Abstract
An Alkanna orientalis leaf and flower extract inhibited the growth of Staphylococcus aureus, a pathogen that causes an estimated 478,000 hospitalizations in the US annually. Bioassay-guided fractionation of A. orientalis resulted in isolation of the flavonoid sarothrin (5,7,4′-trihydroxy-3,6,8-trimethoxyflavone), which inhibited the growth of Mycobacterium smegmatis (MIC 75 μM) and S. aureus (MIC >800 μM), and possessed efflux pump inhibitory activity. This is the first report of antimicrobial or efflux pump inhibitory activity of sarothrin, and of its presence in A. orientalis. Our findings suggest that the effectiveness of A. orientalis extracts is due to a combination of multiple constituents, including sarothrin.
Keywords: Alkanna orientalis, Boraginaceae, antimicrobial, efflux inhibition
Bacterial infections have an estimated $20 billion burden on the US health care system [1]. Botanicals have been suggested as an under-utilized source of antimicrobial agents [2, 3]. With this project, our goals were to evaluate the antimicrobial activity of the plant Alkanna orientalis (L.) Boiss (Boraginaceae) against Staphylococcus aureus and Mycobacterium smegmatis, and to identify compounds that play a role in this activity. A. orientalis was chosen for this study based on antimicrobial activity observed for the crude extract by our laboratory and others [4, 5], and on the ethnobotanical literature. This plant was traditionally employed as a treatment for digestive problems [4] and for wound healing.
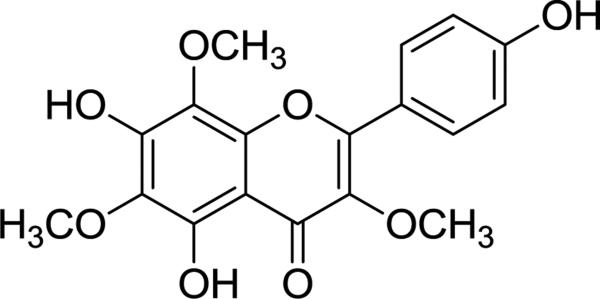
Bioassay-guided fractionation of Alkanna orientalis resulted in the isolation of the flavonoid sarothrin (5,7,4′-trihydroxy-3,6,8-trimethoxyflavone) (Figure 1). Sarothrin is present in other botanicals, including Encelia densifolia (Asteraceae) [6], Ononis rotundifolia (Fabaceae) and Gardenia obtusifolia (Rubiaceae) [7]. However, this is the first report of sarothrin in A. orientalis or any member of the Boraginaceae family.
Fig. 1.
Structure of sarothrin (1) isolated from Alkanna orientalis as a result of bioactivity directed fractionation evaluating antimicrobial activity against Staphylococcus aureus.
Sarothrin was observed to inhibit M. smegmatis (MIC 75 μM), and weakly inhibited S. aureus growth [MIC >800 μM, Table 1, 50% inhibition of growth at 38 μg/mL (100 μM), Figure 3S]. However, the crude A. orientalis leaf and flower extract, which contained only 1.63 ± 0.13% sarothrin, had very similar activity to that of sarothrin alone (Figure 3S). Furthermore, comparisons were made of sarothrin concentrations in various A. orientalis plant parts (Table 2). The highest levels were extracted from leaves and flowers, while very low levels were present in roots and seeds (Table 2). Nonetheless, similar antimicrobial activity (30 to 60% inhibition) was observed from extracts of various plant parts (Figure 3S). Collectively, these findings suggest that additional constituents besides sarothrin are likely to play a role in the antimicrobial activity of A. orientalis.
Table 1.
MIC (concentration required to completely inhibit bacterial growth) measured for purified sarothrin against two pathogenic bacteria.
| Organism | MIC sarothrin (μM) | MIC Ciprofloxacin (μM) |
|---|---|---|
| Staphylococcus aureus NCTC 8325-4 | >800 | 1.5 |
| Mycobacterium smegmatis ATCC 607 | 75 | 6 |
Table 2.
Quantity of the bioactive flavonoid sarothrin in extracts prepared from various plant parts of Alkanna orientalis.
| Plant Part | Sarothrin Concentration (ppm)a ± SD |
|---|---|
| rootb | 0.51 ± 0.40 |
| seed | 0.37 ± 0.12 |
| leaf | 52.9 ±1.4 |
| flower + leaf | 160. ± 13 |
Quantities are reported as mg sarothrin/kg plant material based on LC-MS analysis of extracts prepared from the relevant plant parts. Standard deviations are for triplicate analyses of the same extract. Error bars represent ± the standard deviation of each extract concentration based on linear regression analysis of a 9 point calibration curve of peak area versus concentration with slope (m) = 0.9787 ± 0.0021, intercept (b) = 6.346 ± 0.018 and R2 = 0.9967.
The concentration reported for the root extract is approximate only, as the concentration in the extract at the dilution analyzed was below the lower limit of detection for the method. Concentrations for the seed, leaf, and flower + leaf extract fell within the linear range of the calibration curve.
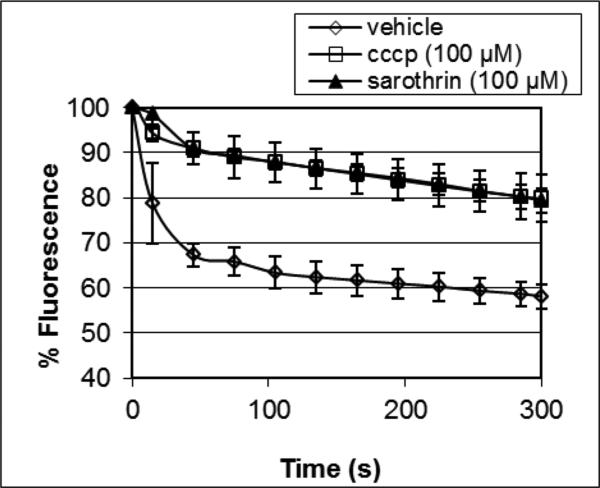
Efflux pump inhibition in combination with antibiotics has been proposed as a potential therapeutic strategy against bacterial infections [3]. Reports indicating efflux pump inhibitory activity of flavonoids [8-11] led us to investigate the efflux pump inhibitory activity of sarothrin. A fluorescence-based assay was utilized, which relies on the efflux of ethidium bromide driven by the S. aureus efflux pump NorA [12]. As is apparent from the data in Figure 2, sarothrin blocked ethidium bromide efflux (data overlaid with the positive control, CCCP). These findings suggest that sarothrin possesses efflux pump inhibitory activity. This finding could be relevant to the overall effectiveness of A. orientalis extracts against bacteria; while sarothrin is only a weak antimicrobial agent alone, it could increase the activity of other antimicrobial compounds in the extracts by blocking bacterial efflux pumps.
Fig. 2.
Percent fluorescence over time for S. aureus (NCTC 8325-4) loaded with ethidium bromide and treated with purified sarothrin. The known efflux pump inhibitor CCCP (carbonyl cyanide m-chlorophenylhydrazone) served as a positive control. Vehicle consisted of 10% DMSO in Müller Hinton broth. Triplicate measurements were made for separate aliquots of solution with different S. aureus pellets, and data points represent the average of these three measurement. Error bars are +/- standard error. Fluorescence measurements were made using λex = 530 nm, λem = 600 nm.
Materials and Methods
Staphylococcus aureus NCTC 8325-4 [13] and Mycobacterium smegmatis (ATCC 607) were employed. Müller Hinton broth, carbonyl cyanide m-chlorophenylhydrazone (CCCP), berberine and ciprofloxacin were purchased from Sigma Aldrich (Saint Louis, MO, USA), all with %purity >98%.
Alkanna orientalis was cultivated at Horizon Herbs (Williams, OR, USA) and identified by Richard Cech. A voucher was deposited in the University of North Carolina Herbarium (NCU 592736). Dried, powdered samples from A. orientalis leaves (2.0 g), roots (2.0 g), leaves + flowers (2060 g) or seeds (10.5 g) were extracted in methanol (1:12.5, w/v). Extracts were stirred for 24 hr, filtered, and rotary evaporated. The residue was separated with liquid/liquid partitioning, as described elsewhere [14]. Final yields of the organic fraction were 17.4 mg, 7.3 mg, 20.3 g and 1.5 mg, respectively for the leaf, root, flower + leaf, and seed extracts.
The flower + leaf extract was fractionated over silica gel with a hexane:chloroform:methanol gradient as described [15]. The most active fraction (strongest inhibition of S. aureus) was separated over silica gel utilizing hexane:ethyl acetate as the gradient [15]. Yellow crystals (52.0 mg, 92% pure) (sarothrin) precipitated and were purified using reversed phase preparative HPLC with a YMC ODS-A column (5 μm, 120Å; 250 × 20 mm, Waters) with a CH3CN:H2O gradient. Sarothrin (Figure 1) eluted at 13.5 min (7.03 mg, 97 % purity, 0.00034 % yield).
Sarothrin (5,7,4′-trihydroxy-3,6,8-trimethoxyflavone) (1): yellow solid, HRESIMS 361.09100 [M+H]+ (calcd for C18H17O8, 361.09180); 1H NMR (500 MHz acetone-d6) (Figure 1S) and 13C NMR (125 MHz, acetone-d6) (Figure 2S) agreed with literature values (Table 1S) [16]. The HRMS and NMR instruments employed were an LTQ-Orbitrap (Thermo, San Jose, CA, USA) and JEOL ECA-500 (Peabody, MA, USA), respectively.
Sarothrin quantitation was performed using a triple quadrupole mass spectrometer (TSQ Access, Thermo, San Jose, CA, USA) with positive ion electrospray coupled to a HP1200 HPLC (Agilent, Santa Clara, CA, USA) with a C-18 Prevail column. An acetonitrile (1% formic acid):water (1% formic acid) gradient was employed at 0.3 mL/min.
Mycobacterium smegmatis was grown in Middlebrook 7H9 medium and MIC values measured after 3 days incubation as described previously [17]. Staphylococcus aureus was grown in Müller Hinton broth, MICs measured using CLSI standard methods [18], and efflux pump inhibitory activity evaluated, as described previously [15, 19].
Supplementary Material
Acknowledgments
Support was provided by Grant Number 1 R15 AT005005-01 from the National Center for Complementary and Alternative Medicine (NCCAM), a component of the National Institutes of Health (NIH), and an undergraduate research grant from the American Society of Pharmacognosy to J. R. Bame. We thank Brandie Ehrmann, Carol Ann McCormick, Myra Williams, Amanda Roffman, Alan Jarmusch, Keivan Ettefagh, Tamam El-Elimat and Adam Brown for technical assistance, and Alexander Horswill for providing S. aureus NCTC 8325-4.
Footnotes
Conflict of Interest
The authors report no conflict of interest
Supporting Information
NMR data for sarothrin and comparison of S. aureus growth inhibition by various A. orientalis extracts are available as Supporting Information.
References
- 1.Roberts RR, Hota B, Ahmad I, Scott RD, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 2.Cowan MM. Plant Products as Antimicrobial Agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis K, Ausubel FM. Prospects for plant-derived antibacterials. Nat Biotechnol. 2006;24:1504–1507. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- 4.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J Ethnopharm. 2000;72:191–205. doi: 10.1016/s0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 5.Mothana RAA, Abdo SAA, Hasson S, Althawab FMN, Alaghbari SAZ, Lindequist U. Antimicrobial, antioxidant and cytotoxic activities and phytochemical screening of some Yemeni medicinal plants. ecam. 2010;7:323–330. doi: 10.1093/ecam/nen004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proksch P, Politt U, Wollenweber E, Wray V, Clark C. Epicuticular flavonoids from Enceli. Planta Med. 1988;54:542–546. doi: 10.1055/s-2006-962543. [DOI] [PubMed] [Google Scholar]
- 7.Yu S, Fang N, Mabry TJ. Flavonoids from Gymnosperma glutinosum. Phytochemistry. 1988;27:171–177. [Google Scholar]
- 8.Belofsky G, Carreno R, Lewis K, Ball A, Casadei G, Tegos GP. Metabolites of the “smoke tree”, Dalea spinosa, potentiate antibiotic activity against multidrug-resistant Staphylococcus aureus. J Nat Prod. 2006;69:261–264. doi: 10.1021/np058057s. [DOI] [PubMed] [Google Scholar]
- 9.Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Nat Acad Sci. 2000;97:1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stermitz FR, Scriven LN, Tegos G, Lewis K. Two flavonols from Artemisia annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002;68:1140–1141. doi: 10.1055/s-2002-36347. [DOI] [PubMed] [Google Scholar]
- 11.Stermitz FR, Tawara-Matsuda J, Lorenz P, Mueller P, Zenewicz LA, Lewis K. 5'Methoxyhydnocarpin-D and phenophorbide A: Berberis species components that potentiate berberine growth inhibition of resistant Staphylococcus aureus. J Nat Prod. 2000;63:1146–1149. doi: 10.1021/np990639k. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh PC, Siegel SA, Rogers B, Davis D, Lewis K. Bacteria lacking a multidrug pump: A sensitive tool for drug discovery. Proc Natl Acad Sci. 1998;95:6602–6606. doi: 10.1073/pnas.95.12.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novick R. Properties of cryptic high-frequency transducing phage of Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 14.Gu J-Q, Graf TN, Lee D, Chai H-B, Mi Q, Kardono LBS, Setyowati FM, Ismail R, Riswan S, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kroll DJ, Falkinham JO, Wall ME, Wani MC, Kinghorn AD, Oberlies NH. Cytotoxic and antimicrobial constituents of the bark of Diospyros maritima collected in two geographical locations in Indonesia. J Nat Prod. 2004;67:1156–1161. doi: 10.1021/np040027m. [DOI] [PubMed] [Google Scholar]
- 15.Junio HA, Sy-Cordero AA, Ettefagh KA, Burns JT, Micko KT, Graf TN, Richter SJ, Cannon RE, Oberlies NH, Cech NB. Synergy Directed Fractionation of Botanical Medicines: A Case Study with Goldenseal (Hydrastis canadensis). J Nat Prod. 2011;74:1621–1629. doi: 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roitman JN, James LF. Chemistry of toxic range plants: Highly oxygenated flavonol methyl ethers from Gutierrezia microcephala. Phytochemistry. 1985;4:835–848. [Google Scholar]
- 17.Sugandhi EW, Macri RV, Williams AA, Kite BL, Slebodnick C, Falkinham JO, Esker AR, Gandour RD. Synthesis, critical micelle concentrations, and antimycobacterial properties of homologous, dendritic amphiphiles. Probing intrinsic activity and the “cutoff” effect. J Med Chem. 2007;50:1645–1650. doi: 10.1021/jm061240d. [DOI] [PubMed] [Google Scholar]
- 18.Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard, Seventh Ed. Vol. 26. Clinical and Laboratory Standards Institute; 2006. pp. M7–A7. [Google Scholar]
- 19.Ettefagh KA, Burns JT, Junio HA, Kaatz GW, Cech NB. Goldenseal (Hydrastis canadensis L.) extracts synergistically enhance the antibacterial activity of berberine via efflux pump inhibition. Planta Med. 2010;77:835–840. doi: 10.1055/s-0030-1250606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.