Abstract
The popular herbal remedy goldenseal (Hydrastis canadensis L.) is traditionally used to treat skin infections. With this study, we show activity of H. canadensis extracts in vitro against methicillin-resistant Staphylococcus aureus (MRSA). An extract from H. canadensis leaves demonstrated more potent antimicrobial activity than the alkaloid berberine alone (MICs of 75 µg/mL and 150 µg/mL, respectively). LC-MS detected alkaloids and efflux-pump inhibitory flavonoids in the extract, and the latter may explain the enhanced efficacy of the extract compared to berberine alone. We also show evidence of anti-virulence activity as a second mechanism by which H. canadensis acts against S. aureus. The H. canadensis leaf extract (but not the isolated alkaloids berberine, hydrastine, and canadine) demonstrated quorum quenching activity against several clinically relevant MRSA isolates (USA300 strains). Our data suggest that this occurs by attenuation of signal transduction through the AgrCA two-component system. Consistent with this observation, the extract inhibited toxin production by MRSA, and prevented damage by MRSA to keratinocyte cells in vitro. Collectively, our results show that H. canadensis leaf extracts possess a mixture of constituents that act against MRSA via several different mechanisms. These findings lend support for the traditional application of crude H. canadensis extracts in the treatment of prevention of infection.
Keywords: Hydrastis canadensis, Ranunculaceae, quorum quenching, berberine, methicillin-resistant Staphylococcus aureus, anti-virulence
Introduction
The European Antimicrobial Surveillance Systems estimates that as many as 52 million people worldwide are colonized with multi-drug resistant bacteria. Among the most prevalent and lethal of these is methicillin-resistant Staphylococcus aureus (MRSA), which now causes more annual deaths in the US than HIV/AIDS [1]. With the growing rates of MRSA levels in community and healthcare settings, and the increased disease burden and higher treatment costs associated with drug-resistant bacterial infections [2], there is a pressing need to devise new strategies to combat problematic pathogens such as MRSA.
Anti-virulence based approaches to disarm drug-resistant bacterial pathogens are increasingly being developed [3]. These agents will be more pathogen-specific than current antibiotics, but the enormity of disease burden caused by S. aureus and MRSA, including an estimated 76% of all skin and soft tissue infections [4], highlights the value of developing MRSA targeted therapeutics. The specificity and the anti-virulence nature of such agents may limit the evolution of widespread drug resistance, facilitate the development of immune responses that will prevent subsequent infection, and avoid negative impacts on the normal microbial flora [3]. One appealing target as a MRSA anti-virulence strategy is the quorum-sensing system [5, 6].
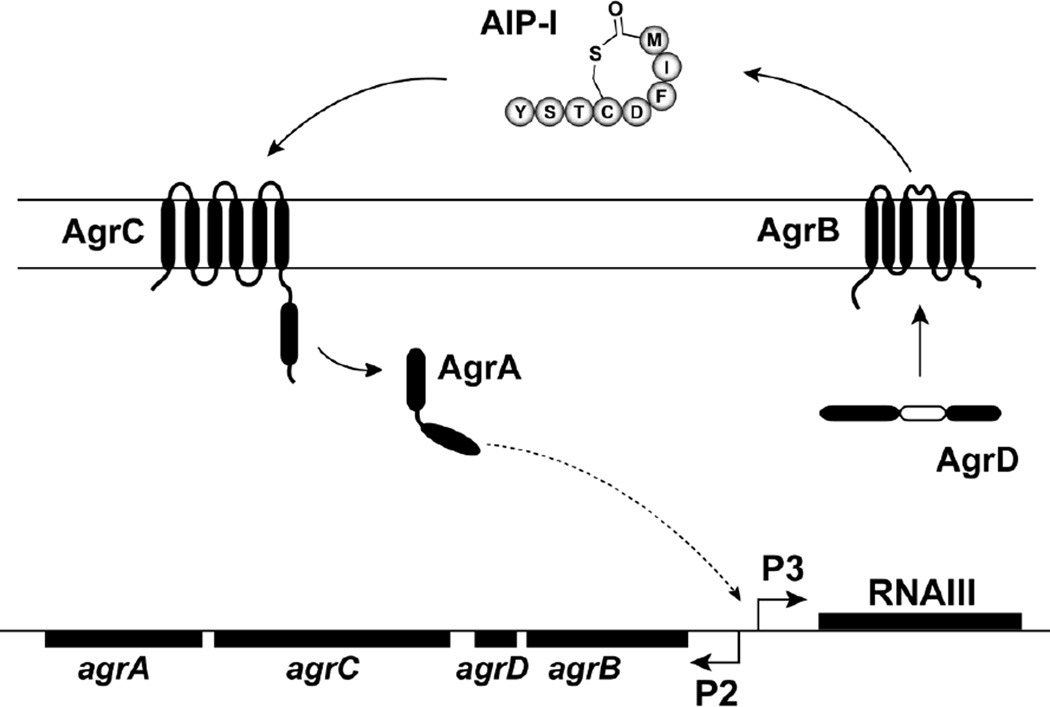
S. aureus quorum-sensing, also called the accessory gene regulator or “agr” system (Figure 1), is a cell density dependent regulatory system that controls the production of many host-damaging agents or “virulence factors”. This system responds to a secreted peptide signal, called an autoinducing peptide (AIP), which is biosynthesized and secreted by S. aureus. Depending on the S. aureus strain, the structure of the secreted AIP can be any one of four types (AIP-1, AIP-2, AIP-3, or AIP-4). Once the extracellular concentration of the AIP signal is high enough, it binds to a surface receptor, called AgrC, activating a signal transduction cascade. The key components of this cascade are the AgrCA two-component regulatory pair, and when activated, they induce the production of extracellular virulence factors. Mutations in the agr system are known to reduce pathogenesis in animal models of infection [5], and numerous studies have shown that small-molecule inhibition of the agr system attenuates skin and soft tissue infections in animal models [5, 7, 8].
Fig. 1.
Schematic of the S. aureus agr quorum sensing system. AgrD is the peptide precursor of the autoinducing peptide (AIP) signal. AgrB is a membrane bound endopeptidase that generates the AIP (AIP-1 sequence is shown). AgrC is a membrane histidine kinase that binds AIP and phosphorylates the response regulator AgrA, which in turn induces transcription from the P2 and P3 promoters in the agr chromosomal locus. This induction autoinduces the system and leads to the production of RNAIII transcript. RNAIII is the primary effector of the system and turns on the secretion of virulence factors.
It was our objective to investigate the in vitro effectiveness of the popular [9] herbal medicine goldenseal (Hydrastis canadensis L.), against several clinical isolates of MRSA. H. canadensis possesses an array of alkaloids, the most abundant of which is berberine [10]. Previously, H. canadensis has demonstrated antimicrobial activity against various Gram positive bacteria [10–13], and berberine has been shown to inhibit the growth of MRSA [14]. However, the antimicrobial activity of crude H. canadensis extracts against MRSA has never been reported, nor has their quorum quenching activity been investigated. With these studies, we tested the central hypothesis that antimicrobial effects of H. canadensis leaf extracts are due to the combined action of multiple constituents acting via different mechanisms. This hypothesis was based in part on seminal work by Stermitz et. al, in which the antimicrobial activity of berberine has been shown to be potentiated by other plant constituents [15, 16]. We focused specifically on H. canadensis leaf extracts in these experiments, because our previous studies have shown leaf extracts to be more effective than root extracts as synergists to the antimicrobial activity of berberine [17]. Although H. canadensis root extracts are most commonly used in traditional medicine applications [18], there is precedent for topical use of goldenseal leaf extracts against infection [19]. Our goals with this study were to evaluate an H. canadensis leaf extract as an antimicrobial agent and quorum quencher for MRSA, and to compare the activity of the crude extract to that of its major alkaloid, berberine. In particular, we investigated activity against USA300 MRSA isolates, which have emerged as the most common strain of MRSA observed in community-acquired soft-tissue infections [4, 20].
Materials and Methods
Test Bacteria, Chemicals, and Biochemicals
Wild type S. aureus strains used in this study were CA-MRSA USA300 TCH1516 [21], LAC [22], AH1263 (also called LAC*) [23], and SA502A [24]. S. aureus quorum-sensing reporter strains containing plasmid pDB59 were also employed, and included representatives of agr group I (CA-MRSA USA300 LAC), agr group II (SA502A), and agr group III (CA-MRSA MW2) [25]. Additional studies to investigate quenching made use of S. aureus lux reporter strain ROJ143 [26, 27]. For the epithelia toxicity tests, alpha-toxin (Δhla::Erm) [28] or agr (Δagr::Tet) [29] mutants of USA300 strain AH1263 (LAC*) were used. Tryptic soy broth (TSB), Mueller Hinton broth, carbonyl cyanide m-chloro-phenylhydrazone (CCCP, purity >98% by TLC), berberine (purity >98% by HPLC), (1R,9S)-(-)-β-hydrastine (purity >98% by HPLC) and DMSO were purchased from Sigma Aldrich (Saint Louis, MO, USA) and canadine (tetrahydroberberine, purity >98% by HPLC, stereochemistry unconfirmed) from Chromadex (Irvine, CA, USA). Sideroxylin, 8-desmethyl-sideroxylin, and 6-desmethyl-sideroxylin were isolated previously from H. canadensis leaves [30] with >98% purity. Acetic acid was purchased from Fisher Chemical (Pittsburgh, PA, USA). Ethanol (95%), HPLC grade acetonitrile, and HPLC grade methanol were obtained from Pharmaco-AAPER (Shelbyville, KY, USA). Water was purified with a nanodiamond system (Barnstead, Dubuque, IA, USA).
Plant material
Cultivated Hydrastis canadensis L. (Ranunculaceae) was harvested in September 2008 from: N 35° 24.277’, W 082° 20.993’, 702.4 m elevation. A voucher specimen is retained at the University of North Carolina Herbarium (NCU583414) and identity was confirmed by Dr. Alan S. Weakly.
Extraction and LC-MS
Extracts were prepared according to standard procedures employed in the manufacture of hydroethanolic Hydrastis canadensis supplements [31], as described elsewhere [17]. A solvent of 50% ethanol:50% water was used, in a ratio of 1:5 (g plant material:mL solvent). The solvent (500 mL) was blended with the entire aerial (above ground) portion of H. canadensis plants (100 g) and macerated for 24 hr, then filtered under vacuum. The resulting extract, which will be referred to as H. canadensis leaf extract throughout this paper, was stored in an amber bottle at room temperature. Biological activity and HPLC profile were consistent for this extract over a three year time period.
The extract was profiled with liquid chromatography – mass spectrometry (LC-MS) to determine alkaloid and flavonoid quantity and identity. Quantitative analysis of alkaloids and flavonoids (Table 1, Supplemental Information) was accomplished using an established method [17, 30]. Constituent identities were confirmed on an LTQ-Orbitrap high resolution mass spectrometer (Thermo, San Jose, CA, USA) coupled to an ultra-performance liquid chromatograph (UPLC) (Waters, Alliance) by comparing accurate mass, fragmentation, and retention time with those of authenticated standards, as reported previously [30].
The following gradient was employed, where A = HPLC grade acetonitrile and B = 0.1% aqueous formic acid: 90%-70% B from 0 to 5.0 min; 70% to 50% B from 5 to 7.0 min; 50% to 45% from 7.0 to 7.5 min, 45% to 0% B from 7.5 to 8.0 min, isocractic at 0% B from 8.0 to 8.5 min and 0 to 90% from 8.5 to 9.0 min. MS analysis was conducted in the positive ion mode for alkaloids and the negative ion mode for flavonoids, with mass range of 100–1000 m/z. Capillary temperature was 300°C, sheath gas pressure was 28 (arbitrary units), and source, capillary, and tube lens voltages were 4.30 kV, 29 V, and 110 V, respectively.
Broth Microdilution MIC Assays
Minimum inhibitory concentration (MIC) was measured according to Clinical Laboratory Standards Institute (CLSI) standard procedures [32]. Briefly, extracts or purified berberine (+ control) were added to 96 well plates in triplicate at concentrations ranging from 4.7 to 300 µg/mL in Mueller Hinton Broth. Vehicle (2% DMSO) was the negative control, and DMSO content was fixed at 2% in all wells. A 24 hr culture of a single colony isolate of S. aureus AH1263, TCH1516, or LAC was grown to log-phase in Mueller Hinton broth and added at a final density of 1.0×105 CFU/mL. Absorbance at 600 nm was measured after 24 hr using a POLARstar Optima microplate reader (BMG Labtech, Inc). MIC was defined as the concentration at which there was no statistically significant difference between the treatment and vehicle control. Absorbance for replicate wells containing all assay components except bacteria was subtracted from the absorbance of assay wells.
Quorum quenching assays with fluorescent reporters
S. aureus quorum-sensing reporter strains containing pDB59 were grown overnight in TSB supplemented with chloramphenicol at 10 µg/mL at 37°C with shaking. For testing extracts or samples, cultures were diluted 500-fold into TSB (approximately 4–10 × 106 CFU/mL after dilution) and 180 µL was dispensed into a microtiter plate. Sample or vehicle control was added (20 µL) to reach the appropriate concentration, and the plates were incubated with shaking (200 rpm) at 37°C. At designated time points, absorbance (600 nM) and fluorescence (excitation 485 nm, emission 530 nm) was measured in a Tecan Infinite M200 plate reader. Reported values are for 30 hr of growth.
Quorum quenching assay with lux reporter
AIP-1 and AIP-2 were obtained by filter sterilizing overnight TSB cultures of S. aureus strains AH1263 and SA502A, respectively [24]. An overnight culture of the lux reporter strain ROJ143 was subcultured 1:50 in TSB containing chloramphenicol (10 µg/mL) and grown at 37°C with shaking to OD600 of 0.8. Aliquots (164 µL) of the lux reporter strain were added to quadruplicate microtiter plate wells that contained 20 µL of AH1263 conditioned medium (i.e. AIP-1) and H. canadensis extract at various sub-inhibitory concentrations, such that all wells contained a final DMSO concentration of 0.8%. Following 90 min of incubation at 37°C with shaking (200 rpm), bioluminescence was measured in a Tecan Infinite M20 plate reader. For comparison, the lux reporter was inhibited with AIP-2 by replacing H. canadensis with SA502A conditioned medium (also in 0.8% DMSO).
Human skin epithelia toxicity assay
USA300 strain AH1263 (LAC*) and derivatives with an alpha-toxin mutation or an agr deletion were grown overnight in TSB. Culture flasks (50 mL) containing 10 mL of TSB, and a range of sub-inhibitory concentrations of H. canadensis (with constant 0.8% DMSO), were inoculated 1:500 with AH1263 overnight culture. TSB aliquots containing DMSO vehicle only were inoculated with the agr and hla mutants. Following 11 hr of growth at 37°C with shaking (225 rpm), cultures were filtered through SpinX filters (0.22 µm, Corning) and α-toxin content of the medium was measured by immunoblotting, as described previously [28]. Human keratinocytes (HaCaT) were cultured in RPMI with 10% fetal calf serum with penicillin/streptomycin [33]. The spent medium was filter sterilized, and diluted 5-fold in HaCaT medium. Aliquots of this spent medium (0.5 mL) were added to confluent HaCaT in 24 well plates and incubated for 24 hr. To assess toxicity, the release lactate dehydrogenase was measured with a commercially available kit (Promega CytoTox 96® Non-Radioactive Cytotoxicity Assay).
Statistics
Significance of means as compared to controls was calculated with two-sample equal variance, 2-tailed student’s t-tests.
Results and Discussion
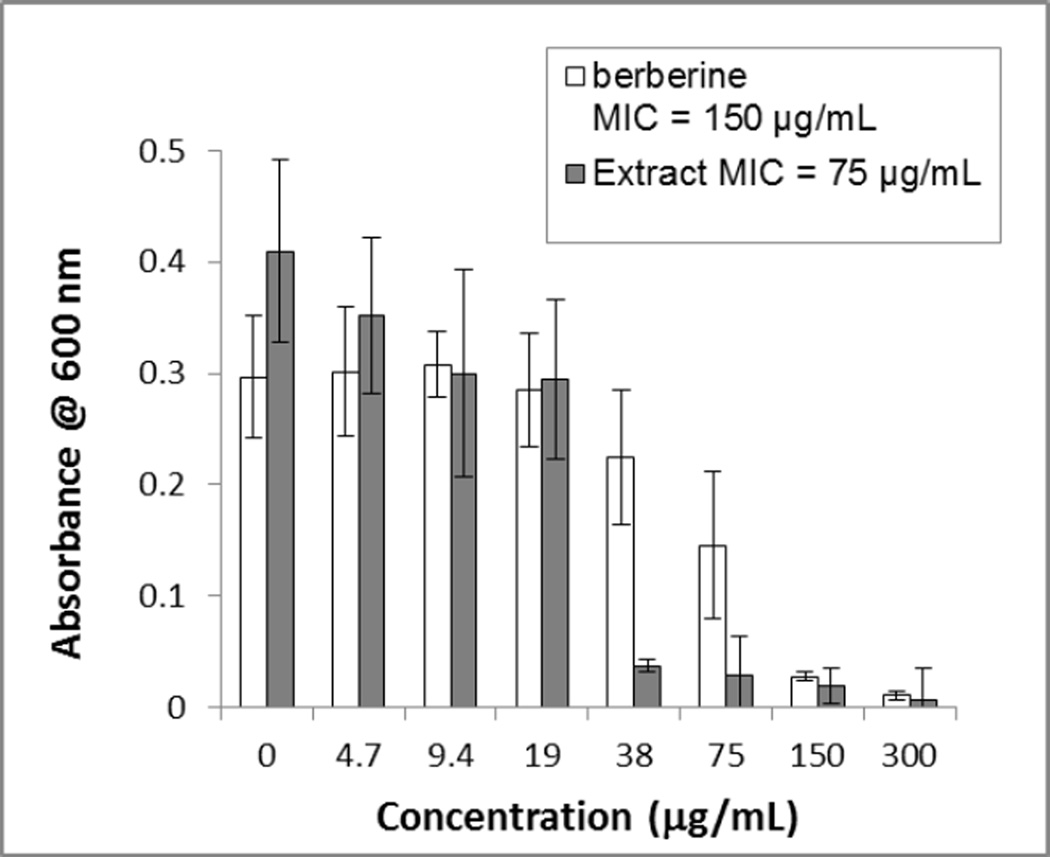
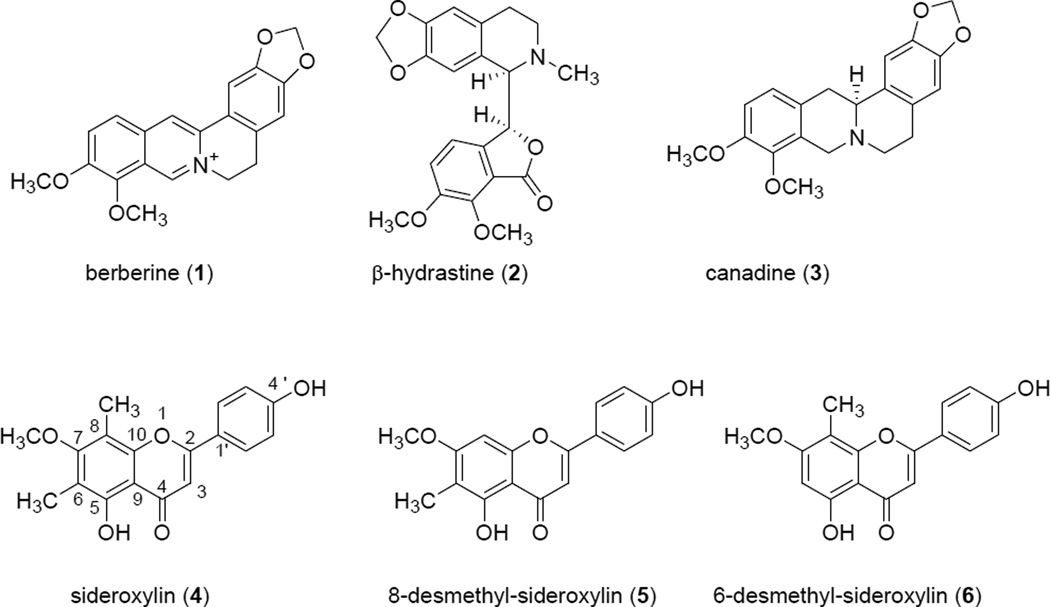
One of the objectives of this study was to compare the antimicrobial activity of an H. canadensis extract against MRSA to that of its major alkaloid berberine. An H. canadensis leaf extract (see chromatogram in Figure 1S, Supporting Information) was standardized to berberine content, and its antimicrobial activity was evaluated against MRSA strain USA300 AH1263 (LAC*) [22] side by side with the same concentrations of berberine alone (Figure 2). The extract displayed a lower MIC value (75 µg/mL, reported as µg berberine/mL assay volume) as compared to that of the pure berberine (150 µg/mL), indicating that constituents other than berberine play a role in its activity against MRSA. Note that berberine serves at the positive control in this assay, and our observed MIC is consistent with numerous previous reports of the activity of this alkaloid against S. aureus [10, 34, 35]. It is very likely that the enhanced activity of the extracts as compared to berberine alone is due to flavonoids such as sideroxylin, 8-desmethyl-sideroxylin, and 6-desmethyl-sideroxylin (Figure 3), which have previously been reported at constituents of H. canadensis [30, 36]. H. canadensis flavonoids have been shown to enhance antimicrobial activity of berberine via efflux pump inhibition [30], and were detected in the H. canadensis extracts under investigation here (Table 1S, Supporting Information).
Fig. 2.
Comparison of antimicrobial activity of goldenseal (Hydrastis canadensis) leaf extract and berberine against MRSA strain USA300 AH1263 [22]. The extract was standardized to berberine content, which is displayed on the x-axis. Different concentrations were prepared via two-fold serial dilution for both test samples. The leaf extract showed an MIC value at 38 µg/mL (expressed as µg berberine/mL assay volume), which is three times lower than the MIC of isolated berberine at 150 µg/mL. Error bars represent standard deviation for OD600 of triplicate assay wells.
Fig. 3.
Structures of major alkaloids, berberine (1), (1R,9S)-(-)-β-hydrastine (2) and canadine (tetrahydroberberine) (3), and flavonoids, sideroxylin (4), 8-desmethyl-sideroxylin (5), and 6-desmethyl-sideroxylin (6), found in Hydrastis canadensis.
Our results show that H. canadensis leaf extracts possess antimicrobial activity. This activity is likely due to the action of the alkaloid berberine , which demonstrated MIC values ranging from 150 µg/mL to 300 µg/mL against three different MRSA strains (Table 2S, Supporting Information). Notably, two other goldenseal alkaloids [10], canadine and hydrastine (Figure 3), did not demonstrate any antimicrobial activity against the three MRSA strains evaluated (Table 2S).
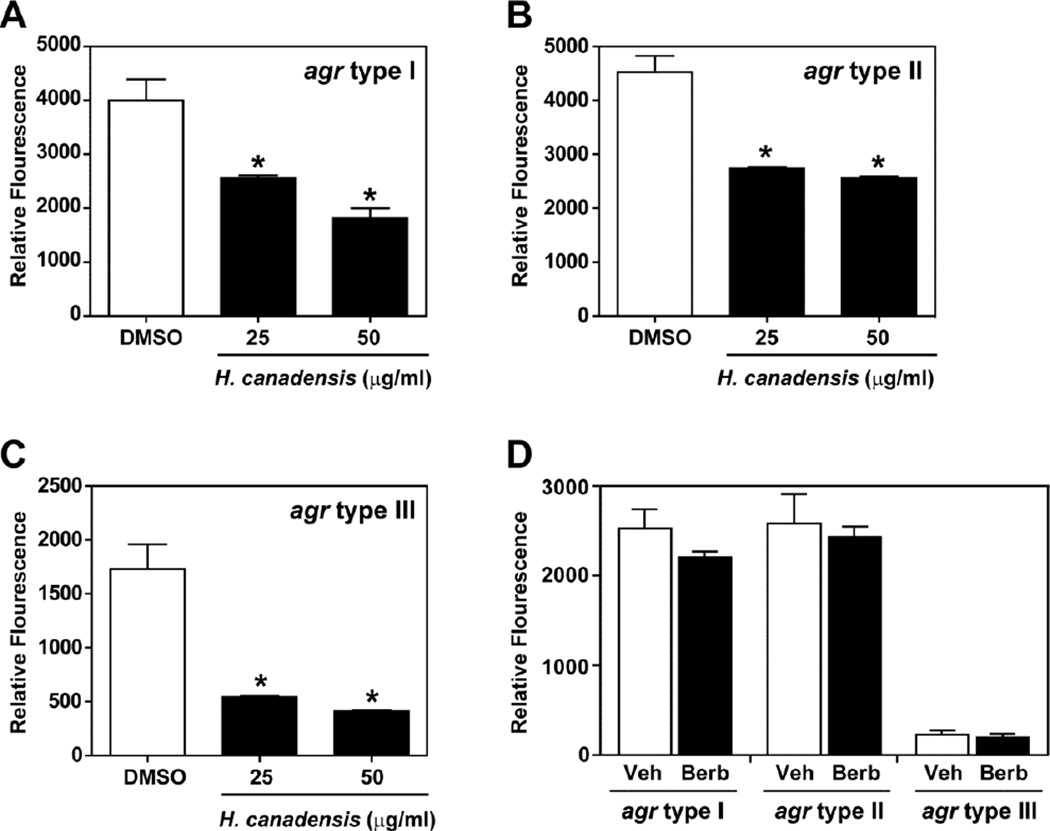
The current study demonstrates that relatively high concentrations of H. canadensis leaf extracts or purified berberine are required to achieve antimicrobial activity; however, there are many anecdotal reports of this botanical being a highly effective treatment for various infections [18]. The S. aureus agr quorum sensing system plays an important role in skin infections. Thus, we hypothesized that quorum quenching activity might partially explain the activity of H. canadensis extracts against MRSA. A reporter assay was employed that has previously been used to characterize other quorum quenching agents [37]. When extracts at sub-inhibitory concentrations were incubated with the reporter strains, we observed a consistent and robust dose-dependent quenching effect with agr type I, II, and III reporters (Figure 4). These three agr classes represent the vast majority of all S. aureus strains [38], indicating that H. canadensis leaf extracts contain a general quorum quenching agent. This quorum quenching effect appeared not to be attributable to the major H. canadensis alkaloid berberine (Figure 4D), which did not inhibit the agr system even at 75 µg/mL. We also failed to observe quenching activity with the other alkaloids hydrastine or canadine (data not shown).
Fig. 4.
Quorum quenching in S. aureus agr P3-GFP reporter strains by H. canadensis leaf extract and berberine. Reporter strains (agr type I, panel A; agr type II, panel B; agr type III, panel C) were grown in tryptic soy broth and extract (standardized to berberine) was tested at 25 and 50 µg/mL. These concentrations were chosen because they were sub-inhibitory under the conditions of this assay. The reporter strains were incubated for 30 hr with the extract and GFP fluorescence was measured. For comparison, a competitive inhibitor of the AIP receptor quenches to ~95% (not shown). In panel D, each agr reporter strain was grown and measured in the same manner and berberine (Berb) was added to 75 µg/mL and compared to sterile water as vehicle control (Veh). * indicates p < 0.01, compared to vehicle (DMSO) in panels A, B. and C. p- values were calculated with a two-tailed student’s t-test, and error bars represent standard deviation of the mean for triplicate measurements. Differences in the results between vehicle and berberine in panel D were not statistically significant.
Further experiments were conducted to gain insight into the H. canadensis leaf extract mechanism of quorum quenching using a S. aureus reporter strain that contains a quorum sensing controlled lux promoter [27]. This lux reporter strain does not make its own AIP, due a deletion in the agrBD genes. When AIP-1 is added to the medium, the lux reporter turns on, generating bioluminescence. This reporter strain was employed to test whether the AgrCA two-component system (Figure 1) could be a target of quenching action. We generated samples containing a constant level of AIP-1, combined them with various sub-inhibitory concentrations of H. canadensis (0–75 µg/mL, standardized to berberine), exposed these mixtures to the reporter strains, and measured bioluminescence (Figure 5). We observed dose dependent inhibition, suggesting that AgrCA is a probable target of some constituent of the extract. In support of this hypothesis, the extract inhibited to the same level as an AgrC competitive antagonist AIP-2 (Figure 5). The most obvious mechanism of inhibition is the AIP receptor on AgrC, which is exposed to the extracellular environment and vulnerable to exogenous agents [5]. Binding to this receptor would cause the observed suppression of bioluminescence by the lux reporter. However, an alternative explanation for the observed effect is that the active compound sequestered or inactivated AIP through a novel interaction, or possibly repressed the agr P3 promoter through a different regulatory mechanism.
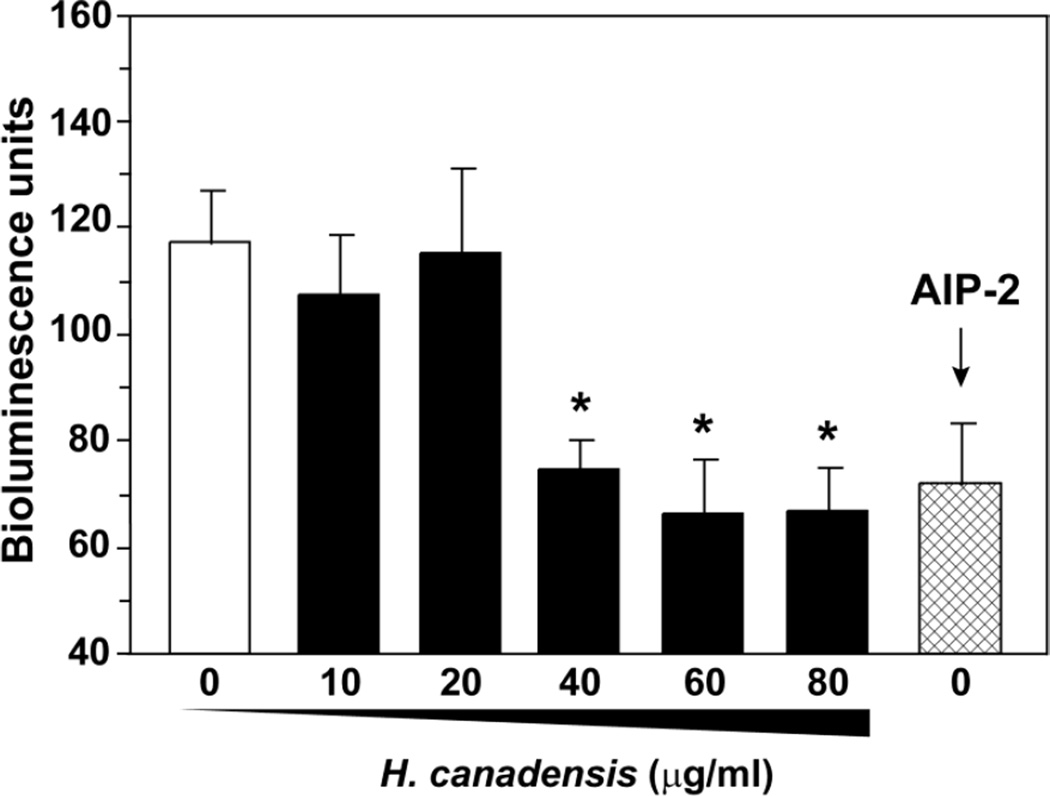
Fig. 5.
Inhibition of S. aureus agr lux reporter with H. canadensis leaf extract. An S. aureus lux reporter strain was used that responds to exogenous AIP-1. A constant level of AIP-1 was mixed with extract, incubated with the lux reporter, and bioluminescence was measured. The extract was standardized to berberine and added to a concentration range of 10–80 µg/mL. AIP-2, a known inhibitor, was used as positive control. Values reported represent means of quadruplicate assay wells, with error bars representing the standard deviation of the mean, and * indicates p <0.0001 compared to vehicle (calculated with a two-tailed student’s t-test).
As further evidence of the relevance of the observed quorum quenching activity of the H. canadensis leaf extract, we investigated its effect on alpha-toxin production by S. aureus. Alpha-toxin is the primary cause of skin abscess dermonecrosis [26] and is, thus, potentially relevant for H. canadensis bioactivity. This toxin is encoded by the hla gene and is under direct control of the agr quorum-sensing system [5]. When the USA300 strain AH1263 was incubated with increasing concentrations of extract, alpha-toxin levels decreased in concert as assessed by immunoblot (Figure 6A). These findings verify the transcriptional reporter assay results (Figures 4 and 5). As expected, the USA300 Δhla mutant, which was included as a control for the immunoblot, did not produce alpha-toxin.
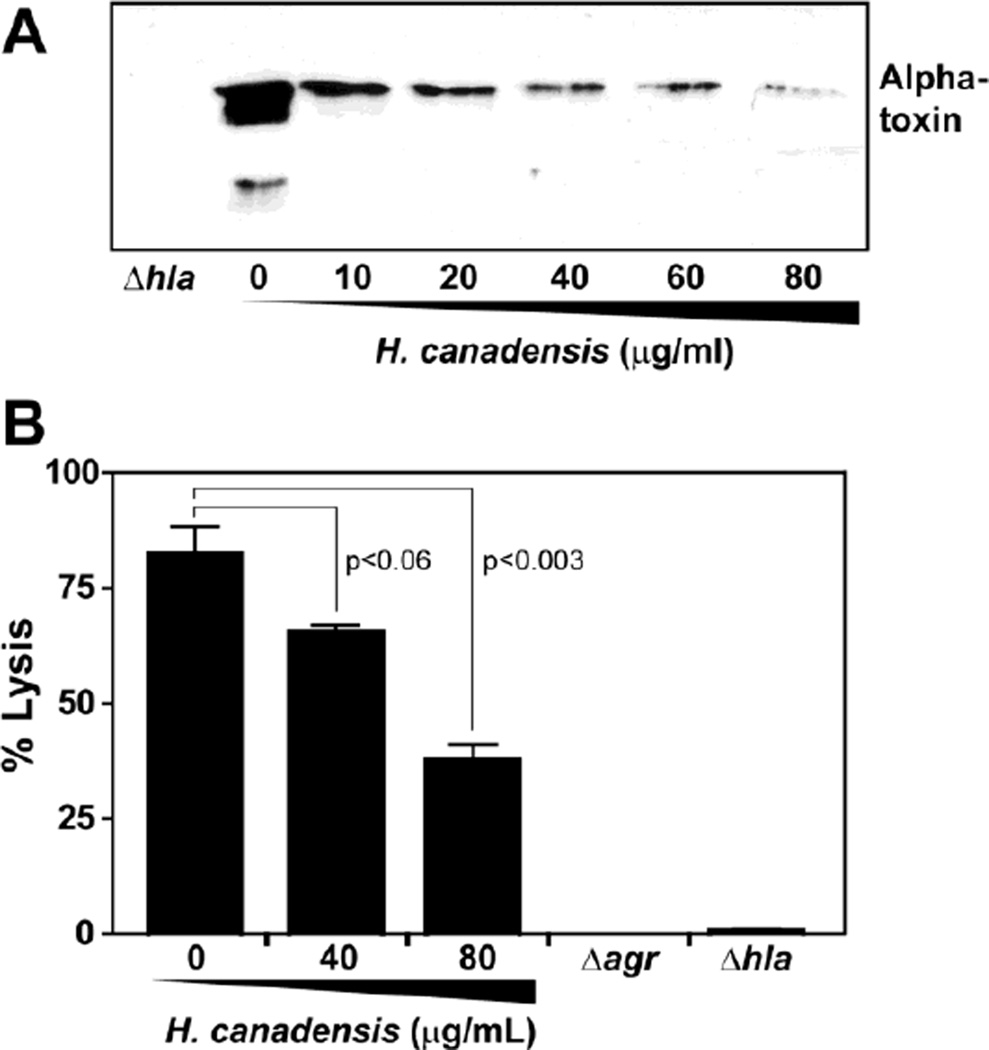
Fig. 6.
A H. canadensis extract inhibits MRSA toxin production and damage to human keratinocyte (HaCaT) cells. MRSA (OD600=0.1) was exposed to extract at berberine-normalized concentrations (10, 20, 40, 60 and 80 µg/mL) of extract or vehicle control and incubated for 11 hr. Spent medium was collected and alpha-toxin immunoblots were performed (A). Confluent layers of HaCaT cells were generated and the same extract treated samples were incubated with the cells to assess damage with a lactate dehydrogenase assay (B). As controls, alpha-toxin (hla) and agr quorum sensing mutants were used. The values reported for % lysis are averages of triplicate assay wells (with error bars representing standard deviation of the mean), and p- values were calculated with a two-tailed student’s t-test.
To relate the H. canadensis leaf extract quorum quenching observations to skin infections, we employed an assay to evaluate MRSA interactions with cultured human keratinocyte cells (HaCaT). Based on the precedent that inhibition of the agr system, or mutations in alpha-toxin, reduced skin abscess formation [5, 28], we tested MRSA wildtype and mutant strains as controls. Spent medium from USA300 strain AH1263, or derivatives with Δagr or Δhla mutation, were exposed to the HaCaT cells, and cell viability was assessed using a lactate dehydrogenase (LDH) release assay (Figure 6B). As anticipated, the wildtype USA300 caused significant damage (83% killing), while the Δagr and Δhla mutants had almost no effect on HaCaT cell integrity. After exposure of the USA300 strain to an H. canadensis extract (80 µg/mL), the USA300 damage to HaCaT cells dropped markedly to 38%. These results demonstrate that H. canadensis inhibition of the agr system results in reduced alpha toxin production, which in turn attenuates damage to human skin keratinocytes.
In conclusion, our data lend support for the traditional use of H. canadensis to treat skin infections. H. canadensis leaf extracts possess direct antimicrobial activity that is due in part to the alkaloid berberine, but not canadine or hydrastine. Importantly, antimicrobial activity is just one of several mechanisms by which H. canadensis appears to act against MRSA. We show that H. canadensis leaf extracts at sub-inhibitory concentrations quench the agr quorum sensing system, and that this activity is not due to the major alkaloids berberine, hydrastine, or canadine. The most likely mechanism by which this quorum quenching effect occurs is attenuation of signal transduction through the AgrCA two-component system. Such attenuation would cause the observed reduction in toxin production by H. canadensis exposed MRSA. Collectively, our results demonstrate that H. canadensis leaf extracts contain several classes of constituents that act against MRSA with different mechanisms. Such a mixture would be expected to demonstrate better efficacy than its components alone in the treatment or prevention of MRSA infections, by virtue of its ability to target the pathogen via multiple pathways. This prediction could be evaluated with future in vivo investigations.
Supplementary Material
Acknowledgments
This publication was supported by 1R15AT005005-01 and 2R44AT003365-02 to N.B.C. from the National Center for Complementary and Alternative Medicine (NCCAM) and by AI078921 to A.R.H. from the National Institute of Allergy and Infectious Diseases (NIAID), both components of the National Institutes of Health (NIH). We thank William Burch for providing H. canadensis, Carol Ann McCormick for assistance with vouchers, and Dr. Brandie M. Ehrmann for mass spectrometry support.
References
- 1.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1711. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RR, Hota B, Ahmad I, Scott D, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. Hospital and societal costs of antimicrobial-resistant infections in a chicago teaching hospital: Implications for antibiotic stewardship. CID. 2009;49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 3.Waldor MK. Disarming pathogens--a new approach for antibiotic development. New Eng J Med. 2006;354:296–297. doi: 10.1056/NEJMcibr054591. [DOI] [PubMed] [Google Scholar]
- 4.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 5.Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. Peptide Signaling in the Staphylococci. Chem Rev. 2011;111:117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antunes LCM, Ferreira RBR, Buckner MMC, Finlay BB. Quorum sensing in bacterial virulence. Microbiology. 2010;156:2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- 7.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick RP, Muir TW. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, Jagasia R, Kaufmann GF, Mathison JC, Ruiz DI, Moss JA, Meijler MM, Ulevitch RJ, Janda KD. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem Biol. 2007;14:1119–1127. doi: 10.1016/j.chembiol.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenthal M. Herb market levels after five years of boom. Herbalgram. 1999;47:64–65. [Google Scholar]
- 10.Scazzocchio F, Cometa MF, Tomassini L, Palmery M. Antibacterial activity of Hydrastis canadensis extract and its major isolated alkaloids. Planta Med. 2001;67:561–564. doi: 10.1055/s-2001-16493. [DOI] [PubMed] [Google Scholar]
- 11.Jung HA, Beavers R, Parekh H, Coulburn J, Wu CD. Goldenseal (Hydrastis canadensis) leaves possess antimicrobial activity against oral pathogens. J Dent Res. 2006;85(Spec Iss A):475. [Google Scholar]
- 12.Mahady GB, Pendland SL, Stoia A, Chadwick L. In vitro suceptibility of helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis. Phytother Res. 2003;17:217–221. doi: 10.1002/ptr.1108. [DOI] [PubMed] [Google Scholar]
- 13.Villinski JR, Dumas ER, Chai HB, Pezzuto JM, Angerhofer CK, Gafner S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm Biol. 2003;41:551–557. [Google Scholar]
- 14.Yu H-H, Kim K-J, Cha J-D, Kim H-K, Lee Y-E, Choi N-Y, You Y-O. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8:454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 15.Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5'-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Nat Acad Sci. 2000;97:1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stermitz FR, Tawara-Matsuda J, Lorenz P, Mueller P, Zenewicz LA, Lewis K. 5'Methoxyhydnocarpin-D and phenophorbide A: Berberis species components that potentiate berberine growth inhibition of resistant Staphylococcus aureus. J Nat Prod. 2000;63:1146–1149. doi: 10.1021/np990639k. [DOI] [PubMed] [Google Scholar]
- 17.Ettefagh KA, Burns JT, Junio HA, Kaatz GW, Cech NB. Goldenseal (Hydrastis canadensis L.) extracts synergistically enhance the antibacterial activity of berberine via efflux pump inhibition. Planta Med. 2010;77:835–840. doi: 10.1055/s-0030-1250606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upton R. Goldenseal root Hydrastis canadensis: Standards of analysis, quality control, and therapeutics. Santa Cruz: American Herbal Pharmacopoeia; 2001. [Google Scholar]
- 19.Moore M. Herb Formulas for Clinic and Home. Albuquerque, NM: Southwest School of Botanical Medicine; 1995. [Google Scholar]
- 20.Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, Ray SM, Blumberg HM. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 21.Highlander SK, Hultén KG, Qin X, Jiang H, Yerrapragada S, Mason EO, Shang Y, Williams TM, Fortunov RM, Liu Y, Igboeli O, Petrosino J, Tirumalai M, Uzman A, Fox GE, Cardenas AM, Muzny DM, Hemphill L, Ding Y, Dugan SB, P R, Buhay CJ, Dinh HH, Hawes AC, Holder M, Kovar CL, Lee SL, Liu W, Nazareth LV, Wang Q, Zhou J, Kaplan SL, Weinstock GM. Subtle genetic changes enhance virulence of methicillin resistant and sensitive Staphylococcus aureus. BMC Microbiol. 2007;7:99. doi: 10.1186/1471-2180-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauderdale KJ, Boles BR, Cheung AL, Horswill AR. Interconnections between Sigma B, agr, and Proteolytic Activity in Staphylococcus aureus Biofilm Maturation. Infect Immun. 2009;77:1623–1635. doi: 10.1128/IAI.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boles BR, M T, Roth AJ, Horswill AR. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. Plos One. 2010;5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malone CL, Boles BR, Horswill AR. Biosynthesis of Staphylococcus aureus Autoinducing Peptides by Using the Synechocystis DnaB Mini-Intein. Appl Environ Microbiol. 2007;73:6036. doi: 10.1128/AEM.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchdoerfer RN, Garner AL, Flack CE, Mee JM, Horswill AR, Janda KD, Kaufmann GF, Wilson IA. Structural basis for ligand recognition and discrimination of a quorum quenching antibody. J Biol Chem. 2011;286:17351–17358. doi: 10.1074/jbc.M111.231258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy AD, Wardenburg JB, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. Targeting of Alpha-Hemolysin by Active or Passive Immunization Decreases Severity of USA300 Skin Infection in a Mouse Model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoendel M, Horswill AR. Identification of Staphylococcus aureus AgrD residues required for autoinducing peptide biosynthesis. J Biol Chem. 2009;284:21828–21838. doi: 10.1074/jbc.M109.031757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, Nauseef WM. agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun. 2010;2:546–559. doi: 10.1159/000319855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE. 2011;6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Junio HA, Sy-Cordero AA, Ettefagh KA, Burns JT, Micko KT, Graf TN, Richter SJ, Cannon RE, Oberlies NH, Cech NB. Synergy Directed Fractionation of Botanical Medicines: A Case Study with Goldenseal (Hydrastis canadensis) J Nat Prod. 2011;74:1621–1629. doi: 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cech RA. Making Plant Medicine. Williams: Horizon Herbs; 2000. p. 276. [Google Scholar]
- 32.Methods for Dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd edition. Villanova, PA: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 33.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol. 1969;15:1067–1076. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]
- 35.Hyeon-Hee Y, Kang-Ju K, Jeong-Dan C, Hae-Kyong K, Young-Eun L, Na-Young C, Yong-Ouk Y. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8:454–461. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 36.Coulburn J, Chin YW, Parekh H, Yildiz S, Beaver R, Kinghorn AD, Wu CD. Oral Antimicrobial Compounds from Hydrastis canadensis L. (Goldenseal) Leaves. J Dent Res. 2007;86(Spec Iss A):2156. [Google Scholar]
- 37.Distribution and abundance of Hydrastis canadensis L. (Ranunculaceae) and Panax quinquefolius L. (Araliaceae) in the central Appalachian region.
- 38.Holtfreter S, Grumann D, Schmudde M, Nguyen HTT, Eichler P, Strommenger B, Kopron K, Kolata J, Giedrys-Kalemba S, Steinmetz I, Witte W, Bröker BM. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J Clin Microbiol. 2007;45:2669–2680. doi: 10.1128/JCM.00204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.