Abstract
Previous studies have demonstrated that healthy young people typically have side-to-side differences in knee strength of about 10% when the peak torque generated by the stronger leg is contrasted with that of the weaker leg. However, the mechanisms responsible for side-to-side differences in knee strength have not been clearly defined. The current study tested the hypothesis that side-to-side knee extensor strength differences are explained by inter-limb variations in voluntary activation, antagonistic hamstrings activity, and electrically evoked torque at rest. Twenty-two volunteers served as subjects. Side-to-side differences in quadriceps activation and electrically evoked knee extensor torque explained 69% of the strength differences by side. Antagonistic hamstrings activity did not contribute significantly. The results suggest both central and peripheral mechanisms contribute to inter-limb variations in strength.
Keywords: Central activation, Antagonist Muscle Activity, Knee Strength, Contractile Properties, ITT
Introduction
Side-to-side strength ratios are commonly used to assess knee strength in human performance testing, clinical practice, and in research related to knee disorders (Palmieri-Smith et al. 2008; Petterson et al. 2008; Williams et al. 2005). Side-to-side knee extensor strength differences of 6% to 19% have been reported when the peak torque generated by the stronger legs of healthy young people was contrasted with the peak torque generated by their weaker legs (Goslin and Charteris 1979; Kvist 2004; Ostenberg et al. 1998). The mechanisms underlying these side-to-side strength differences are unclear. Elucidating these mechanisms is expected to further our understanding of quadriceps muscle function and to have meaningful implications for knee extensor strength assessment in human performance testing, clinical practice, and research.
The net torque generated at a joint during maximal contractions is largely determined by the anatomical, physiological and biomechanical parameters of the musculotendinous units spanning the joint (Enoka 1988, 1997; Lieber 1992; Macaluso et al. 2002). Accordingly, joint strength is determined not only by size of the contracting muscles, but may also be influenced by activation levels of the agonist muscles and coactivation of antagonist muscle groups. This is substantiated by evidence demonstrating disproportionate loss in muscle strength relative to muscle mass following aging, disuse, immobilization, and joint trauma (Berg et al. 1991; Macaluso et al. 2002; Narici et al. 2003; Stevens et al. 2006; Williams et al. 2005). Moreover, higher voluntary activation of the agonist muscles and decreased coactivation of the antagonist muscles have been shown to parallel training-induced gains in muscle strength (Carolan and Cafarelli 1992; Hakkinen et al. 2000; Hakkinen et al. 1998). Together, these results suggest that side-to-side differences in strength are a product of neuromuscular variability across sides. Therefore, the purpose of this study was to test the hypothesis that inter-limb variations in voluntary activation level of the quadriceps muscle, electrically evoked tetanic knee extensor torque at rest, and antagonistic hamstrings muscle activity are significant determinants of side-to-side knee extensor strength differences in healthy young people.
Methods
Subjects
Twenty-two subjects with no history of serious lower extremity injury volunteered to participate in this study. The sample consisted of 11 males (mean age: 24.0 ± 2.19 yrs, height: 1.80 ± 0.07 m, weight: 80.24 ± 8.40 kg) and 11 females (mean age: 24.36 ± 2.84 yrs, height: 1.65 ± 0.06 m, weight: 63.63 ± 4.96 kg) who were regular participants in sports or fitness activities (Tegner Activity Scores: male 6.45 ± 0.69, female 6.0 ± 1.0). Subjects were asked to refrain from any strenuous physical activity for 24 hours prior to testing. All subjects provided written consent to participation by signing an informed consent document approved by the University of Iowa Human Subjects Research Institutional Review Board.
Quadriceps Muscle Strength and Activation Testing
Knee extensor strength and voluntary quadriceps muscle activation were assessed with the knee joint fixed at 60° of flexion using a tetanic superimposed interpolated twitch technique (Behm et al. 2001). The tests were performed with subjects seated on a HUMAC NORM Testing and Rehabilitation System (Computer Sports Medicine, Inc., Stoughton, MA, USA) with the lateral epicondyle of the femur aligned with the axis of rotation of the servo motor. Subjects performed four sub-maximal contractions (50% to 85% maximum perceived effort) and one 5-second maximum voluntary isometric contraction (MVIC) to familiarize with the testing condition and potentiate the quadriceps muscles. After a two-minute rest period, subjects performed three 5-second MVICs of the knee extensors and flexors in an alternating fashion with three minutes of rest between each like trial. Loud verbal encouragement and visual feedback of real-time torque development were provided to facilitate maximal effort during the MVICs. Approximately three seconds after the onset of each knee extensor MVIC, a supramaximal train of electrical pulses (10 pulse, 100 Hz, 200 µs pulse duration, 400 V) at a predetermined current intensity was superimposed on the subjects’ maximal voluntary efforts through two adhesive stimulating electrodes (2.75 × 5.00 inches, Dura-Stick II, Chattanooga Group, Hixon, TN, USA) that were applied over the proximal and distal surface of the quadriceps muscles. The electrode leads were connected to a constant current muscle and nerve stimulator (model DS7AH, Digitimer Ltd, Hertfordshire, England). The subject specific current intensities used during testing were determined while the subjects were seated at rest by sequentially stimulating the quadriceps muscle using pulse trains with the electrical characteristics described above in current steps of 100 mA until the torque associated with the stimulus-induced contractions no longer increased, but decreased. Current was then reduced by 50 mA and a final stimulus was provided. The current intensity producing the greatest torque (evoked tetanic knee extensor torque at rest) was used during testing. Voluntary activation of the quadriceps muscle was determined for each leg using the following formula (Bampouras et al. 2006; Behm et al. 2001; Merton 1954; O'Brien et al. 2008):
| [1] |
Torque signals were corrected for the weight of the leg (gravity corrected). Both legs were tested independently in an identical fashion and the order in which the subjects’ legs were tested was randomized a priori in order to minimize potential effects of test order. The trial that produced the highest voluntary torque was used in analysis.
Antagonistic Hamstrings Muscle Activity
Antagonistic hamstrings muscle activity during knee extension trials was evaluated using two surface EMG preamplifiers (model 544, Therapeutics Unlimited, Iowa City, IA; 35x differential gain, 22 mm inter-electrode distance, 8 mm electrode diameter, 87 dB common-mode rejection at 60 Hz, input impedence > 25 MΩ, noise < 2 µV RMS) placed over the muscle bellies of semitendinosus and biceps femoris longus. Electrode placement sites were standardized according to the recommendations of Perotto (Perotto 1994). The potential for noise associated with pressure on the EMG preamplifers was minimized by seating the subjects on a small platform placed on the testing system’s chair, which caused the subjects’ weight to be transferred through the proximal third of their thigh and left the electrode placement sites unloaded. All signals were sampled at 1000 Hz using a PC with a 16-bit data acquisition card (PCI-6032E, National Instruments Corp., Austin, TX, USA) and LabVIEW software (version 7.0, National Instruments Corp., Austin, TX, USA). EMG signals were conditioned using an 8th order analog Butterworth lowpass filter (SCXI-1143, National Instruments Corp., Austin, TX, USA) with a 500 Hz cut-off. After removing baseline offset values, the EMG signals were processed by calculating root mean square (RMS) amplitudes over the 500 ms window immediately preceding peak torque. The magnitudes of medial and lateral hamstrings activity recorded during peak knee extensor trials were normalized to peak RMS values obtained from the respective muscles during flexion MVICs. The activation values of the medial and lateral hamstrings muscles were later averaged to determine the overall hamstrings activity during knee extension.
Data Analysis
Statistical analyses were performed using SPSS for Windows version 16.0 (SPSS Inc., Chicago, IL, USA). Doubly Multivariate Analysis of Variance was used to evaluate if there were significant differences in the magnitudes of voluntary activation, evoked tetanic torque, and hamstrings activity between stronger and weaker legs. Multiple linear regression with backward elimination was performed to determine the most significant factors (side-to-side differences in evoked tetanic torque, voluntary activation, and hamstrings activity) predicting side-to-side differences in knee extensor strength. The threshold for significance was set at α = 0.05.
Results
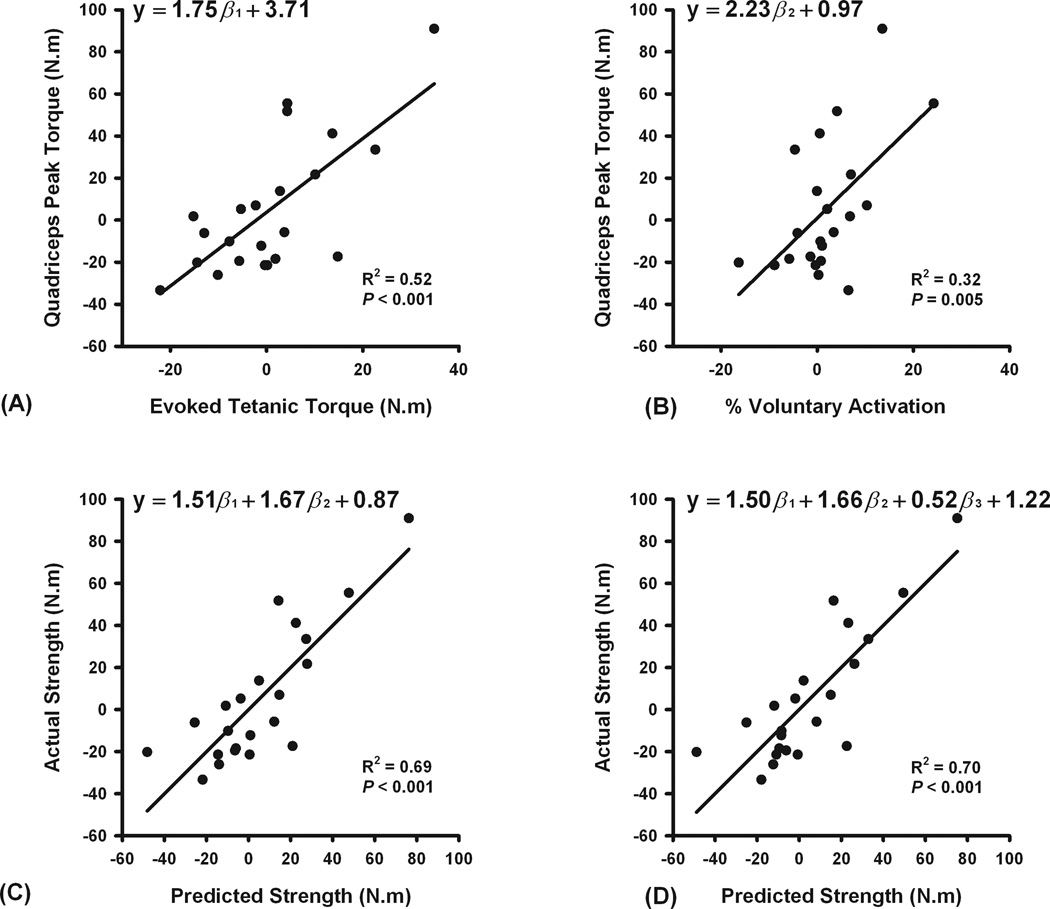
The subjects’ mean side-to-side knee extensor strength ratio (peak torque of weaker leg / peak torque of stronger leg * 100) was 91%. Approximately half the sample was stronger on each side (right leg: 10 subjects, left leg: 12 subjects). The side of dominance (preferred leg for kicking a ball) was not associated with which side was stronger (P = .892, Φ = −.029). The mean current intensity used in the burst superimposition tests was similar between legs (330 ± 73 mA for stronger legs, 320 ± 73 mA for weaker legs; median = 300 mA for each leg). Quadriceps muscle activation and evoked tetanic torque were significantly greater in the subjects’ stronger legs (voluntary activation: 94.2 ± 5.5% vs. 90.2 ± 8.0%, P = .017; evoked tetanic torque: 140.1 ± 39.0 N·m vs. 134.5 ± 36.6 N·m, P = 0.036). There were no significant differences, however, in the magnitude of hamstrings muscle activity between legs (10.9 ± 6.2% vs. 9.6 ± 5.6%, P = 0.305). Regression analyses revealed that side-to-side differences in electrically evoked tetanic torque at rest explained 52% of the knee extensor strength differences by side (P < 0.001, Figure 1A), whereas side-to-side voluntary activation differences explained 32% of the knee extensor strength differences by side (P = 0.005, Figure 1B). A regression model including both evoked tetanic torque and voluntary activation of the quadriceps muscle indicated that together these factors explain 69% of the side-to-side variation in knee extensor peak torque (P < 0.001, Figure 1C). Side-to-side differences in antagonistic hamstrings muscle activity did not contribute significantly to the observed strength differences by side (P = 0.458, Figure 1D).
Figure 1.
Scatterplots depicting the relative contributions of side-to-side differences (right leg values − left leg values) in electrically evoked tetanic torque (A), voluntary activation of the quadriceps muscle (B), electrically evoked tetanic torque and voluntary activation (C), and electrically evoked tetanic torque, voluntary activation, and antagonist hamstrings activity (D) to the observed strength differences by side (right leg values − left leg values). y = side-to-side differences in quadriceps peak torque, β1 = side-to-side differences in tetanic evoked torque, β2 = side-to-side differences in voluntary activation of the quadriceps muscle, and β3 = side-to-side differences in antagonist hamstrings activity. Note that inclusion of antagonist hamstrings activity in the model did not significantly affect the slopes of the equation or the coefficient of regression (1 C & 1 D).
Discussion
The purpose of this study was to determine the extent to which inter-limb variations in voluntary activation of the quadriceps muscle, antagonistic hamstrings muscle activity, and electrically evoked tetanic torque of the knee extensors at rest explain the side-to-side differences in knee extensor strength commonly observed in healthy young people. The main findings of this study are 1) inter-limb variations in voluntary activation and evoked tetanic torque of the quadriceps muscle explained 69% of the observed strength differences by side, and 2) antagonistic hamstrings activity did not contribute significantly to the observed side-to-side knee extensor strength discrepancies.
We found a 9% difference in strength between stronger and weaker legs. This finding is consistent with the results (6% to 19%) in previous studies (Goslin and Charteris 1979; Ostenberg et al. 1998). Side-to-side thigh muscle strength discrepancies have been suggested as a risk factor for lower-extremity injury (Knapik et al. 1992; Myer et al. 2006). Accordingly, scientists have recommended incorporating strength training programs that normalize muscle imbalances in lower extremity injury prevention programs (Hewett et al. 2001; Knight 1980; Myer et al. 2006). An understanding of the mechanisms underlying side-to-side strength differences may lead to more effective and efficient intervention. The current study adds to the knowledgebase by providing insight into the mechanisms contributing to side-to-side strength differences in knee extensor strength. The findings suggest that both central and peripheral mechanisms contribute to inter-limb variations in knee extensor strength. The observation of greater evoked tetanic torque and higher voluntary activation of the quadriceps muscle in the stronger legs of the subjects indicates that healthy young people have side-to-side differences in their neuromotor control and muscle morphology/architecture. The results of this study also provide evidence that reinforces the importance of taking quadriceps activation levels into consideration when administering knee extensor strength tests (Stevens et al. 2006; Williams et al. 2005).
Side-to-side differences in evoked tetanic torque can be attributed to several factors. Electrically evoked torque provides information on the physiological performance (force output) of a muscle/muscle group and is considered a surrogate measure of skeletal muscle strength and mechanical function (Brass et al. 1996; Lieber 1992; Spector et al. 1980). Evoked torque measurements may also provide insight on peripheral adaptations such as changes in muscle morphology or musuclotendinous architecture as this testing approach bypasses the central inputs to the muscle (Davies et al. 1985; Narici et al. 2003). Given the plasticity of the neuromuscular system, side-to-side differences in physiological stress may lead to different neuromuscular adaptations by side. Tate et al (Tate et al. 2006) have demonstrated that young athletic people usually have significant differences in quadriceps muscle morphology (e.g., the volumes of the individual muscles) between sides. Hence, it is expected that morphological differences in the individuals’ quadriceps muscles contributed to the observed side-to-side differences in evoked tetanic torque. Differences in the architecture and physiological function of the muscle fibers and connective tissues of the subjects’ right and left quadriceps muscle groups may also have contributed to the observed side-to-side variance in evoked torque as these factors are known to influence tetanic tension (Lieber 1992; Roos et al. 1999; Vivodtzev et al. 2005).
Several researchers have indicated that voluntary activation deficits and muscle atrophy contribute substantially to the observed weakness following aging, joint injury, or surgery (Morse et al. 2005; Stackhouse et al. 2001; Stevens et al. 2006; Stevens et al. 2003; Williams et al. 2005). However, there is little evidence that provides insight on whether antagonist muscle activity affects side-to-side strength indices. In theory, coactivation of the antagonist muscles during agonist muscle contractions will result in reduced net joint torque (Kellis 1998). Accordingly, it is believed that antagonist muscle activity reduces the magnitude of agonist strength estimates (Carolan and Cafarelli 1992; Hakkinen et al. 2000; Macaluso et al. 2002; Stackhouse et al. 2005). Experimental evidence demonstrating strength gains associated with training related reductions in agonist-antagonist coactivation support this idea (Carolan and Cafarelli 1992; Hakkinen et al. 2000; Hakkinen et al. 1998). Conversely, the results of the present study indicate that antagonist activity, though measurable and notable in magnitude, does not in fact have a significant effect on knee extensor strength in healthy young people. In fact, the magnitude of hamstrings muscle activity recorded from the subjects’ stronger legs during the maximal knee extension trials was slightly higher than the values recorded from their weaker legs. This suggests that the observed coactivity is either a byproduct of a generalized increase in neural drive during MVICs or it serves another purpose such as providing a stable proximal “platform” that facilitates maximal torque generation at the knee joint.
In conclusion, this study provides insight into the mechanisms underlying strength differences between sides in healthy young people. Side-to-side differences in electrically evoked tetanic knee extensor torque at rest and voluntary activation of the quadriceps muscles together explained 69% of knee extensor strength differences by side, whereas side-to-side differences in antagonistic hamstrings activity had little effect on the subjects’ knee extensor strength. From a clinical perspective, the findings indicate that integrating electrically evoked torque measurements at rest with strength and activation testing provides meaningful insight into the peripheral and central contributions of muscular weakness.
Acknowledgements
This work was supported in part by NIH-NCMRR Grant # 1 K12 HD055931.
References
- Bampouras TM, Reeves ND, Baltzopoulos V, Maganaris CN. Muscle activation assessment: effects of method, stimulus number, and joint angle. Muscle Nerve. 2006;34:740–746. doi: 10.1002/mus.20610. [DOI] [PubMed] [Google Scholar]
- Behm D, Power K, Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve. 2001;24:925–934. doi: 10.1002/mus.1090. [DOI] [PubMed] [Google Scholar]
- Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol. 1991;70:1882–1885. doi: 10.1152/jappl.1991.70.4.1882. [DOI] [PubMed] [Google Scholar]
- Brass TJ, Loushin MK, Day JW, Iaizzo PA. An improved method for muscle force assessment in neuromuscular disease. J Med Eng Technol. 1996;20:67–74. doi: 10.3109/03091909609008382. [DOI] [PubMed] [Google Scholar]
- Carolan B, Cafarelli E. Adaptations in coactivation after isometric resistance training. J Appl Physiol. 1992;73:911–917. doi: 10.1152/jappl.1992.73.3.911. [DOI] [PubMed] [Google Scholar]
- Davies CT, Dooley P, McDonagh MJ, White MJ. Adaptation of mechanical properties of muscle to high force training in man. J Physiol. 1985;365:277–284. doi: 10.1113/jphysiol.1985.sp015771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM. Muscle strength and its development. New perspectives. Sports Med. 1988;6:146–168. doi: 10.2165/00007256-198806030-00003. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Neural adaptations with chronic physical activity. J Biomech. 1997;30:447–455. doi: 10.1016/s0021-9290(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Goslin BR, Charteris J. Isokinetic dynamometry: normative data for clinical use in lower extremity (knee) cases. Scand J Rehabil Med. 1979;11:105–109. [PubMed] [Google Scholar]
- Hakkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ. Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol. 2000;83:51–62. doi: 10.1007/s004210000248. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Malkia E, Kraemer WJ, Newton RU, Alen M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol. 1998;84:1341–1349. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR. Prevention of anterior cruciate ligament injuries. Curr Womens Health Rep. 2001;1:218–224. [PubMed] [Google Scholar]
- Kellis E. Quantification of quadriceps and hamstring antagonist activity. Sports Med. 1998;25:37–62. doi: 10.2165/00007256-199825010-00004. [DOI] [PubMed] [Google Scholar]
- Knapik JJ, Jones BH, Bauman CL, Harris JM. Strength, flexibility and athletic injuries. Sports Med. 1992;14:277–288. doi: 10.2165/00007256-199214050-00001. [DOI] [PubMed] [Google Scholar]
- Knight KL. Strength imbalance and knee injury. Phys Sportsmed. 1980;8:140. doi: 10.1080/00913847.1980.11948551. [DOI] [PubMed] [Google Scholar]
- Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34:269–280. doi: 10.2165/00007256-200434040-00006. [DOI] [PubMed] [Google Scholar]
- Lieber RL. Skeletal Muscle Structure and Function: Implications for Rehabilitation and Sports. Baltimore: Medicine Williams & Wilkins; 1992. [Google Scholar]
- Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve. 2002;25:858–863. doi: 10.1002/mus.10113. [DOI] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol. 2005;99:1050–1055. doi: 10.1152/japplphysiol.01186.2004. [DOI] [PubMed] [Google Scholar]
- Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36:385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- Narici M, Kayser B, Barattini P, Cerretelli P. Effects of 17-day spaceflight on electrically evoked torque and cross-sectional area of the human triceps surae. Eur J Appl Physiol. 2003;90:275–282. doi: 10.1007/s00421-003-0955-7. [DOI] [PubMed] [Google Scholar]
- O'Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. Assessment of voluntary muscle activation using magnetic stimulation. Eur J Appl Physiol. 2008;104:49–55. doi: 10.1007/s00421-008-0782-y. [DOI] [PubMed] [Google Scholar]
- Ostenberg A, Roos E, Ekdahl C, Roos H. Isokinetic knee extensor strength and functional performance in healthy female soccer players. Scand J Med Sci Sports. 1998;8:257–264. doi: 10.1111/j.1600-0838.1998.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27:405–424. vii–ix. doi: 10.1016/j.csm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Perotto A. Anatomical guide for the electromyographer. 3rd ed. Springfield: 1994. [Google Scholar]
- Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. 2008;40:422–427. doi: 10.1249/MSS.0b013e31815ef285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–1103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Spector SA, Gardiner PF, Zernicke RF, Roy RR, Edgerton VR. Muscle architecture and force-velocity characteristics of cat soleus and medial gastrocnemius: implications for motor control. J Neurophysiol. 1980;44:951–960. doi: 10.1152/jn.1980.44.5.951. [DOI] [PubMed] [Google Scholar]
- Stackhouse SK, Binder-Macleod SA, Lee SC. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve. 2005;31:594–601. doi: 10.1002/mus.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackhouse SK, Stevens JE, Lee SC, Pearce KM, Snyder-Mackler L, Binder-Macleod SA. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81:1102–1109. [PubMed] [Google Scholar]
- Stevens JE, Pathare NC, Tillman SM, Scarborough MT, Gibbs CP, Shah P, Jayaraman A, Walter GA, Vandenborne K. Relative contributions of muscle activation and muscle size to plantarflexor torque during rehabilitation after immobilization. J Orthop Res. 2006;24:1729–1736. doi: 10.1002/jor.20153. [DOI] [PubMed] [Google Scholar]
- Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve. 2003;27:99–101. doi: 10.1002/mus.10279. [DOI] [PubMed] [Google Scholar]
- Tate CM, Williams GN, Barrance PJ, Buchanan TS. Lower extremity muscle morphology in young athletes: an MRI-based analysis. Med Sci Sports Exerc. 2006;38:122–128. doi: 10.1249/01.mss.0000179400.67734.01. [DOI] [PubMed] [Google Scholar]
- Vivodtzev I, Wuyam B, Flore P, Levy P. Changes in quadriceps twitch tension in response to resistance training in healthy sedentary subjects. Muscle Nerve. 2005;32:326–334. doi: 10.1002/mus.20374. [DOI] [PubMed] [Google Scholar]
- Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33:402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]