Abstract
Complement activation has been implicated in ischemia/reperfusion injury. This study aimed to determine whether mild hypothermia (HT) inhibits systemic and cerebral complement activation after resuscitation from cardiac arrest. Sixteen minipigs resuscitated from 8 minutes of untreated ventricular fibrillation were randomized into two groups: HT group (n=8), treated with HT (33°C) for 12 hours; and normothermia group (n=8), treated similarly as HT group except for cooling. Blood samples were collected at baseline and 0.5, 6, 12, and 24 hours after return of spontaneous circulation (ROSC). The brain cortex was harvested 24 hours after ROSC. Complement and pro-inflammatory markers were detected using enzyme-linked immunosorbent assay. Neurologic deficit scores were evaluated 24 hours after ROSC. C1q, Bb, mannose-binding lectin (MBL), C3b, C3a, C5a, interleukin-6, and tumor necrosis factor-α levels were significantly increased under normothermia within 24 hours after ROSC. However, these increases were significantly reduced by HT. Hypothermia decreased brain C1q, MBL, C3b, and C5a contents 24 hours after ROSC. Hypothermic pigs had a better neurologic outcome than normothermic pigs. In conclusion, complement is activated through classic, alternative, and MBL pathways after ROSC. Hypothermia inhibits systemic and cerebral complement activation, which may provide an additional mechanism of cerebral protection.
Keywords: cardiac arrest, complement, mild hypothermia, neuroprotection, pig
Introduction
Cardiac arrest and resuscitation represent a pathophysiologic state of whole-body ischemia/reperfusion, also described as postcardiac arrest syndrome.1, 2 The morbidity and mortality of patients who are resuscitated from cardiac arrest can be partly attributed to the immediate and delayed effects of reperfusion injury to vital organs, especially the brain and heart.2 Ischemia/reperfusion triggers systemic activation of the innate immune system, including a systemic inflammation response via direct effects on leukocytes, endothelium, and other tissues, and this has many features in common with sepsis.1, 3 This systemic inflammatory response may further contribute to injury of vital organs. Growing evidence has shown that the inflammatory response is associated with complement activation.4, 5, 6, 7 Recently, some clinical studies have showed activation of the complement system in cardiac arrest survivors.4, 8
The complement system is one of the major components of innate immunity. Activation of the complement system contributes to a variety of protective responses, including opsonophagocytosis, coordinating various events during inflammation, and killing pathogens and removal of damaged host cells.9 Complement is activated via three main pathways or the recently described extrinsic pathway. (1) The classic pathway is triggered by the binding of C1q to microorganisms, immune complexes, and apoptotic and necrotic cells to initiate the stepwise activation of C1r and C1s. (2) The alternative pathway is triggered by deposition of spontaneously activated complement components directly on microbial surfaces. (3) The mannose-binding lectin (MBL) pathway is initiated by the binding of MBL and serum ficolins to the terminal mannose, fucose, glucose, and N-acetyl-d-glucosamine moieties on pathogens or necrotic and apoptotic cells to activate MBL-associated serine proteases-2.4, 10 All of these pathways converge at the point of C3-convertase formation, which cleaves C3 into C3a and C3b.4 Increasing evidence has shown that circulating complement components can enter brain parenchyma via a damaged blood–brain barrier.11, 12 In addition, complements and their receptors can be expressed by astrocytes, microglia, neurons, and oligodendrocytes.11, 12
Currently, mild hypothermia is the only effective therapy for improving neurologic outcome and survival in cardiac arrest patients and animals.1, 13, 14, 15, 16, 17 Mild hypothermia has been documented to alter the immune response and reduce pro-inflammatory cytokines.18, 19, 20, 21 There are inconsistent results regarding the effect of mild hypothermia on the complement system. Recently, an in vitro study showed increased classic complement activation during mild hypothermia.22 In contrast, blunted complement activation was observed in postcardiac arrest patients during 24 or 72 hours of whole-body mild hypothermia.23, 24 However, these two observational in vivo studies lacked a normothermic (NT) control group owing to ethical considerations. Therefore, a causal effect between mild hypothermia and complement activation could not be established in these studies.
In the present study, we investigated (1) the effect of mild hypothermia on systemic and cerebral complement activation, and (2) the associations of complement activation with inflammation and neurologic outcome in a swine model of cardiac arrest.
Materials and Methods
Preparation of Animals
This study was conducted in strict accordance with the guideline for animal care and use established by the Capital Medical University Animal Care and Use Committee. The protocol was approved by the Committee on the Ethics of Animal Experiments of Capital Medical University.
Twenty male inbred Chinese Wuzhishan minipigs (Permit Number: SYXK (Beijing) 2008-0007, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China) that were aged 4 to 6 months and weighed 27.3±2.0 kg were fasted overnight, except for free access to water. Anesthesia was initiated by ketamine (20 mg/kg intramuscularly) and completed by propofol (2 mg/kg intravenously) in an ear vein. After premedication, pigs were supinely secured on the operating table with a ‘V' bedding and transfused with normal saline (2 to 10 mL/kg/h intravenously guttae) to maintain a central venous pressure of 5 to 12 mm Hg. Pentobarbital (8 mg/kg intravenously) was administered at intervals of ~1 hour to maintain anesthesia until 18 hours after return of spontaneous circulation (ROSC), followed by injection of propofol (0.4 mg/kg intravenously) when necessary. Propofol was used before evaluation of neurologic deficit scores because recovery from propofol is more rapid and complete compared with pentobarbital. Fentanyl (5 μg/kg/h intravenously guttae) was administered for analgesia. After endotracheal intubation, the pigs were ventilated with a volume-controlled ventilator (Servo 900c, Siemens, Munich, Germany) with a tidal volume of 15 mL/kg, a fraction of inspiration O2 of 0.21, and a ventilation rate of 12 to 20 breaths/min. End-tidal CO2 was monitored with an in-line infrared capnograph (CO2SMO Plus monitor, Respironics, Pittsburgh, PA, USA). The ventilation rate and tidal volume were adjusted to maintain normocapnia (35 to 45 mm Hg). Arterial blood gases (ABL80, Radiometer, Copenhagen, Denmark) were analyzed to confirm adequate baseline ventilation.
To record the standard lead II electrocardiogram, three adhesive electrodes were applied to the shaved skin of two upper limbs and left lower limb. Mean aortic pressure was measured with a fluid-filled 7F catheter (Edwards Life Sciences, Irvine, CA, USA) advanced from the left femoral artery into the thoracic aorta. To measure right atrial pressure and cardiac output, a Swan–Ganz catheter (Edwards Life Sciences) was advanced from the left femoral vein and flow directed into the pulmonary artery. The electrocardiogram, aortic and right atrial pressure, and cardiac output were continuously monitored during the experiment with a monitor (Vigilance II; Edwards Life Sciences). To induce ventricular fibrillation (VF), a 5F pacing catheter was advanced from the right external jugular vein into the right ventricle. To cool pigs, a central venous cooling catheter (Icy; Alsius Corp., Irvine, CA, USA) was advanced from the right external jugular vein into the superior vena cava after induction of VF was finished and the 5Fr pacing catheter was pulled out. A temperature-sensing Foley catheter (Integral Medical Products, Shaoxing, China) was inserted into the bladder after fistulation. The central venous catheter and the temperature-sensing Foley catheter (Integral Medical Products) were connected to an external cooling device (CoolGard 3000 system, Alsius Corp.). The operation was performed using aseptic surgical techniques. All catheters were flushed and filled with heparinized normal saline (5 U/mL) to prevent clotting. Prior to induced VF, intrabladder temperature (core temperature) was adjusted to 37°C to 38°C using a heating lamp and warm packs if the temperature was lower, or an electric fan and ice bags if the temperature was higher.
Experimental Protocol
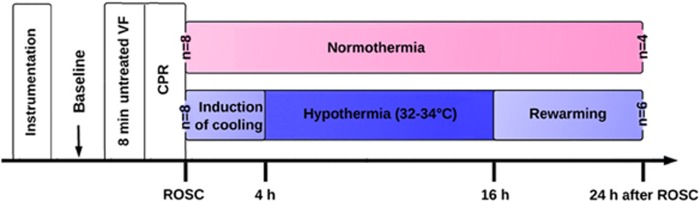
After instrumentation, the pigs were allowed to equilibrate for 30 minutes to achieve a stable resting level, and then baseline values were obtained (Figure 1). Ventricular fibrillation was induced by programmed electric stimulation and confirmed by the VF wave in the electrocardiogram. Mechanical ventilation was discontinued after the onset of VF. After 8 minutes of untreated VF, cardiopulmonary resuscitation was started, which included chest compressions performed by the same investigator and ventilation conducted using a bag respirator attached to an endotracheal tube with room air. The compression-to-ventilation ratio was 30:2. After 2 minutes of cardiopulmonary resuscitation, a single 150-J biphasic electrical shock was attempted with a Smart Biphasic defibrillator (Philips Medical Systems, Andover, MA). If the shock failed, another 2 minutes of cardiopulmonary resuscitation was resumed, followed by epinephrine (30 μg/kg intravenously) via the femoral vein. Additional doses of epinephrine were administered, if necessary, every 3 minutes until ROSC was achieved. The 200- J shocks were used for the second and all subsequent attempts. Return of spontaneous circulation was defined as an organized cardiac rhythm with a mean arterial pressure of >60 mm Hg, which was sustained continuously for at least 10 minutes.15, 16 Cardiopulmonary resuscitation was terminated if animals had no ROSC for 20 minutes. Immediately after ROSC, mechanical ventilation was resumed with the same setting as baseline values. The pigs were randomized using the envelope method into the mild hypothermic (HT) group or NT group (see details below).
Figure 1.
Experimental procedure. Blood samples were collected at baseline and 0.5, 6, 12, and 24 hours after ROSC. CPR, cardiopulmonary resuscitation; ROSC, restoration of spontaneous circulation; VF, ventricular fibrillation.
These animals received postresuscitation HT or NT intensive care. Induction of mild hypothermia was initiated immediately after ROSC in the HT group. Pigs were actively cooled to a targeted body temperature of 33°C (1.0°C/h), maintained at this temperature for 12 hours, and then actively rewarmed (0.5°C/h) to ~37°C using the CoolGard 3000 system (Alsius Corp.) according to the landmark study by Bernard et al.14 Hypothermia-induced shivering was suppressed through administration of pentobarbital and warming the skin with a quilt. The NT pigs were treated identically to the HT pigs, except for cooling with the CoolGard 3000 system (Alsius Corp.). Obvious hyperthermia was prevented as much as possible by using an electric fan and ice bags.
Blood samples were collected in 0.109 mol/L trisodium citrate tubes (9:1 vol/vol) via the left femoral arterial catheter at baseline and 0.5, 6, 12, and 24 hours after ROSC. The initial 5 mL of each blood sample was discarded because of containing heparinized normal saline. The subsequent 5 mL of blood sample was immediately centrifuged at 1500 g for 10 minutes (4°C). The plasma was stored at −80°C until analysis. At 24 hours after ROSC, neurologic deficit scores were evaluated and then the pigs were euthanized by 10 mL of potassium chloride (10 mol/L intravenously) after injecting propofol (3 mg/kg intravenously). Frontal cortex samples of the left hemisphere were rapidly harvested, immediately snap-frozen in liquid nitrogen, and subsequently stored at −80°C until later use. The cerebral cortex was chosen for study because it is closely related to functional outcomes.
Determination of Plasma and Brain Complement Components and Plasma Pro-Inflammatory Mediators
Frozen cerebral cortex samples were homogenized in buffer containing 2 mmol/L ethylene diamine tetraacetic acid (EDTA), 10 mmol/L ethylene glycol tetraacetic acid (EGTA), 20 mmol/L Tris, 0.25 mmol/L sucrose, and 1% Triton X-100. A protease inhibitor cocktail tablet (Sigma-Aldrich, St Louis, MO, USA) was added to each 10 mL of the homogenization buffer. The homogenate was then centrifuged at 100,000g for 45 minutes (4°C) and supernatants were collected. Total protein content was determined using the Bradford method. The levels of C1q, Bb, MBL, C3b, C3a, and C5a (BlueGene Biotech, Shanghai, China) in plasma and in the homogenate of the cerebral cortex, as well as plasma tumor necrosis factor (TNF)-α and interleukin (IL)-6 (BlueGene Biotech) levels were detected using an enzyme-linked immunosorbent assay according to the manufacturer's instructions. The brain contents of complement components were expressed in pg/mg (C1q), μg/mg (MBL and C3b), and ng/mg (Bb, C3a, and C5a) of total protein content. Each sample was tested in triplicate and then averaged.
Neurologic Deficit Scores
Sedation and analgesia were discontinued at 23 hours after ROSC. Neurologic deficit scores were evaluated 24 hours after ROSC, as described previously.16, 25 Specifically, neurologic deficit scores included the level of consciousness, motor and sensory function, respiratory patterns, and behavior (See Appendix A). The scores from each category were summed. A minimum score of zero indicated that pigs were normal, whereas a total score of 400 represented brain death. Investigators were masked to the pigs' respective treatments.
Appendix A. Neurologic deficit scores for pigs.25.
| Level of consciousness | |
| 0 | Normal; complete awareness of auditory stimuli |
| 30 | Clouded; conscious, but drowsy or irritable |
| 60 | Stupor; motor response only to painful stimuli |
| 100 | Coma; no motor response to painful stimuli |
| Motor and sensory function | |
| Motor response to pinch hoof pad | |
| 0 | Normal; brisk withdrawal |
| 10 | Sluggish response |
| 25 | Very sluggish response |
| 50 | No response |
| Muscle tone: pick up and release extremity | |
| 0 | Normal tone |
| 25 | One or two extremities stiff or flaccid |
| 50 | Three or four extremities stiff or flaccid |
| Respiratory pattern | |
| 0 | Normal |
| 50 | Abnormal spontaneous breathing |
| 100 | Apnea |
| Behavior | |
| Standing | |
| 0 | Can stand |
| 20 | Cannot stand |
| Walking | |
| 0 | Normal |
| 10 | Unsteady gait |
| 20 | Very unsteady or ataxic gait |
| 30 | Cannot walk |
| Restraint: attempt to hold down pig from behind | |
| 0 | Normal: vigorously resists |
| 20 | Sluggish; resists |
| 40 | Very sluggish; resists minimally |
| 50 | No resistance |
Values are assigned for deficits in neurological function (e.g., a score of 0 is normal and a score of 400 reflects brain death).
Statistical Analysis
Data are presented as the mean±s.d. or median (interquartile range 25th to 75th percentile). After confirmation of normal distribution with the Kolmogorov–Smirnov test, variables among time points were compared using repeated-measure analysis of variance, followed by the Bonferroni test for multiple comparisons. Mauchly's sphericity test was used to validate spherical distribution, and Greenhouse–Geisser corrections were used when indicated. Student's t-test was used to assess differences between both groups at each time point. Spearman's rank correlation coefficient method was used for correlation analysis. The 24-hour survival rate was compared using Fisher's exact test. Cumulative survival curves as a function of time were generated using the Kaplan–Meier method and compared using the log-rank test. The Mann–Whitney U-test was used if the data were not normally distributed. A P-value of <0.05 was considered statistically significant. Analysis was performed using the software package SPSS 16.0 (SPSS, Chicago, IL, USA).
Results
Resuscitation Data, Baseline Characteristics, and Hemodynamics
Sixteen of the 20 (80.0%) pigs were successfully resuscitated from cardiac arrest. Cardiopulmonary resuscitation time (3.2±1.2 minutes versus 3.3±1.1 minutes, P>0.05), the number of defibrillation attempts (1.4±0.6 versus 1.5±0.5, P>0.05), and the total amount of epinephrine used (0.9±0.4 mg versus 0.8±0.3 mg, P>0.05) were not significantly different between the HT (n=8) and NT (n=8) groups.
At baseline, there were no significant differences in weight (27.3±2.1 kg versus 27.2±1.9 kg, P>0.05), bladder temperature (37.9°C±0.3°C versus 37.9°C±0.2°C, P>0.05; Figure 2), arterial lactate levels (0.7±0.2 mmol/L versus 0.8±0.3 mmol/L, P>0.05), or PaO2 (91±8 mm Hg versus 92±7 mm Hg, P>0.05), nor in hemodynamic parameters (Table 1) between the groups. At 6 and 12 hours after ROSC, heart rate and cardiac output were significantly lower in the HT group compared with the NT group (all P<0.05; Table 1).
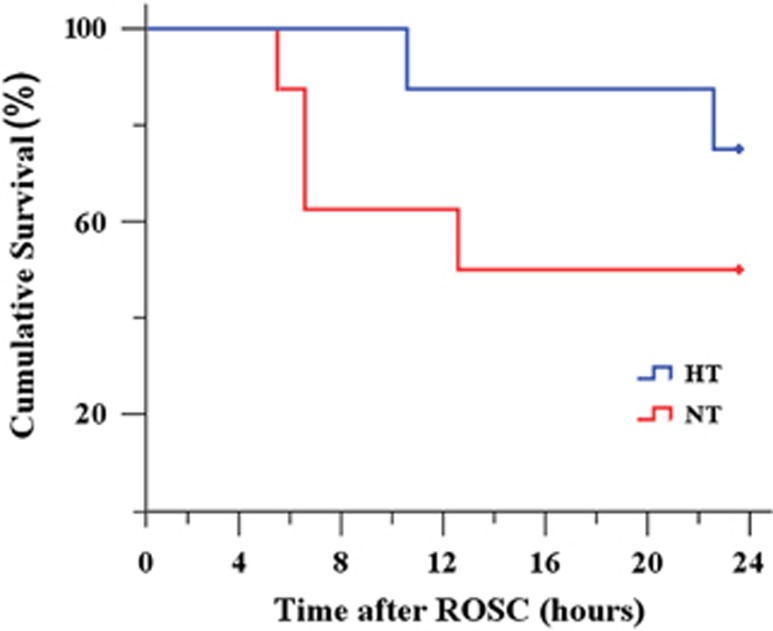
Figure 2.
Kaplan–Meier survival curves. The survival curves showed no significant difference between the NT and HT groups (P=0.24) when compared using the log-rank test. HT, mild hypothermia; NT, normothermia; ROSC, restoration of spontaneous circulation.
Table 1. Hemodynamic data.
| Baseline |
After ROSC |
||||
|---|---|---|---|---|---|
| 0.5 hours | 6 hours | 12 hours | 24 hours | ||
| Heart rate (beats/min) | |||||
| NT | 94±8 | 99±11 | 95±8 | 96±10 | 97±9 |
| HT | 95±9 | 98±10 | 83±4* | 82±3* | 95±8 |
| MAP (mm Hg) | |||||
| NT | 113±8 | 94±9 | 102±10 | 107±7 | 111±12 |
| HT | 112±9 | 93±8 | 98±6 | 102±7 | 109±11 |
| CPP (mm Hg) | |||||
| NT | 76±3 | 68±5 | 71±3 | 74±4 | 75±2 |
| HT | 77±3 | 65±4 | 68±4 | 71±5 | 77±4 |
| CO (L/min) | |||||
| NT | 4.5±0.3 | 3.1±0.4 | 3.2±0.5 | 3.4±0.3 | 3.6±0.3 |
| HT | 4.4±0.5 | 3.1±0.5 | 2.5±0.6* | 2.6±0.4* | 3.9±0.3 |
Abbreviations: CPP, coronary perfusion pressure (CPP=diastolic aortic pressure-right atrial pressure); CO, cardiac output; HT, mild hypothermia; MAP, mean aortic pressure; NT, normothermia; ROSC, restoration of spontaneous circulation.
*P<0.05 versus the NT group.
Core Temperature
In the NT group, the core temperature ranged from 37.9°C to 38.4°C after ROSC, and was significantly higher at 10, 12, 20, 22, and 24 hours after ROSC than at baseline (all P<0.05). In the HT group, the core temperature gradually decreased to the targeted temperature (32°C to 34°C) at ~4 hours after ROSC, and then was maintained at this temperature for 16 hours after ROSC, followed by slow rewarming. At 24 hours after ROSC, the core temperature returned to 37.2°C±0.3°C.
Survival Data and Neurologic Outcomes
Four out of eight resuscitated (50.0%) pigs in the NT group and six out of eight resuscitated (75%) pigs in the HT group survived for 24 hours, but this difference (25%) in survival rate did not reach significance (P>0.05). The log-rank test showed no significant difference between the two survival curves (P=0.24; Figure 3). Specifically, in the NT group, three pigs died between 6 to 12 hours after ROSC and one pig died at 13 hours after ROSC. In the HT group, one pig died at 11 hours after ROSC and one pig died at 23 hours after ROSC. They all died of refractory shock.
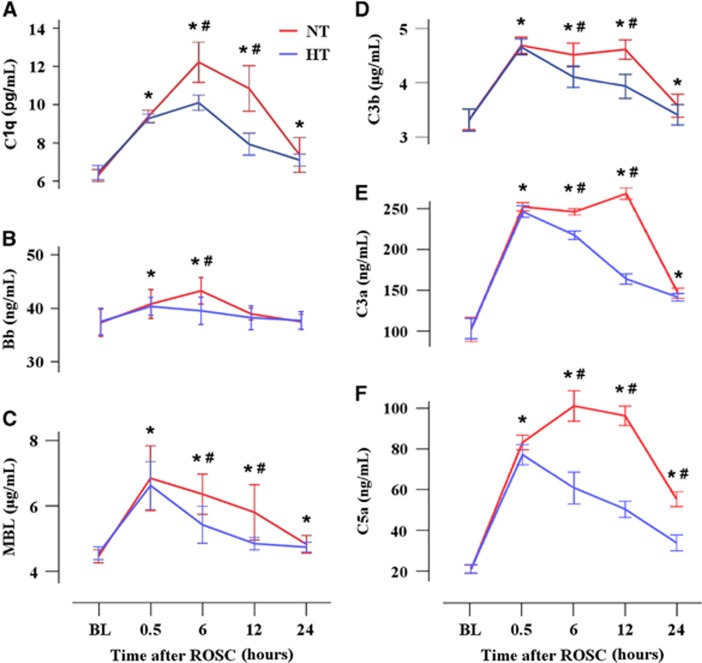
Figure 3.
Mild hypothermia inhibits complement activation in plasma and brain parenchyma. Complement fragments C1q (A), Bb (B), MBL (C), C3b (D), C3a (E), and C5a (F) were detected using ELISA. *P<0.05 compared with baseline values; #P<0.05 compared with the HT group. ELISA, enzyme-linked immunosorbent assay; HT, mild hypothermia; MBL, mannose-binding lectin; NT, normothermia; ROSC, restoration of spontaneous circulation.
Pigs in the HT group showed significantly lower neurologic deficit scores compared with pigs in the NT group at 24 hours after ROSC (113.3±13.7 versus 203.7±18.9, P<0.05).
Mild Hypothermia Inhibits Complement Activation in Plasma and Brain Parenchyma
Pigs in the NT group showed higher plasma levels of C1q, MBL, C3a, and C5a at 0.5, 6, 12, and 24 hours (all P<0.05), higher plasma C3b levels at 0.5, 6, and 12 hours (P<0.05), and higher plasma Bb levels at 0.5 hours and 6 hours after ROSC (P<0.05) compared with baseline values (Figure 3). However, pigs in the HT group had lower plasma Bb levels only at 6 hours (P<0.05), lower plasma levels of C1q, C3b, MBL, C3a, and C5a at 6 and 12 hours (all P<0.05), and lower plasma C5a levels at 6, 12, and 24 hours after ROSC (all P<0.05) compared with the NT group. In addition, pigs in the HT group had lower brain contents of C1q, MBL, C3b, and C5a at 24 hours after ROSC compared with the NT group (all P<0.05, Table 2). No significant differences in the brain contents of Bb and C3a at 24 hours after ROSC were observed between the NT and HT groups (both P>0.05).
Table 2. Comparison of complement components in the brain cortex at 24 hours after ROSC between the NT and HT groups.
| C1q (pg/mg) | Bb (ng/mg) | MBL (μg/mg) | C3b (μg/mg) | C3a (ng/mg) | C5a (ng/mg) | |
|---|---|---|---|---|---|---|
| NT | 1.44±0.41 | 10.83±1.58 | 1.01±0.09 | 1.03±0.09 | 40.30±9.45 | 12.60±0.77 |
| HT | 0.97±0.15* | 9.78±1.17 | 0.72±0.15* | 0.84±0.12* | 37.57±6.61 | 10.23±1.45* |
Abbreviations: HT, mild hypothermia; MBL, mannose-binding lectin; NT, normothermia; ROSC, restoration of spontaneous circulation.
The concentrations of complement components are expressed in pg/mg (C1q), μg/mg (MBL and C3b), and ng/mg (Bb, C3a and C5a) of total protein content.
*P<0.05 versus brain content in the NT group.
Mild Hypothermia Decreases Plasma Interleukin-6 and Tumor Necrosis Factor-α Levels
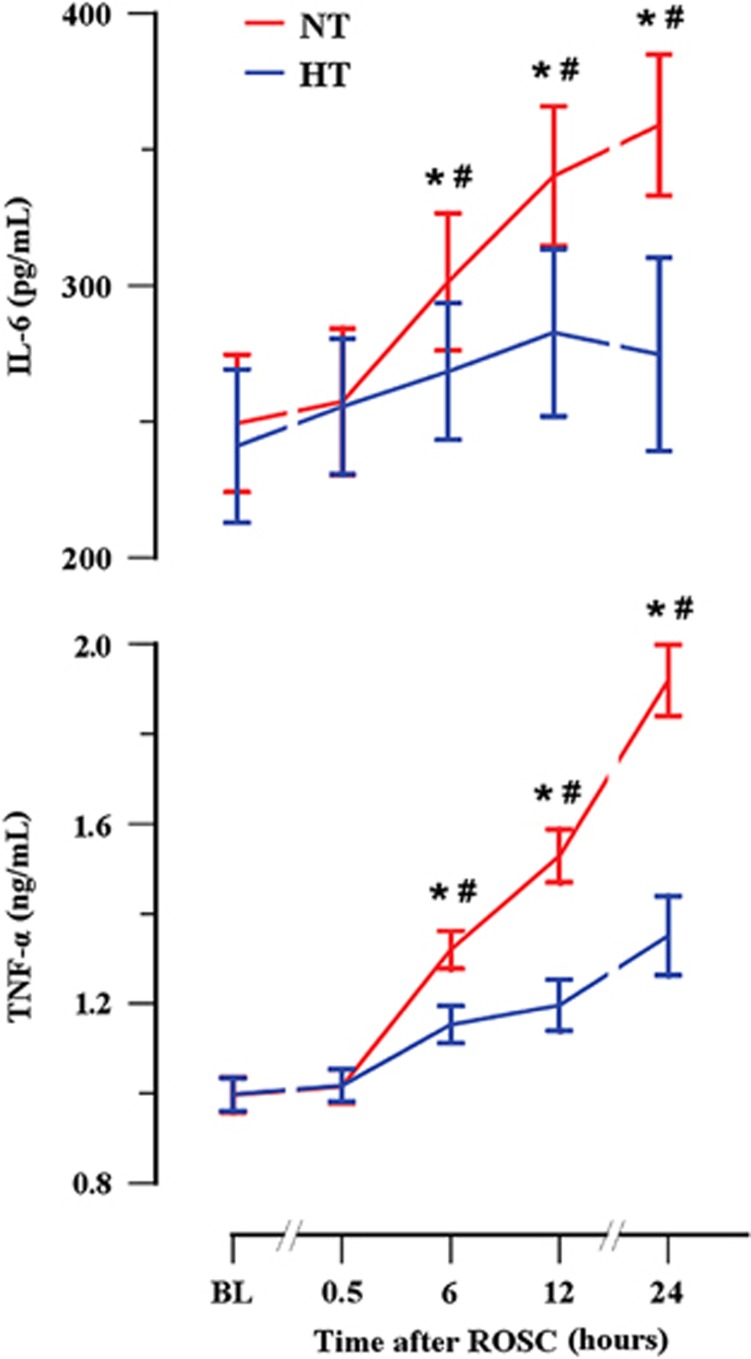
Pigs in the NT group had higher plasma IL-6 and TNF-α levels at 6, 12, and 24 hours after ROSC (all P<0.05) compared with baseline values (Figure 4). However, pigs in the HT group had lower plasma IL-6 and TNF-α levels at 6, 12, and 24 hours after ROSC (all P<0.05) compared with the NT group.
Figure 4.
Mild hypothermia inhibits plasma levels of pro-inflammatory mediators. Plasma IL-6 and TNF-α levels were detected using ELISA. *P<0.05 compared with baseline values; #P<0.05 compared with the HT group. ELISA, enzyme-linked immunosorbent assay; HT, mild hypothermia; IL-6, interleukin-6; NT, normothermia; ROSC, restoration of spontaneous circulation; TNF-α, tumor necrosis factor-α.
Plasma IL-6 levels were weakly positively related to C1q (r=0.381, P=0.003), Bb (r=0.339, P=0.008), C3b (r=0.270, P=0.037), and C3a levels (r=0.371, P=0.004). Plasma IL-6 levels were not correlated with MBL (r=0.204, P=0.118) and C5a levels (r=0.219, P=0.093). Tumor necrosis factor-α levels were not significantly related to C1q (r=0.142, P=0.278), Bb (r=−0.020, P=0.876), MBL (r=−0.091, P=0.489), C3b (r=−0.026, P=0.844), C3a (r=0.040, P=0.759), and C5a levels (r=0.124, P=0.085).
Discussion
The main findings of the present study were as follows. (1) Plasma levels of MBL, the complement fragments C1q, Bb, C3b, C3a, and C5a, and the pro-inflammatory mediators IL-6 and TNF-α were significantly increased within 24 hours after ROSC. (2) This increase in markers was significantly reduced by whole-body mild hypothermia, which was induced after ROSC. (3) Mild hypothermia also decreased the brain contents of C1q, MBL, C3b, and C5a at 24 hours after ROSC. (4) Plasma C1q, Bb, C3b, and C3a levels were weakly positively related to IL-6 levels, but not to TNF-α levels.
Complement activation occurs immediately when the system encounters appropriate stimuli (pathogen-associated molecular patterns and/or damage-associated molecular patterns).9, 11 Considering various activating mechanisms, complement can independently be triggered by ischemic tissues in the absence of an infection after cardiac arrest.9 In the present pig experiment, we found that complement was activated via three pathways after ROSC. Our study showed significantly increased plasma C3b level as a marker for the converging point of the three pathways,4 C1q as a marker for activation of the classic complement pathway,26 Bb as a marker for activation of the alternative complement pathway,27, 28 and MBL as a marker for complement activation of the MBL pathway.29 Recently, two clinical studies on out-of-hospital cardiac arrest patients also suggested that the complement cascade is activated through certain pathways after ROSC.8, 23 Significantly increased blood complement C3a and SC5b-9 levels during cardiopulmonary resuscitation and within 48 hours after ROSC were observed by Bottiger et al.8 In addition, Bisschops et al.23 found that activation of blood complement C4d, Bb, C3a, and terminal complement complex was increased on admission. However, in the study by Bisschops et al.,23 changes at subsequent time points were not measured because all the patients were treated with mild hypothermia and an NT control group was lacking because of ethical considerations.
Complement activation after ROSC might play a vital role in the pathogenesis of ischemia/reperfusion injury.4, 10 Complement activation has been considered as a system that orchestrates and connects various responses during immune and inflammatory reactions, and is not merely a killer of bacteria.9 Under some pathophysiologic conditions, an excess of complement is deleterious to the host.9, 12 Previous studies have showed that excessive or inappropriate complement activation has been implicated in the most pathologic inflammatory events,9, 30 including upregulation of adhesion molecules, activation of polymorphonuclear leukocytes, chemotaxis, expression of IL-8, and monocyte chemoattractant protein-1 by endothelial cells, which occur shortly after the ischemic insult.31 In addition, complement activation directly generates the inflammatory mediators C3a and C5a, and leads to cellular injury by insertion of the cytolytic membrane attack complex C5b-9.32 The systemic inflammatory response occurring after ROSC has been characterized as a part of postcardiac arrest syndrome.1 Our finding of a significant increase in the pro-inflammatory mediators IL-6 and TNF-α 24 hours after ROSC is consistent with previous studies.19, 23, 33 In addition, we found that IL-6, but not TNF-α levels, were weakly positively related to the plasma complement fragments C1q, Bb, C3b, and C3a. This relation may be because of the regulation of complement fragments on expression of pro-inflammatory mediators.34, 35 Fischer et al.34 showed that C3a increases the expression of IL-6 and enhances lipopolysaccharide-induced nuclear factor-kappaB, and activates protein-1 binding activity in vitro.34 Production of IL-6 and IL-8 triggered by C1q has also been showed in human umbilical vein endothelial cells.35 Therefore, based on the above-mentioned observations, we conclude that complement activation after ROSC may be correlated with the initiation and development of the systemic inflammatory response, which synergistically contributes to postresuscitation ischemia/reperfusion injury.
Considering that excessive complement activation contributes to ischemia/reperfusion injury, inhibition of complement as a neuroprotective strategy after cardiac arrest has been proposed.4 Interestingly, we found that whole-body mild hypothermia was also associated with inhibited activation of plasma C1q, C3b, Bb, MBL, C3a, and C5a levels after ROSC, which indicated inhibited complement activation of all the three pathways (classic, alternative, and MBL). Inhibited activation of complement, as shown by a decrease in the C1r-C1s-C1 inhibitor complex, C4bc, C3bPBb, C3bc, and soluble terminal complement complex, was also recently observed in postcardiac arrest patients during 72 hours of mild hypothermia.24 This inhibition of hypothermia on complement activation may be because of decreased enzymatic activity at lower temperatures, which is a common property of enzymatic reactions.36 However, recently, Shah et al.22 observed that hypothermia increased classic complement activation in vitro.22 One of the main reasons for this inconsistence with our results could be because of the complicated mechanisms of complement activation in vivo, which are different from those in the in vitro study that only showed an independent effect of hypothermia on antibody-initiated complement activation.22 In addition, a shorter duration (1 hour) of hypothermia and the nonischemia/reperfusion model in the in vitro study are limitations that could partly explain the discrepancy between studies.
In the present study, mild hypothermia was associated with an improved neurologic outcome, which is consistent with previous studies.13, 14, 17 Moreover, a lesser decrease in the apparent diffusion coefficient and N-acetyl aspartate/creatinine and a greater increase in choline/creatinine, which were detected by magnetic resonance spectroscopy, and an improvement in microscopic and ultrastructural abnormalities, and mitochondrial dysfunction in brain tissue after ROSC during mild hypothermia have been shown in our previous studies.15, 16, 37 In the present study, we also observed a significant decrease in the brain contents of C1q, MBL, C3b, and C5a after treatment with mild hypothermia, which indicated inhibited complement activation through classic and MBL pathways, even at 24 hours after ROSC. Therefore, based on the above findings we speculate that inhibition of mild hypothermia on complement activation may be associated with postresuscitation ischemia/reperfusion injury, thus improving neurologic outcome after ROSC.
The inhibition of mild hypothermia on pro-inflammatory mediators in the plasma and tissues has been documented in many animal19, 38 and human18 studies, despite some contradictory results.39, 40 The present study further showed this inhibitory effect of mild hypothermia on pro-inflammatory mediators. Because of regulation of complement activation on inflammation as discussed above, inhibition of mild hypothermia on the systemic inflammatory response and complement activation after ROSC could synergistically contribute to neuroprotection.
However, there are some limitations in this study. First, we focused on changes in the complement system in brain tissues only after rewarming (at 24 hours after ROSC), but the changes during mild hypothermia were not investigated. Second, the effects of different magnitudes and different onset times of hypothermia on complement activation were also not investigated. These factors need to be examined in future studies. Third, the experiment was performed only in healthy young male pigs, whereas most individuals (males or females) with cardiac arrest are old and have underlying diseases, especially heart diseases. Fourth, we failed to find a significant effect of mild hypothermia on survival rate because of the small sample size. Finally, a high tidal volume (15 mL/kg), which was used to maintain normocapnia, may induce lung injury with subsequent inflammation. Nevertheless, all the pigs were ventilated using the same procedure, regardless of their group assignment.
In conclusion, the complement system is activated through classic, alternative, and MBL pathways after ROSC. Whole-body mild hypothermia is associated with inhibited systemic and cerebral complement activation, which may provide an additional mechanism of cerebral protection.
Acknowledgments
The authors thank the technical assistance provided by Xuefeng Xu, Xianfei Ji, Zhiyu Su, Junyuan Wu, and Shuo Wang.
The authors declare no conflict of interest.
Footnotes
This work was supported by a Liaoning Province Nature Science Foundation Grant (2013023020) and a Training Programs of Talents in Science and Technology Grant (2013D003).
References
- Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123:1428–1435. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a ‘sepsis-like' syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- Zacharia BE, Hickman ZL, Grobelny BT, DeRosa PA, Ducruet AF, Connolly ES. Complement inhibition as a proposed neuroprotective strategy following cardiac arrest. Mediators Inflamm. 2009;2009:124384. doi: 10.1155/2009/124384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvington A, Atkinson C, Zhu H, Yu J, Takahashi K, Stahl GL, et al. The alternative complement pathway propagates inflammation and injury in murine ischemic stroke. J Immunol. 2012;189:4640–4647. doi: 10.4049/jimmunol.1201904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, et al. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiger BW, Motsch J, Braun V, Martin E, Kirschfink M. Marked activation of complement and leukocytes and an increase in the concentrations of soluble endothelial adhesion molecules during cardiopulmonary resuscitation and early reperfusion after cardiac arrest in humans. Crit Care Med. 2002;30:2473–2480. doi: 10.1097/00003246-200211000-00012. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FH, Anderson AJ, Taylor SM, Woodruff TM, Ruitenberg MJ. Complement activation in the injured central nervous system: another dual-edged sword. J Neuroinflammation. 2012;9:137. doi: 10.1186/1742-2094-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini F, De Blasio D, Zangari R, Zanier ER, De Simoni MG. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci. 2014;8:380. doi: 10.3389/fncel.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Gong P, Hua R, Zhang Y, Zhao H, Tang Z, Mei X, et al. Hypothermia-induced neuroprotection is associated with reduced mitochondrial membrane permeability in a swine model of cardiac arrest. J Cereb Blood Flow Metab. 2013;33:928–934. doi: 10.1038/jcbfm.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Li CS, Hua R, Zhao H, Tang ZR, Mei X, et al. Mild hypothermia attenuates mitochondrial oxidative stress by protecting respiratory enzymes and upregulating MnSOD in a pig model of cardiac arrest. PLoS One. 2012;7:e35313. doi: 10.1371/journal.pone.0035313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128. doi: 10.1002/14651858.CD004128.pub3. [DOI] [PubMed] [Google Scholar]
- Meybohm P, Gruenewald M, Zacharowski KD, Albrecht M, Lucius R, Fosel N, et al. Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Crit Care. 2010;14:R21. doi: 10.1186/cc8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Li CS, Gong P, Tang ZR, Hua R, Mei X, et al. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation. 2012;83:913–920. doi: 10.1016/j.resuscitation.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- Shah TA, Mauriello CT, Hair PS, Sandhu A, Stolz MP, Bass WT, et al. Clinical hypothermia temperatures increase complement activation and cell destruction via the classical pathway. J Transl Med. 2014;12:181. doi: 10.1186/1479-5876-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisschops LL, Hoedemaekers CW, Mollnes TE, van der Hoeven JG. Rewarming after hypothermia after cardiac arrest shifts the inflammatory balance. Crit Care Med. 2012;40:1136–1142. doi: 10.1097/CCM.0b013e3182377050. [DOI] [PubMed] [Google Scholar]
- Bisschops L, van der Hoeven JG, Mollnes TE, Hoedemaekers C. Seventy-two hours of mild hypothermia after cardiac arrest is associated with a lowered inflammatory response during rewarming in a prospective observational study. Crit Care. 2014;18:546. doi: 10.1186/s13054-014-0546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enna B, Wenzel V, Schocke M, Krismer AC, Voelckel WG, Klima G, et al. Excellent coronary perfusion pressure during cardiopulmonary resuscitation is not good enough to ensure long-term survival with good neurologic outcome: a porcine case report. Resuscitation. 2000;47:41–49. doi: 10.1016/s0300-9572(00)00200-8. [DOI] [PubMed] [Google Scholar]
- Wouters D, Wiessenberg HD, Hart M, Bruins P, Voskuyl A, Daha MR, et al. Complexes between C1q and C3 or C4: novel and specific markers for classical complement pathway activation. J Immunol Methods. 2005;298:35–45. doi: 10.1016/j.jim.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, et al. Activation of the alternative pathway of complement is a feature of pre-term parturition but not of spontaneous labor at term. Am J Reprod Immunol. 2010;63:318–330. doi: 10.1111/j.1600-0897.2009.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto E, Romero R, Vaisbuch E, Erez O, Mazaki-Tovi S, Kusanovic JP, et al. Fragment Bb: evidence for activation of the alternative pathway of the complement system in pregnant women with acute pyelonephritis. J Matern Fetal Neonatal Med. 2010;23:1085–1090. doi: 10.3109/14767051003649870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommett RM, Klein N, Turner MW. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MM, Fries LF. The role of complement in inflammation and phagocytosis. Immunol Today. 1991;12:322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- Szeplaki G, Szegedi R, Hirschberg K, Gombos T, Varga L, Karadi I, et al. Strong complement activation after acute ischemic stroke is associated with unfavorable outcomes. Atherosclerosis. 2009;204:315–320. doi: 10.1016/j.atherosclerosis.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Glovsky MM, Warner RL, Horvath SJ, Ward PA. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation. 1996;20:1–9. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- Samborska-Sablik A, Sablik Z, Gaszynski W. The role of the immuno-inflammatory response in patients after cardiac arrest. Arch Med Sci. 2011;7:619–626. doi: 10.5114/aoms.2011.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer WH, Jagels MA, Hugli TE. Regulation of IL-6 synthesis in human peripheral blood mononuclear cells by C3a and C3a(desArg) J Immunol. 1999;162:453–459. [PubMed] [Google Scholar]
- van den Berg RH, Faber-Krol MC, Sim RB, Daha MR. The first subcomponent of complement, C1q, triggers the production of IL-8, IL-6, and monocyte chemoattractant peptide-1 by human umbilical vein endothelial cells. J Immunol. 1998;161:6924–6930. [PubMed] [Google Scholar]
- Bouma HR, Carey HV, Kroese FG. Hibernation: the immune system at rest. J Leukoc Biol. 2010;88:619–624. doi: 10.1189/jlb.0310174. [DOI] [PubMed] [Google Scholar]
- Tang ZR, Li CS, Zhao H, Gong P, Zhang MY, Su ZY, et al. Effects of hypothermia on brain injury assessed by magnetic resonance imaging after cardiopulmonary resuscitation in a porcine model of cardiac arrest. Am J Emerg Med. 2013;31:86–93. doi: 10.1016/j.ajem.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Han HS, Karabiyikoglu M, Kelly S, Sobel RA, Yenari MA. Mild hypothermia inhibits nuclear factor-kappaB translocation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Rittenberger JC, Logue ES, McMichael MJ. Hypothermia after cardiac arrest does not alter serum inflammatory markers. Crit Care Med. 2008;36:2607–2612. doi: 10.1097/CCM.0b013e318184443b. [DOI] [PubMed] [Google Scholar]
- Fries M, Stoppe C, Brucken D, Rossaint R, Kuhlen R. Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J Crit Care. 2009;24:453–457. doi: 10.1016/j.jcrc.2008.10.012. [DOI] [PubMed] [Google Scholar]