Abstract
The common functional single-nucleotide polymorphism (rs324420, C385A) of the endocannabinoid inactivating enzyme fatty acid amide hydrolase (FAAH) has been associated with anxiety disorder relevant phenotype and risk for addictions. Here, we tested whether the FAAH polymorphism affects in vivo binding of the FAAH positron emission tomography (PET) probe [11C]CURB ([11C-carbonyl]-6-hydroxy-[1,10-biphenyl]-3-yl cyclohexylcarbamate (URB694)). Participants (n=24) completed one [11C]CURB/PET scan and were genotyped for rs324420. Relative to C/C (58%), A-allele carriers (42%) had 23% lower [11C]CURB binding (λk3) in brain. We report evidence that the genetic variant rs324420 in FAAH is associated with measurable differences in brain FAAH binding as per PET [11C]CURB measurement.
Keywords: [11C]CURB; fatty acid amide hydrolase; FAAH polymorphism (rs324420, C385A); positron emission tomography
Introduction
Endogenous cannabinoids (endocannabinoids) are lipid neurotransmitters primarily known for ‘mimicking' the effects of cannabis on cannabinoid receptors (CB1); their critical roles in modulating human behaviors and physiologic processes, as well as their highly valued anxiolytic, analgesic, and anti-inflammatory properties, are increasingly being recognized (and investigated for therapeutic applications). Fatty acid amide hydrolase (FAAH) is a single-pass membrane protein of 579 amino acids and the primary catabolic enzfyme for the major endocannabinoid N-arachidonoylethanolamine (anandamide) and other fatty acid amides including N-palmitoylethanolamine and N-oleoylethanolamine and as such is a key regulator of endocannabinoid tone/signalling (evidenced by mice lacking FAAH, FAAH−/−) and is implicated in a number of human behaviors and has been a major target for drug development.1, 2, 3
A single-nucleotide polymorphism in the human FAAH gene has been associated with increased risk for addictions.4 This single-nucleotide polymorphism involves a transversion that changes a cytosine to an adenine (C385A) in the FAAH gene and results in the conversion of a conserved proline residue at amino-acid position 129 to threonine (P129T). Studies using T-lymphocytes from human peripheral blood,5 transfected COS-7 cells,4, 5 and FAAH knock-in mice3 to investigate the functional consequence of the variant enzyme indicate that relative to the wild-type variant, the variant FAAH has markedly reduced FAAH protein expression and steady-state activity, due to a posttranslational mechanism that precedes the productive folding of the enzyme. As a result those homozygous for the A allele have elevated plasma N-arachidonoylethanolamine (and related N-acylethanolamides) levels.3, 6 Approximately 38% of individuals of European descent have one or more copies of the variant form of the enzyme (AC and AA genotypes).4
Genetic association studies have thus far shown that the enzyme variant that leads to a reduced expression and functionality of FAAH; i.e., higher N-arachidonoylethanolamine (N-palmitoylethanolamine and N-oleoylethanolamine), is associated, in Caucasian individuals, with higher rates of cannabis sampling, higher reward reactivity (in functional magnetic resonance imaging task)7 and trait impulsiveness, but lower risk of development of substance use disorder diagnosis (reduced drug-craving, withdrawal symptoms and self-reported drug liking in cannabis and methamphetamine users) as well as lower anxiety-relevant traits (lower amygdala reactivity to threat during functional magnetic resonance imaging task and self-reported anxiety).1, 2, 3, 8
We recently developed [11C]CURB ([11C-carbonyl]-6-hydroxy-[1,10-biphenyl]-3-yl cyclohexylcarbamate (URB694)) the first available positron emission tomography (PET) radiotracer for imaging FAAH9 and described kinetic analyses in which the composite parameter λk3 (λ=K1/k2) derived from a two-tissue compartment model with irreversible trapping was recommended as the preferred index for PET quantification of FAAH activity.10 The aim of the current study was to test the hypothesis that having the variant form of the FAAH enzyme (A/A+A/C) would be associated with lower binding (λk3) of [11C]CURB than in those without the variant (C/C homozygotes).
Materials and methods
Subjects
All procedures were approved by the Centre for Addiction and Mental Health Research Ethics Board. Subjects were recruited from the local community in Toronto, Canada using Internet advertisements to participate in a single PET scan study with [11C]CURB. After provision of written informed consent, subjects completed a comprehensive medical/screening interview to rule out past or present significant medical conditions, neurologic illnesses or head trauma, Axis I psychiatric disorders, MR and PET counterindication, use of medication that may affect the central nervous system, or positive drug screening for drugs of abuse.
Image Acquisition and Reconstruction
The radiosynthesis of [11C]CURB has been described in our previous publication.9 The PET scanning was performed using a 3D HRRT brain tomograph (CPS/Siemens, Knoxville, TN, USA); details of the image acquisition are given in Rusjan et al.10 In brief, after lying down on the scanning table with head held in place with a thermoplastic mask to reduce movement, a short transmission scan was acquired followed by injection of ~370±40 MBq (~10±1 mCi) of [11C]CURB. Radioactivity in brain was measured during sequential frames of increasing duration. Scanning time was 60 minutes. The images were reconstructed from the 2D sinograms using a 2D filtered-back projection algorithm, with a HANN filter at Nyquist cutoff frequency.
Arterial samples were taken from a radial artery manually at time 3, 7, 12, 20, 30, 45, and 60 minutes after injection and automatically for 22.25 minutes (automatic blood sampling system, Model PBS-101, Veenstra Instruments, Joure, The Netherlands). A metabolite corrected plasma curve was generated and used as the input function for the kinetic analysis (details in Rusjan et al10). Blood-to-plasma radioactivity ratios were interpolated by a biexponential function and the fraction of parent compound in plasma by a Hill function.
Subjects underwent standard proton density weighted brain magnetic resonance imaging on a Discovery MR750 3 T MRI scanner (General Electric, Mississauga, ON, Canada) for the purpose of region of interest (ROI) delineation.
Region of Interest Kinetic and Statistical Analysis
Time-activity curves acquired over 60 minutes (as per Rusjan et al10) in each ROI (listed in Figure 1) were extracted using ROMI (details in Rusjan et al11) and analyzed using a two-tissue compartment model with irreversible binding to the second compartment described in Rusjan et al.10 The parameters of interest to quantify FAAH activity are the composite parameter λk3 (λ=K1/k2).
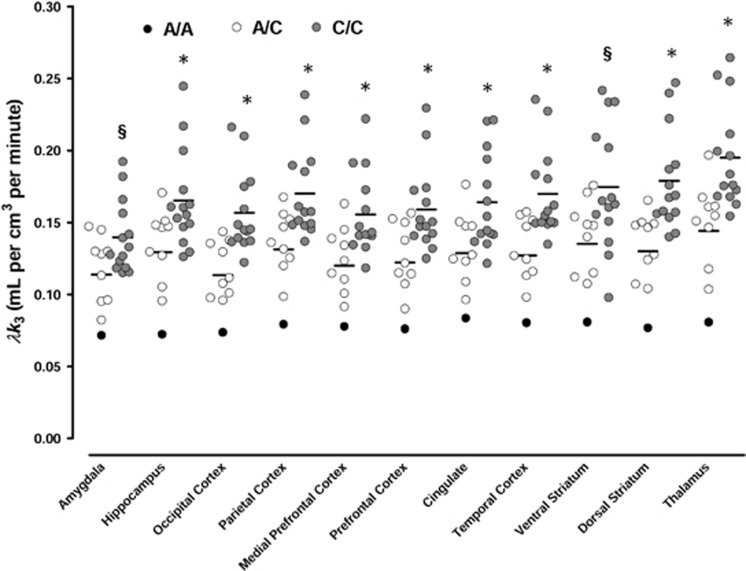
Figure 1.
Scattergram of [11C]CURB ([11C-carbonyl]-6-hydroxy-[1,10-biphenyl]-3-yl cyclohexylcarbamate (URB694)) λk3 in A/A+A/C and CC homozygotes for the fatty acid amide hydrolase C385A variants. *Significant at P<0.05; §P=0.1.
Differences in [11C]CURB λk3 in each ROI between the genotype groups was investigated with repeated-measures ANOVAs (SPSS 20.0, SPSS Inc., Chicago, IL, USA). When appropriate, least significant difference t-tests, Bonferroni corrected, were applied to determine the significance of regional differences in [11C]CURB binding between genotype groups (AA+A/C vs CC).
FAAH Genotyping
The FAAH genotype (rs324420) was determined using the Taqman SNP genotyping assay set performed on a ViiA7 thermal cycler (Life Technologies, Burlington, Ontario, Canada) with appropriate controls. Briefly 5 μl of 2x GTXpress Master mix (cat#4401892, Life Technologies) is mixed with 10 ng of DNA and the 40 × probe (cat#C_1897306_10, Life Technologies) in a final volume of 10 μl and run for 50 cycles of 95°C for 1 second and 60°C for 20 seconds.
Results
Healthy volunteers (n=25) were recruited and 24 completed the full study (one subject's genotype data were not available). Subject characteristics are reported in Table 1. Groups were matched with respect to gender and ethnicity and their body mass index was within normal range; however, we noted a difference in age between A/A+A/C vs C/C homozygotes. All subjects tested negative for drugs of abuse at the interview (and on scan day) and only one subject smoked nicotine cigarettes occasionally. There were no differences in scan parameters between groups (all P>0.5).
Table 1. Demographic and clinical characteristics of subjects.
| A/A+A/C (n =10) | C/C homozygotes (n=14) | P value | |
|---|---|---|---|
| Age, mean±s.d. (range) | 41±12 (22–56) | 30±8 (19–42) | 0.024 |
| Gender (M, F) | 4, 6 | 8, 6 | (χ2=0.69) 0.41a |
| Ethnicity (White, Asian, Black) | 6, 3, 1 | 9, 4, 1 | (χ2=0.08) 0.96a |
| Education (years) | 17±3 | 15±2 | 0.144 |
| Body mass index (kg/m2) | 24±4 | 23±2 | 0.489b |
| Cigarette smokers (# of smokers) | 1 | 0 | (χ2=1.46) 0.23a |
| Alcohol use (# of non-drinkers) | 1 | 3 | (χ2=0.55) 0.46a |
| Alcohol drinks a week | 0.44 | 1.64 | 0.227 |
| Cannabis ‘ever used' | 5 | 1 | (χ2=5.71) 0.05a |
| Dose injected (mCi) | 9.35±0.77 | 9.36±0.81 | 0.971 |
| Specific activity (mCi/μmol) | 3,110±1123 | 3,317±1325 | 0.693 |
| Mass injected (μg) | 1.07±0.45 | 1.04±0.46 | 0.840 |
Unless otherwise indicated, P values were obtained using independent sample t-tests.
Comparisons of proportions were carried out using Chi-Square tests.
Non-significant (P<0.05) when adjusted for age differences between groups.
Repeated measure ANOVA with age as a co-variate indicated a significant effect of group (genotype C/C vs A/A+A/C) on [11C]CURB binding (λk3, F(1, 21)=7.2, P=0.014, Cohen's d 1.17) and a significant ROI by genotype group interaction (F(10, 210)=2.3, P=0.016). The magnitude of the difference in (11) selected ROIs was −23%. Follow-up pairwise comparisons revealed that all regions with the exception of amygdala and ventral striatum showed significantly lower [11C]CURB binding in A/A+A/C genotype vs C/C homozygotes. The effects in the ventral striatum and amygdala were marginal (P=0.1). There was only one participant with A/A genotype; this subject (Caucasian, female, 52 years) had the lowest [11C]CURB binding across all ROIs, specifically the mean difference between C/C homozygotes and the single A/A subject was −53% (Figure 1). Taking into account ethnicity did not change the findings. There were no correlations between [11C]CURB λk3 and age, gender or body mass index (taking into account genotype). The genetic variants did not significantly affect plasma metabolism of the radiotracer (main genotype effect: F(1, 22)=2.0, P=0.17; time-by-genotype interaction: F(6, 132)=0.69, P=0.66) although there was a trend for decreased metabolism in A/A+A/C genotype vs C/C genotype at the end of the scan (%unmetabolized [11C]CURB=33±9% and 40±8%, respectively; P=0.06).
Discussion
We provide, for the first time, in vivo evidence for a variant A-allele dose-dependent reduction in [11C]CURB/PET binding (−20% in A/C, −50A/A) in the brains of living humans. This finding is in agreement with the previous data in human peripheral blood T-lymphocytes,5 in transfected COS-7 cells,4, 5 and in FAAH knock-in mice,3 which had suggested lower levels of activity and protein of the enzyme. The impact of the P129T variant on human brain FAAH levels, previously inferred from peripheral measures, is now demonstrated.
Based on preclinical studies investigating the impact of the P129T variation on FAAH, we suggest that changes in [11C]CURB/PET binding reflect reduced FAAH protein levels (i.e., Bmax) rather than affinity to the radioligand. This variant, in the conserved P129 amino acid, did not impact gene expression or catalytic activity (Km and kcat) of the enzyme but rather was shown to destabilize its local structure and increase proteolytic sensitivity leading to reduced FAAH protein levels and activity.4, 5 However, given that P129T is localized on the cytoplasmic loop close to Lys142 that is part of the Ser-Ser-Lys catalytic triad of FAAH,12 we cannot exclude the possibility that this variation might affect the binding affinity of [11C]CURB.
[11C]CURB is a recently developed PET radiotracer for FAAH and our blocking studies with a highly specific FAAH inhibitor PF-04457845 confirmed that brain [11C]CURB binding is very specific and sensitive to FAAH inhibition (JCBFM, submitted). This genetic report again demonstrated the sensitivity of this PET ligand to endogenous FAAH activity changes and serves to validate the modelling parameter λk3 for FAAH quantification by PET.
The reported findings have important implications for interpretation and designing of studies measuring FAAH activity with [11C]CURB/PET. Genotyping the FAAH rs324420 (C385A) polymorphism and genetic profiling during statistical analysis will insure adequate comparison in cross-sectional studies aimed at quantifying FAAH activity in different clinical population, which may or may not be otherwise matched with respect to genotype. In this regard, a number of gene association studies have suggested that different frequencies of the FAAH variant may occur in certain neuropsychiatric conditions (including addictions and generalized anxiety disorder).2, 3, 4, 7, 8 In our sample, the prevalence of FAAH variants (C/C, 58% A/C, 38% A/A, 4%) was similar to that in the literature for healthy controls. Another generic point to consider was the possibility that FAAH activity driven by racial differences in the impact of the gene variant has a race-specific effect. However, we did not observe an effect of race on [11C]CURB/PET binding: our genetic groups were matched with regard to race and composed, for the most part of Caucasian subjects; this suggests a similar impact of the FAAH genetic variant among racial groups; however, future studies may need to further confirm this observation.
The limitations of this report include a small sample size and that the genotypes were not matched for age; the A/A+A/C group was significantly older than C/C genotype. However, animal studies reported either no effect13, 14 or an age-related increase13, 15 in FAAH activity or protein and we did not observe a significant effect of age on [11C]CURB/FAAH binding in the limited sample. Future comprehensive studies are needed to confirm this and further to address the relationship between FAAH genetic variation, brain [11C]CURB/PET binding and peripheral measures of FAAH activity and protein or endocannabinoid levels. Examination of autopsied human brain for FAAH genotype and the association with activity/protein would also help confirm our PET findings.
In conclusion, we show for the first time that FAAH activity in brain is dependent on a common gene variant and that genotyping for FAAH rs324420 variants during [11C]CURB/PET will allow better interpretation of data.
The authors declare no conflict of interest.
Footnotes
Author Contributions
IB and JT contributed to study concept and design, analysis and interpretation of data, drafting/revising the manuscript for content, study coordination, and acquisition of data. RFT and QZ contributed to genetic data analysis, interpretation of data, and revising the manuscript for content. BW, EM, and DW contributed to acquisition and analysis of data and revising the manuscript for content. PR contributed to data analysis/kinetic modelling, interpretation of data, and revising the manuscript for content. BLF, RM, and VDL contributed to study medical supervision, study concept and design, and revising the manuscript for content. AAW contributed to tracer radio-synthesis, study concept and design, revising manuscript for content, and interpretation of data. SH contributed to study concept and design, revising manuscript for content, interpretation of data, and study medical supervision. SJK contributed to study concept and design, revising manuscript for content, and interpretation of data.
This study was supported in part by Canada Foundation for Innovation, the Ontario Ministry of Research and Innovation (SH), The National Institute of Health and National Institute of Drug Abuse (NIH/NIDA R21 DA036024 (IB)), CIHR TMH109787 (RFT), and an Endowed Chair in Addictions (Psychiatry Department, RFT).
References
- Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013;34:637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Echeverry-Alzate V, Buhler KM. The genetic basis of the endocannabinoid system and drug addiction in humans. J Psychopharmacol. 2012;26:133–143. doi: 10.1177/0269881111416689. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Scott TM, Murray S, Harismendy O, Simon GM, Cravatt BF, et al. Biomarkers of endocannabinoid system activation in severe obesity. PLoS One. 2010;5:e8792. doi: 10.1371/journal.pone.0008792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:660–666. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Parkes J, Houle S, Tong J, Vasdev N. [11C]CURB: evaluation of a novel radiotracer for imaging fatty acid amide hydrolase by positron emission tomography. Nucl Med Biol. 2011;38:247–253. doi: 10.1016/j.nucmedbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Wilson AA, Mizrahi R, Boileau I, Chavez SE, Lobaugh NJ, et al. Mapping human brain fatty acid amide hydrolase activity with PET. J Cereb Blood Flow Metab. 2013;33:407–414. doi: 10.1038/jcbfm.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science. 2002;298:1793–1796. doi: 10.1126/science.1076535. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Attina M, Bari M, Cartoni A, Ledent C, Finazzi-Agro A. Anandamide degradation and N-acylethanolamines level in wild-type and CB1 cannabinoid receptor knockout mice of different ages. J Neurochem. 2001;78:339–348. doi: 10.1046/j.1471-4159.2001.00413.x. [DOI] [PubMed] [Google Scholar]
- Bishay P, Haussler A, Lim HY, Oertel B, Galve-Roperh I, Ferreiros N, et al. Anandamide deficiency and heightened neuropathic pain in aged mice. Neuropharmacology. 2013;71:204–215. doi: 10.1016/j.neuropharm.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Pascual AC, Martin-Moreno AM, Giusto NM, de Ceballos ML, Pasquare SJ. Normal aging in rats and pathological aging in human Alzheimer's disease decrease FAAH activity: modulation by cannabinoid agonists. Exp Gerontol. 2014;60:92–99. doi: 10.1016/j.exger.2014.10.011. [DOI] [PubMed] [Google Scholar]