Abstract
Traumatic brain injury causes progressive brain atrophy and cognitive decline. Surprisingly, an early treatment with erythropoietin (EPO) prevents these consequences of secondary neurodegeneration, but the mechanisms have remained obscure. Here we show by advanced imaging and innovative analytical tools that recombinant human EPO, a clinically established and neuroprotective growth factor, dampens microglial activity, as visualized also in vivo by a strongly attenuated injury-induced cellular motility.
Keywords: ATP, brain injury, migration, process protrusion, Rac1
Introduction
Microglia are essential mediators of innate immunity in brain providing constant surveillance and protection via rapid reaction to homeostatic disturbances. Enduring microglia (over)activation, however, may foster neurodegeneration.1, 2, 3, 4 Recently, we reported a bilateral increase in activated microglia in the brain 24 hours after a small, standardized, unilateral parietal cortical cryolesion in juvenile mice, which persisted for at least 1 year and was associated with progressive global brain atrophy and cognitive decline. Intriguingly, early treatment with recombinant human erythropoietin (EPO) prevented this increase as well as the downstream neurodegenerative consequences.5, 6
Erythropoietin has been recognized for nearly two decades in both preclinical and clinical studies as a potent neuroprotective and neuroregenerative growth factor with a multifaceted, hematopoiesis-independent action profile.6 The present paper has been designed to test the hypothesis that EPO might modulate microglial motility.
Materials and methods
Materials and methods with associated references as well as four videos with legends are provided in the Supplementary information on the JCBFM website.
Results and discussion
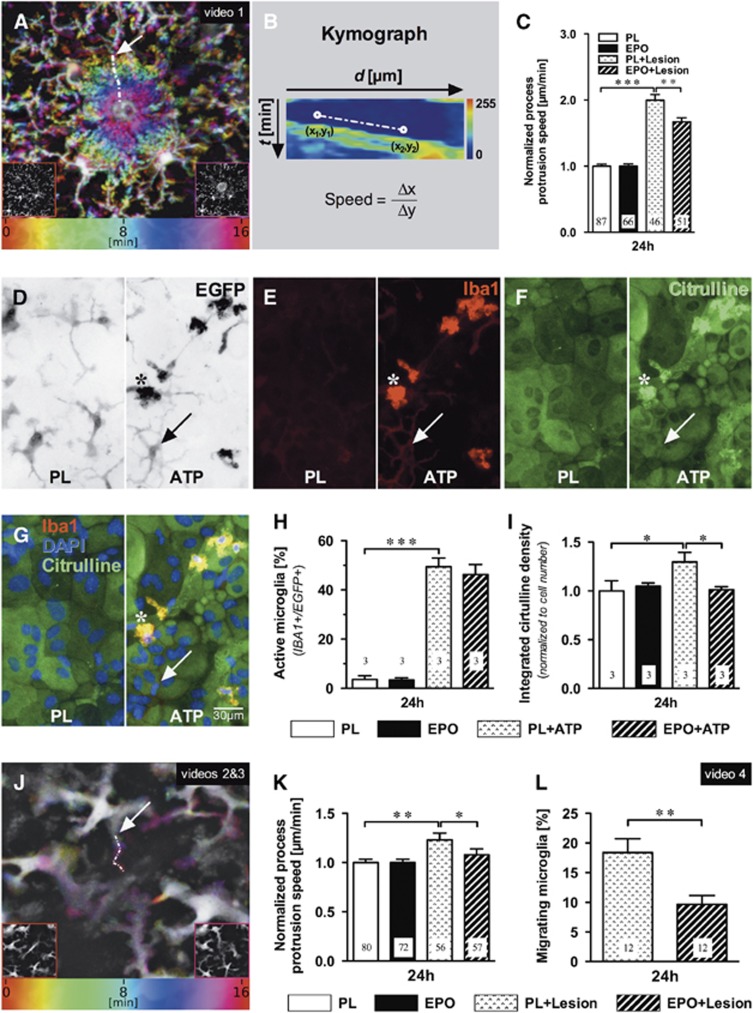
We first tested in vivo the hypothesis that EPO might modulate microglial motility. Using CX3CR1+/EGFP mice, we performed our standardized right parietal cortical cryolesion and injected EPO or placebo intraperitoneally immediately thereafter. At 24 hours later, we compared in vivo microglial process protrusion before (‘basal') and upon laser-induced microinjury in the contralateral (left) parietal cortex. Microglia respond strongly with goal-directed process movement to laser injury (Supplementary video 1).7, 8 This intense response was dampened under EPO compared with placebo. In contrast, basal movements were similar between treatment groups (Figures 1A–C).
Figure 1.
Erythropoietin (EPO) effects on cortical microglia in vivo and on microglia in mixed cultures. In vivo: (A) Microglial processes converge onto a central laser lesion (colors represent time). Lower left and right insets illustrate pre- and post-laser lesion views (see Supplementary Video 1). (B) Process movements were traced (arrow in A shows exemplary path) and sampled to create kymographs, whose slope corresponds to the process protrusion speed. (C) Laser lesion-induced process protrusion was higher than basal motility, but reduced by 24 hours EPO pretreatment (5 IU/g intraperitoneally), compared with placebo (PL). Numbers of analyzed processes from 4 to 5 independent experiments each are given in the bars. Mean±s.e.m. presented, **P⩽0.01, ***P⩽0.001 (two-tailed t-test). In vitro: (D to G) Representative images of surveilling (placebo, PL) and ATP-stimulated microglia: EGFP fluorescence (D, inverse signal), Iba1 (E), L-citrulline (F), overlay plus DAPI (G); arrows denote a weakly Iba1-stained, L-citrulline-negative ramified microglia; stars designate a strongly Iba1+/L-citrulline+ cell. (H) Increase in strongly Iba1+/EGFP+ microglia under ATP (300 μmol/L, 2 hours) and (I) reduction of ATP-stimulated L-citrulline by EPO pretreatment (3 IU/mL, 24 hours); numbers of independent experiments are given in the bars. (J) Microglia send processes to a central laser lesion (colors represent time). Insets correspond to pre- and post-laser lesion time points. (K) Kymograph-derived speed measurements (arrow in J shows exemplary path, see Supplementary videos 2 and 3) indicate increased process protrusion rate upon laser lesion, which was reduced under EPO (3 IU/mL, 24 hours). (L) Under EPO, a lower percentage of microglia migrates toward the laser lesion (Supplementary video 4). Numbers in bars refer to analyzed processes (K) or independent cultures (L). Mean±s.e.m. presented, *P⩽0.05, **P⩽0.01, ***P⩽0.001 (one- or two-tailed t-tests).
Next, we wondered whether the effect of systemically injected EPO on microglia would be reproducible in a less complex system, i.e., primary murine microglia-astrocyte mixed cultures, and thus be clearly independent of hematopoiesis. We developed an in vitro method of quantifying microglia movements upon laser-induced microinjury. To first characterize the activity state of the cultures under our experimental conditions, immunocytochemistry was performed with EPO or placebo, again added 24 hours before the planned time point of injury. Under placebo conditions, mainly ramified microglia in surveilling state (94% with only weak Iba1 staining) were seen. ATP, added for 2 hours as in vitro lesion equivalent7, 9, 10 provoked strong Iba1 and L-citrulline staining (Figures 1D–H). Expectedly, the L-citrulline signal, readout of inducible nitric oxide formation, was reduced under EPO (Figure 1I).11 Both microglial process motility (Figures 1J and 1K; Supplementary video 2) and cell migration (Figure 1L; Supplementary video 4), observed upon in vitro laser microinjury in this coculture, were attenuated by EPO.
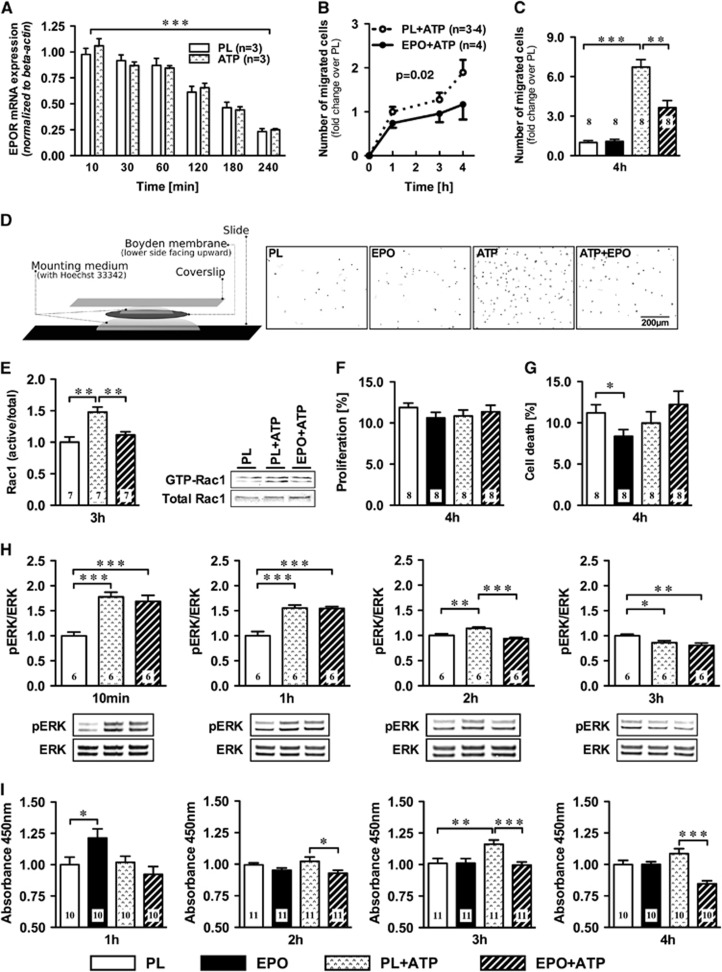
Multiple cell types in the brain are known to express EPOR (EPO receptor) and may contribute to the measured EPO effect on microglial motility. To prove a direct effect of EPO on microglia, we further reduced complexity by using pure microglia cultures (⩾99%). This allowed us to quantify the effect of EPO on microglial migration in the Boyden chamber under the influence of ATP (again as injury model). The EPOR mRNA expression declined from very prominent expression at seeding throughout the 4-hour experiment, likely related to the settling of the initially highly stressed cells (Figure 2A). Again, EPO acted on microglial motility, diminishing ATP-stimulated migration by up to 50% (Figures 2B–D).
Figure 2.
Erythropoietin (EPO) effects on pure microglia cultures. (A) EPO receptor (EPOR) mRNA levels decrease over time, independent of ATP. (B) Increasing number of ATP stimulated microglia (expressed in fold change of the placebo condition) passing through Boyden chamber membranes after 1, 3, and 4 hours, with EPO-treated cells showing less migration (area under the curve P=0.02), (C) most obvious at 4 hours. (D) Boyden membrane slide mount with representative fields-of-view of Boyden chamber membranes of the Hoechst-33342-stained nuclei (inverted) at 4 hours of placebo (PL) or EPO-pretreated microglia ±ATP exposure. (E) EPO pretreatment reduces ATP-stimulated Rac1 GTPase activation at 3 hours; representative western blots. (F) Proliferation or (G) cell death in these cultures. (H) MAPK signaling, investigated by monitoring pERK/ERK after 10 minutes, 1 hour, 2 hours, and 3 hours of 300 μmol/L ATP and 3 IU/mL EPO or PL, shows accelerated inactivation by EPO. (I) EPO decreased microglial water soluble tetrazolium-1 (WST-1) conversion starting with the 2-hour ATP stimulus. Numbers of experiments are given in the bars. Mean±s.e.m. presented, *P⩽0.05, **P⩽0.01, ***P⩽0.001 (two-tailed t-test).
We next searched for first hints regarding molecular mediators of this striking motility reduction. Taking into consideration that active microglial Rac1 is associated with numerous neurodegenerative diseases,12 we determined the activation state of this Rho GTPase 3 hours after seeding, the time point at which the migration curves under EPO versus placebo start dissociating (Figure 2B). Rac1 signaling is a critical component in the formation of lamellipodia, one of the earliest structural events in microglia motility.13, 14 ATP led to the expected increase in active/total Rac1,15 which was attenuated by EPO (Figure 2E). Erythropoietin did not influence proliferation of microglia in this context but slightly reduced cell death in the basal (nonATP) condition (Figures 2F and 2G).
Since EPO was shown to inhibit overactivation of MAPK in astrocytes,16 and MAPK signaling in turn is associated with Rac1 activity,12, 17 we determined the pERK/ERK ratio over time. Under EPO, the inactivation of the ERK pathway after ATP stimulation was accelerated, leading to a reduced pERK/ERK ratio at the 2-hour time point (Figure 2H). This acceleration could be a consequence of EPO-induced Rac1 inhibition, or indicate a mediator role of ERK for the reduced Rac1 activity under EPO.12, 17 We also noted that EPO decreased ATP-stimulated WST-1 (water soluble tetrazolium-1) conversion in microglia (Figure 2I), suggesting mitigated metabolic activity and lower superoxide production under EPO in conditions of injury.18
At three different levels of experimental complexity, ranging from in vivo to in vitro, we showed the capacity of EPO to reduce motility as an important feature of microglial (over)reaction to damage. This mechanism may underlie in part the beneficial effects observed for EPO in neurodegenerative diseases.
HE holds user patents for EPO in stroke, schizophrenia, and multiple sclerosis. The remaining authors state no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Author Contributions
MM executed all microscopy data analyses, including the development of novel imaging and analytical approaches. LD, with the help of MaM and SG, performed all cell culture work including confocal microscopy. MM conducted the TPLSM experiments. HE and KAN developed study concept and design, supported in respective parts by MM, LD, UKH, RH, and JS. HE, MM, and LD wrote the manuscript, had full access to all data in the study and take responsibility for data integrity and accuracy of analyses. All authors have seen and approved the manuscript.
This study was supported by the Max Planck Society and the DFG Research Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB), the SFB TRR 43, as well as by the Niedersachsen-Research Network on Neuroinfectiology (N-RENNT) of the Ministry of Science and Culture of Lower Saxony.
Supplementary Material
References
- Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia. Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano AM, Peri F. Microglia: multitasking specialists of the brain. Dev Cell. 2015;32:469–477. doi: 10.1016/j.devcel.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Siren AL, Radyushkin K, Boretius S, Kammer D, Riechers CC, Natt O, et al. Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain. 2006;129:480–489. doi: 10.1093/brain/awh703. [DOI] [PubMed] [Google Scholar]
- Sargin D, Hassouna I, Sperling S, Siren AL, Ehrenreich H. Uncoupling of neurodegeneration and gliosis in a murine model of juvenile cortical lesion. Glia. 2009;57:693–702. doi: 10.1002/glia.20797. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, et al. The p2y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vairano M, Dello Russo C, Pozzoli G, Battaglia A, Scambia G, Tringali G, et al. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16:584–592. doi: 10.1046/j.1460-9568.2002.02125.x. [DOI] [PubMed] [Google Scholar]
- Stankiewicz TR, Linseman DA. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 2014;8:314. doi: 10.3389/fncel.2014.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Persson AK, Estacion M, Ahn H, Liu S, Stamboulian-Platel S, Waxman SG, et al. Contribution of sodium channels to lamellipodial protrusion and Rac1 and ERK1/2 activation in ATP-stimulated microglia. Glia. 2014;62:2080–2095. doi: 10.1002/glia.22728. [DOI] [PubMed] [Google Scholar]
- Tang Z, Sun X, Huo G, Xie Y, Shi Q, Chen S, et al. Protective effects of erythropoietin on astrocytic swelling after oxygen-glucose deprivation and reoxygenation: mediation through AQP4 expression and MAPK pathway. Neuropharmacology. 2013;67:8–15. doi: 10.1016/j.neuropharm.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of rac1 and rhoa for tumor cell motility. Cancer Cell. 2003;4:67–79. doi: 10.1016/s1535-6108(03)00162-4. [DOI] [PubMed] [Google Scholar]
- Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, wst-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.