCD monitor placed in front of them. To match the targets, subjects attempted to position the real-time torque curves associated with force they generated against the dynamometer's torque arm over a linear torque target displayed on the monitor in front of them. Subjects were provided with two practice trials to familiarize them with the test methods and minimize the effects of task novelty and learning. Subjects then matched targets at the four moment magnitudes. The moment targets were presented in random order to minimize systematic error associated with the presentation of target loads. Five seconds of data were collected during each trial once subjects achieved relative stability in their torque curves. Two trials were performed at each target magnitude. The average of the two trials was used in analysis.
Both legs of the ACL reconstructed subjects were tested in an identical fashion and the order of testing subjects' legs was randomized a priori in order to minimize effects associated with the order of testing. The control group subjects underwent testing on one side. The side selected for testing was based on the reconstructed leg of the subject that the control subject was matched to.
Antagonist Hamstrings Muscle Activity
Hamstrings activity during knee extension trials was evaluated using two surface EMG-preamplifiers (model 544, Therapeutics Unlimited, Iowa City, IA) applied over the bellies of semitendinosus and biceps femoris longus muscles. EMG signals were low-pass filtered at 500 Hz using an 8th order analog Butterworth filter (SCXI-1143, National Instruments Corporation, Austin, TX, USA). After removing the baseline offset values, EMG signals were rectified and smoothened using a recursive 8th order digital Butterworth filter with a 6 Hz cut-off. The mean activity over the 250 ms window immediately preceding peak torque was used in the analysis. The magnitudes of medial and lateral hamstrings activity recorded during peak knee extensor trials were normalized to peak values obtained from the respective muscles during flexor trials. The activation values of the medial and lateral hamstrings muscles were later averaged to determine the overall hamstrings activity during knee extension.
Modeling of Antagonist Muscle Moment
The contribution of medial and lateral hamstrings muscles to the total agonist flexor moment was partitioned based on the relative size, activation, and moment arm of the muscles.16,17 The details of the muscle moment modeling are described in the supplementary material. We examined the effects of antagonistic hamstrings muscle activity on side-to-side knee extensor torque ratios by correcting the observed voluntary peak knee extensor torque values for predicted moments generated by the hamstrings muscles during strength testing. The following equation was used in this analysis:
| [3] |
where corrected torque is the peak knee extensor torque after correcting for the antagonist torque generated by the hamstrings muscles, observed torque is the observed peak extensor torque during isometric knee strength testing, and antagonist torque is the calculated antagonist torque generated by the hamstrings muscles.
Data Analysis
Statistical analyses were performed using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were calculated for each variable. The 95% confidence intervals (CI) for the torque and activation measurements were estimated by Bootstrap resampling with replacements (5000 Bootstrap iterations).18 Paired t-tests were used to evaluate if significant differences existed between the ACL reconstructed and the non-reconstructed legs. Two sample t-tests were used to evaluate whether or not significant differences existed in the demographic profiles of the ACL reconstructed and control subjects and also to detect significant inter-group differences (reconstructed vs. control legs). Multiple linear regression was performed to determine the variables (side-to-side differences in triplet evoked torque, voluntary activation, and antagonist hamstrings moment) that significantly explained side-to-side differences in knee extensor strength. Pearson product moment correlation coefficient was used to evaluate the association between side-to-side triple evoked torque ratio and knee extensor peak torque ratio. A significance level of α = 0.05 was used for statistical analyses.
Results
The mean age, height, weight, and activity level between groups were similar indicating that the subjects were adequately matched (Table 1). The clinical characteristics of the subjects are provided in S-Table 1. The ACL reconstructed subjects had significantly lower scores than the control group in all the five subscales of the KOOS (Table 2).
Table 1.
Physical characteristics of the subjects.
| Variable | Sex | n | Mean | SD | P |
|---|---|---|---|---|---|
| Age (years) | ACLR | 15 | 24.73 | 4.98 | 1.000 |
| Control | 15 | 24.73 | 3.71 | ||
| Total | 30 | 24.73 | 4.32 | ||
|
| |||||
| Height (m) | ACLR | 15 | 1.75 | 2.81 | 0.951 |
| Control | 15 | 1.75 | 3.06 | ||
| Total | 30 | 1.75 | 2.89 | ||
|
| |||||
| Weight (Kg) | Female | 15 | 77.99 | 27.42 | 0.338 |
| ACLR | 15 | 74.15 | 19.46 | ||
| Control | 30 | 76.07 | 23.75 | ||
|
| |||||
| BMI (Kg/m2) | ACLR | 15 | 25.49 | 3.41 | 0.259 |
| Control | 15 | 24.3 | 2.10 | ||
| Total | 30 | 24.9 | 2.85 | ||
|
| |||||
| Activity (Hrs/Week) | ACLR | 15 | 8.77 | 5.64 | 0.777 |
| Control | 15 | 9.29 | 4.23 | ||
| Total | 30 | 9.03 | 4.90 | ||
|
| |||||
| Tegner Activity Score | ACLR | 15 | 5.73 | 0.70 | 0.407 |
| Control | 15 | 5.93 | 0.59 | ||
| Total | 30 | 5.83 | 0.65 | ||
|
| |||||
| Marx Activity Score | ACLR | 15 | 10.13 | 2.17 | 0.217 |
| Control | 15 | 11.13 | 2.17 | ||
| Total | 30 | 10.63 | 2.19 | ||
|
| |||||
| Laxity (mm) | ACLR | 15 | 2.18 | 1.39 | 0.001* |
| Control | 15 | 0.73 | 0.70 | ||
| Total | 30 | 1.46 | 1.31 | ||
Height in meters, Body weight in kilograms, Activity in hours/week, Laxity in millimeters of tibial translation (absolute side-to-side differences) measured during manual maximal tests using KT-2000™ ligament arthrometer.
Table 2. Knee Injury and Osteoarthritis Outcomes Survey scoresa.
| KOOS Domain | ACLR | Control | P-value |
|---|---|---|---|
| Pain | 95.9 ± 3.2 | 99.5 ± 2.1 | 0.002 |
| Symptom | 90.0 ± 9.6 | 96.3 ± 4.9 | 0.030 |
| ADL | 99.2 ± 1.7 | 100.0 ± 0.0 | 0.048 |
| Sports & Recreation | 91.0 ± 6.6 | 99.3 ± 2.6 | < 0.001 |
| Quality of Life | 81.4 ± 15.1 | 100.0 ± 0.0 | < 0.001 |
Data are presented as mean ± SD. Higher scores in KOOS denote greater levels of function and lower knee symptoms.
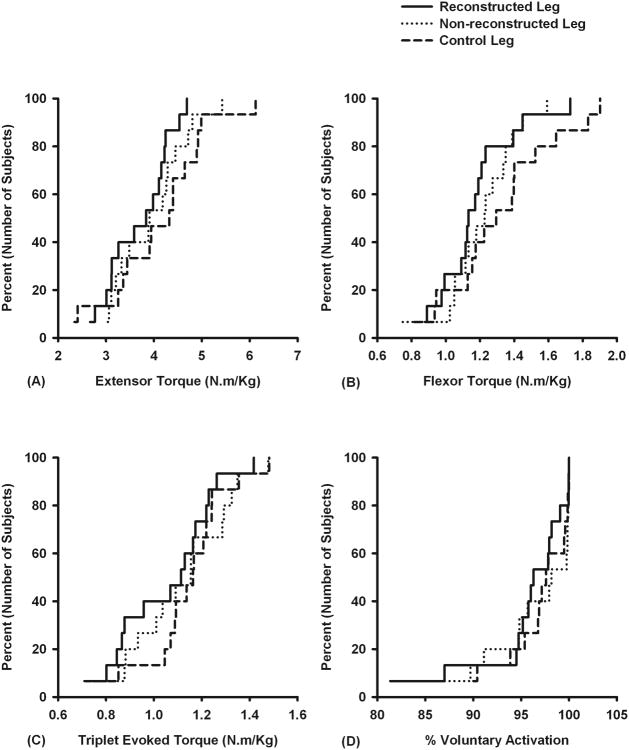
The knee extensor torque and potentiated evoked torque of the subjects' ACL reconstructed legs were significantly lower than their non-reconstructed legs (P = 0.023 & P = 0.016, Table 3). The mean estimates of voluntary quadriceps muscle activation were similar between subjects' ACL reconstructed and non-reconstructed legs (P = 0.445, Table 3 and Figure 2D). The magnitudes of antagonistic hamstrings activity and associated estimates of hamstrings torque during knee extensor strength testing observed in subjects' ACL reconstructed and non-reconstructed legs were not significantly different (P = 0.662 and P = 0.391, Table 3). The side-to-side knee extensor torque ratios of the ACL reconstructed group did not change significantly when antagonistic hamstrings torque values were accounted for (93.9 ± 8.9% vs. 93.6 ± 9.3%, P = 0.488). There were also no side-to-side differences in the mean normalized rate of torque rise (0.0097 ± 0.0012 ms-1 vs. 0.0097 ± 0.0013 ms-1, P = 0.645) and torque fall (0.0058 ± 0.0014 ms-1 vs. 0.0056 ± 0.0011 ms-1, P = 0.287). No significant differences were observed between the values of the subjects' ACL reconstructed legs and those from the legs of the control subjects for any of the variables (P = 0.156 to P = 0.803, Table 3). Cumulative distribution functions for peak knee extensor torque, peak knee flexor torque, and triplet evoked torque showed a reduction in the torque (leftward shift) when the functions for the subjects' reconstructed legs were compared with those for their non-reconstructed legs and those for the control subjects legs (Figures 2A, 2B, & 2C).
Table 3. Strength and activation profiles of ACL reconstructed and control subjectsa.
| Variable | ACLR | Non-ACLR | Control |
|---|---|---|---|
| Extensor Peak Torque | 287.2 ± 66.2 (253.7 – 319.9) | 307.3 ± 74.1 (270.7 – 344.4) | 304.1 ± 84.2 (261.1 – 345.7) |
| Flexor Peak Torque | 90.5 ± 17.6 (82.2 – 99.7) | 93.7 ± 18.5 (84.4 – 103.2) | 97.6 ± 25.9 (85.0 – 110.8) |
| Triplet Evoked torque | 82.7 ± 20.9 (72.3 – 93.3) | 88.2 ± 22.8 (76.8 – 100.0) | 84.5 ± 17.2 (76.0 – 93.0) |
| Normalized Extensor Torque | 3.67 ± 0.66 (3.35 – 4.02) | 3.94 ± 0.73 (3.58 – 4.32) | 4.09 ± 1.02 (3.59 – 4.61) |
| Normalized Flexor Torque | 1.17 ± 0.22 (1.07 – 1.29) | 1.21 ± 0.21 (1.10 – 1.31) | 1.32 ± 0.32 (1.16 – 1.48) |
| Normalized Triplet Evoked Torque | 1.06 ± 0.20 (0.96 – 1.16) | 1.12 ± 0.20 (1.03 – 1.23) | 1.14 ± 0.19 (1.04 – 1.23) |
| Voluntary Activation | 95.6 ± 5.1% (92.7 – 97.8%) | 96.5 ± 4.4% (94.3 – 98.6%) | 97.0 ± 3.3% (95.2 – 98.5%) |
| Antagonist Activity | 10.3 ± 6.4% (6.9 – 12.6%) | 9.5 ± 5.0% (7.0 – 11.9%) | 12.9 ± 7.5% (9.3 – 17.1%) |
| Antagonist Moment | 11.5 ± 7.9% (7.2 – 14.7%) | 13.4 ± 8.5% (9.0 – 17.1%) | 15.9 ± 9.2% (11.8 – 21.0%) |
Data are presented as mean ± SD. Values on parentheses represent 95% confidence intervals obtained from bootstrap resampling method. Units = Peak torque, N·m; Normalized peak torque, N·m/Kg. Abbreviations = ACLR, reconstructed legs; Non-ACLR, non-reconstructed legs; Control, control subjects' legs
Figure 2.
Cumulative distribution functions of the normalized peak knee extensor torque (A), flexor torque (B), triplet evoked knee extensor torque at rest (C), and voluntary quadriceps muscle activation (D). Torque values were normalized to subject's body mass (continuous lines: ACL reconstructed legs; dotted lines: ACL non-reconstructed legs; broken lines: control legs)
Regression analyses revealed that side-to-side differences in evoked knee extensor torque at rest explained 71% of the knee extensor strength differences by side (P < 0.001, Figure 3A), whereas side-to-side differences in voluntary quadriceps activation and antagonistic hamstrings moment did not contribute significantly to observed strength differences by side (P = 0.931 and P = 0.578). A strong linear relationship was observed between the side-to-side evoked torque ratio and voluntary knee extensor peak torque ratio (Figure 3B).
Figure 3.
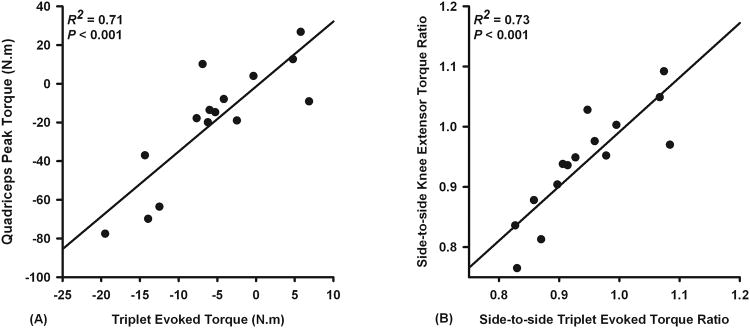
Scatterplots demonstrating that quadriceps weakness after ACL reconstruction (2 to 15 years) is related to the peripheral changes in the quadriceps muscle-tendon unit. Regression analysis revealed that side-to-side differences in electrically evoked torque at rest explained 71% of variations in knee extensor strength differences by side (A). Scatterplot demonstrating a linear relationship between side-to-side evoked torque and peak voluntary torque ratios (B). Side-to-side ratios are obtained by expressing values of reconstructed legs as a ratio of non-reconstructed legs.
Discussion
This study adds to the existing knowledgebase in at least three distinct ways. First, it provides evidence that chronic quadriceps weakness is minimal in the majority of people who undergo ACL reconstruction and related to peripheral changes in the muscle. Second, it provides evidence indicating that voluntary quadriceps muscle activation returns to normal levels and contributes little to persistent quadriceps weakness after ACL reconstruction, which is contrary to the existing belief that quadriceps activation failure is the source of persistent quadriceps weakness after ACL reconstruction. Finally, the detailed neuromuscular testing performed in this sample provides a clearer picture of long-term neuromuscular adaptations that occur after ACL reconstruction.
Quadriceps weakness is commonly reported after ACL injury and reconstruction.3 This weakness and associated dysfunction have been implicated as a source of reduced knee related quality of life, increased risk of knee osteoarthritis, and long-term functional disability.4,19-21 Our sample displayed a mean of 6% side-to-side quadriceps weakness in their reconstructed legs. Seventy-three percent of the subjects (11 out of 15) had side-to-side knee extensor strength ratios of ≥ 90%. These findings are consistent with the results of previous studies incorporating isokinetic testing.19,22,23 The clinical significance of this strength difference remains uncertain. Our data and those from the recent literature19,22 suggest that quadriceps muscle strength returns to near normal levels in the majority (about 70%) of people who undergo modern ACL reconstruction procedures. It is important, however, to consider the 30% of the population who have more substantial weakness. It is unclear why this subset of the population has greater quadriceps weakness and whether these individuals are more prone to poorer long term knee health outcomes.
The findings from this study provide new insight into mechanisms contributing to chronic quadriceps weakness after ACL reconstruction. The data for evoked knee extensor torque at rest suggest that most of the observed strength deficits are related to peripheral changes in the muscle-tendon units of the quadriceps muscle. These peripheral changes may include chronic atrophy, changes in the compliance of the series elastic components of the muscle-tendon units, and alterations in the architectural structure and composition (fiber type) of the quadriceps muscle. We observed significant side-to-side differences in the magnitude of evoked quadriceps muscle torque at rest, but not in contractile speed properties. This suggests that the observed quadriceps weakness was likely a result of persistent quadriceps atrophy and associated changes in the muscle-tendon units rather than metabolic or enzymatic adaptations. Unfortunately, we cannot partition the contributions of quadriceps atrophy from other changes in the muscle-tendon units as such an analysis would require detailed information on each subjects' muscle morphology.
Quadriceps activation failure has been implicated as a source of lingering quadriceps weakness after ACL injury.3 This is understandable based on the large body of evidence demonstrating severe voluntary activation deficits early after ACL injury and reconstruction.1,9 However, there is little evidence regarding whether chronic quadriceps activation failure is a problem for patients in the years after ACL reconstruction. Such activation failure has been implicated as a potential contributor to the pathoetiology of post-traumatic knee osteoarthritis after ACL injury.4 Some support for this idea is provided by a prospective study indicating that recovery of voluntary quadriceps muscle activation is incomplete two years after surgery despite significant improvement over time.9 Our results, however, indicate that voluntary quadriceps activation recovered to normal levels in our sample and did not contribute significantly to the residual quadriceps weakness observed. The finding of “normal” levels of quadriceps muscle activation in our ACL reconstructed sample is not surprising considering that the subjects did not report knee pain or display signs of knee effusion, which are postulated to be primary sources of arthrogenic reflex inhibition.3,24,25 It remains unclear if chronic quadriceps muscle activation failure is present in people with poorer outcomes following ACL reconstruction.
Coactivation of antagonist muscle groups is a common feature associated with various types of joint loading.26 Hamstrings muscles are believed to assume the role of joint stabilizers in people with ACL pathology.27,28 Altered hamstrings activity during maximal knee extension has been reported in subjects with ACL reconstruction.27,29 Such altered activity, though important for stability, may produce a countermoment that would affect knee strength test results. Researchers have reported that the countermoment associated with hamstrings muscle activity during isometric knee extensor strength tests is about 10% of the peak torque values produced when the muscles are agonists.16,17 Although this countermoment may lead to an underestimation of actual knee extensor strength, the accuracy of side-to-side comparisons of knee strength tests would not be affected unless significant differences in antagonist muscle activity were present across sides. A recent research report in healthy young people indicates that although the magnitude of antagonistic hamstrings activity recorded during strength tests is sufficient to warrant analysis, the effect on observed side-to-side differences in strength measurements is negligible.30 The literature lacks evidence on whether antagonist activity plays a role in side-to-side differences in knee strength after knee trauma. Our study helps fill this gap. We used an established EMG-moment model16 to estimate the countermoments associated with antagonistic hamstrings activity during knee extensor strength testing. Limb symmetry indices did not change significantly when countermoments associated with hamstrings coactivation were accounted for. Regression analysis indicated side-to-side differences in antagonistic hamstrings moment contributed little to observed strength differences by side.
One of the strengths of this study is its inclusion of a matched control group. Previous studies that have investigated knee strength in patients several years post ACL reconstruction have used contralateral limb strength alone as the reference for evaluating muscle weakness.19,21,22 However, evidence indicates that unilateral ACL injury results in bilateral quadriceps weakness and activation failure.9 This bilateral phenomenon has been shown to persist for at least two years after ACL reconstruction.9 Therefore, the use of contralateral limb strength as a reference to measure knee strength is less than ideal. The inclusion of a matched control group allowed us to further examine the strength and neuromuscular profile of our sample. In general, the non-reconstructed legs' results fell between those of the reconstructed and control legs and were similar to those of the control legs. This suggests that the non-reconstructed legs may serve as a stable reference when evaluation is performed two or more years after ACL reconstruction.
This study has some potential limitations that warrant discussion. We only included active people with little knee pain who were active in fitness activities or sports (Tegner Activity Level ≥ 4). Potential subjects who reported injuries to the reconstructed knee and those who had additional surgery to that knee following their primary ACL reconstruction were excluded from participation. We felt that these were important control procedures considering our study was to be performed at a single site and would therefore have relatively a small sample. This approach resulted in a sample of relatively high functioning individuals, which may limit the generalizability of the results. We note, however, that our knee strength results are consistent with those of other studies in the literature.19,21,22 Regardless of this fact, we acknowledge that people with symptomatic knees may belong to a different subset of the ACL reconstruction population than our sample and have greater strength and activation deficits than were observed in the present study.
Strength and activation testing was performed at 90° of knee flexion. Our results are specific to this angle and may not be characteristic of findings at other angles as knee joint position influences quadriceps strength and voluntary activation.8 We did not exclude subjects based on graft types as the primary aim of this study was to provide insight into central and peripheral contributions of muscle weakness. There is currently no consensus regarding the best graft choice for ACL surgery. It is reasonable that different surgical approaches may have different effects on muscle. The current investigation was not large enough to allow an appropriate analysis of the effect of ACL graft choice on long-term outcome and neuromuscular status. Finally, the muscle parameter inputs to the mathematical model16 used to predict antagonistic hamstrings torque were values in the literature from healthy individuals similar to the typical ACL injury population rather than values from our sample. Hence, the results of the model are reasonable estimates rather than specific estimates.
In conclusion, the results of this study indicated that side-to-side differences in electrically evoked torque at rest explained a large proportion of variation (∼71%) in knee strength differences by side, suggesting that residual quadriceps weakness after ACL reconstruction is primarily associated with peripheral adaptations in the quadriceps muscle. Quadriceps activation deficits were small and did not contribute significantly to our sample's strength deficits, calling into question the commonly held view that quadriceps activation failure is the primary source of chronic quadriceps weakness in people with ACL reconstruction. Antagonistic hamstrings muscle activity was similar across legs (reconstructed and non-reconstructed), between groups (ACL reconstructed vs. control), and did not affect side-to-side quadriceps strength ratios.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Abby Erion, April Hoffmeyer, Daniel Ludgate, and Kelly Michaels for their assistance with data collection. This work was supported in part by NIH grant K12 HD055931 and the Davee Foundation.
References
- 1.Williams GN, Buchanan TS, Barrance PJ, et al. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33:402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 2.Yasuda K, Ohkoshi Y, Tanabe Y, Kaneda K. Muscle weakness after anterior cruciate ligament reconstruction using patellar and quadriceps tendons. Bull Hosp Jt Dis Orthop Inst. 1991;51:175–185. [PubMed] [Google Scholar]
- 3.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27:405–424. vii–ix. doi: 10.1016/j.csm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37:147–153. doi: 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- 5.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45:87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enoka RM. Neural adaptations with chronic physical activity. J Biomech. 1997;30:447–455. doi: 10.1016/s0021-9290(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 7.Williams GN, Barrance PJ, Snyder-Mackler L, et al. Specificity of muscle action after anterior cruciate ligament injury. J Orthop Res. 2003;21:1131–1137. doi: 10.1016/S0736-0266(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 8.Becker R, Awiszus F. Physiological alterations of maximal voluntary quadriceps activation by changes of knee joint angle. Muscle Nerve. 2001;24:667–672. doi: 10.1002/mus.1053. [DOI] [PubMed] [Google Scholar]
- 9.Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris a prospective twitch interpolation study. J Bone Joint Surg Br. 2001;83:1104–1110. doi: 10.1302/0301-620x.83b8.11618. [DOI] [PubMed] [Google Scholar]
- 10.Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan C, Williams GN. Sex differences in quadriceps and hamstrings EMG-moment relationships. Med Sci Sports Exerc. 2009;41:1652–1660. doi: 10.1249/MSS.0b013e31819e8e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmielewski TL, Stackhouse S, Axe MJ, Snyder-Mackler L. A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res. 2004;22:925–930. doi: 10.1016/j.orthres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan C, Allen EJ, Williams GN. Torque-based triggering improves stimulus timing precision in activation tests. Muscle Nerve. 2009;40:130–133. doi: 10.1002/mus.21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oskouei MA, Van Mazijk BC, Schuiling MH, Herzog W. Variability in the interpolated twitch torque for maximal and submaximal voluntary contractions. J Appl Physiol. 2003;95:1648–1655. doi: 10.1152/japplphysiol.01189.2002. [DOI] [PubMed] [Google Scholar]
- 15.Dudley-Javoroski S, Littmann AE, Iguchi M, Shields RK. Doublet stimulation protocol to minimize musculoskeletal stress during paralyzed quadriceps muscle testing. J Appl Physiol. 2008;104:1574–1582. doi: 10.1152/japplphysiol.00892.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellis E, Katis A. Hamstring antagonist moment estimation using clinically applicable models: Muscle dependency and synergy effects. J Electromyogr Kinesiol. 2008;18:10. doi: 10.1016/j.jelekin.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan C, Williams GN. Error associated with antagonist muscle activity in isometric knee strength testing. Eur J Appl Physiol. 2010;109:527–536. doi: 10.1007/s00421-010-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efron B. Bootstrap methods:Another look at the jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 19.Ageberg E, Thomee R, Neeter C, et al. Muscle strength and functional performance in patients with anterior cruciate ligament injury treated with training and surgical reconstruction or training only: a two to five-year followup. Arthritis Rheum. 2008;59:1773–1779. doi: 10.1002/art.24066. [DOI] [PubMed] [Google Scholar]
- 20.Morrissey MC, Hooper DM, Drechsler WI, Hill HJ. Relationship of leg muscle strength and knee function in the early period after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2004;14:360–366. doi: 10.1046/j.1600-0838.2003.366.x. [DOI] [PubMed] [Google Scholar]
- 21.Moisala AS, Jarvela T, Kannus P, Jarvinen M. Muscle strength evaluations after ACL reconstruction. Int J Sports Med. 2007;28:868–872. doi: 10.1055/s-2007-964912. [DOI] [PubMed] [Google Scholar]
- 22.Lautamies R, Harilainen A, Kettunen J, et al. Isokinetic quadriceps and hamstring muscle strength and knee function 5 years after anterior cruciate ligament reconstruction: comparison between bone-patellar tendon-bone and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2008;16:1009–1016. doi: 10.1007/s00167-008-0598-7. [DOI] [PubMed] [Google Scholar]
- 23.Lund-Hanssen H, Gannon J, Engebretsen L, et al. Isokinetic muscle performance in healthy female handball players and players with a unilateral anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 1996;6:172–175. doi: 10.1111/j.1600-0838.1996.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 24.Fahrer H, Rentsch HU, Gerber NJ, et al. Knee effusion and reflex inhibition of the quadriceps. A bar to effective retraining. J Bone Joint Surg Br. 1988;70:635–638. doi: 10.1302/0301-620X.70B4.3403614. [DOI] [PubMed] [Google Scholar]
- 25.Konishi Y, Aihara Y, Sakai M, et al. Gamma loop dysfunction in the quadriceps femoris of patients who underwent anterior cruciate ligament reconstruction remains bilaterally. Scand J Med Sci Sports. 2007;17:393–399. doi: 10.1111/j.1600-0838.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- 26.Kellis E. Quantification of quadriceps and hamstring antagonist activity. Sports Med. 1998;25:37–62. doi: 10.2165/00007256-199825010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Osternig LR, Caster BL, James CR. Contralateral hamstring (biceps femoris) coactivation patterns and anterior cruciate ligament dysfunction. Med Sci Sports Exerc. 1995;27:805–808. [PubMed] [Google Scholar]
- 28.Tibone JE, Antich TJ. Electromyographic analysis of the anterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1993:35–39. [PubMed] [Google Scholar]
- 29.Grabiner MD, Weiker GG. Anterior cruciate ligament injury and hamstrings coactivation. Clin Biomech (Bristol, Avon) 1993;8:215–219. doi: 10.1016/0268-0033(93)90017-C. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan C, Williams GN. Evoked tetanic torque and activation level explain strength differences by side. Eur J Appl Physiol. 2009;106:769–774. doi: 10.1007/s00421-009-1057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tate CM, Williams GN, Barrance PJ, Buchanan TS. Lower extremity muscle morphology in young athletes: an MRI-based analysis. Med Sci Sports Exerc. 2006;38:122–128. doi: 10.1249/01.mss.0000179400.67734.01. [DOI] [PubMed] [Google Scholar]
- 32.Buford WL, Jr, Ivey FM, Jr, Malone JD, et al. Muscle balance at the knee--moment arms for the normal knee and the ACL-minus knee. IEEE Trans Rehabil Eng. 1997;5:367–379. doi: 10.1109/86.650292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.