Abstract
Multitrauma is a common medical problem worldwide, and often involves concurrent traumatic brain injury (TBI) and bone fracture. Despite the high incidence of combined TBI and fracture, preclinical TBI research commonly employs independent injury models that fail to incorporate the pathophysiologic interactions occurring in multitrauma. Here, we developed a novel mouse model of multitrauma, and investigated whether bone fracture worsened TBI outcomes. Male mice were assigned into four groups: sham-TBI+sham-fracture (SHAM); sham-TBI+fracture (FX); TBI+sham-fracture (TBI); and TBI+fracture (MULTI). The injury methods included a closed-skull weight-drop TBI model and a closed tibial fracture. After a 35-day recovery, mice underwent behavioral testing and magnetic resonance imaging (MRI). MULTI mice displayed abnormal behaviors in the open-field compared with all other groups. On MRI, MULTI mice had enlarged ventricles and diffusion abnormalities compared with all other groups. These changes occurred in the presence of heightened neuroinflammation in MULTI mice at 24 hours and 35 days after injury, and elevated edema and blood–brain barrier disruption at 24 hours after injury. Together, these findings indicate that tibial fracture worsens TBI outcomes, and that exacerbated neuroinflammation may be an important factor that contributes to these effects, which warrants further investigation.
Keywords: animal model, cytokines, DTI, MRI, polytrauma
Introduction
Multitrauma involves significant injury to at least two body regions, and is a common consequence of motor vehicle accidents, warzone injuries, as well as slips and falls.1, 2, 3 Among multitraumas, two common injury components are traumatic brain injury (TBI) and bone fracture.3, 4, 5
Traumatic brain injury is a neurodegenerative condition that is induced by biomechanical forces applied to the brain, and is a leading cause of death and chronic disability worldwide.6, 7 Brain damage post-TBI is often classified as resulting from either primary or secondary injury mechanisms.6, 7 Primary injury involves tissue damage caused directly by mechanical forces at the moment of impact, and may include blood–brain barrier (BBB) damage, edema, ischemia, necrosis, and axonal shearing.6, 7 Secondary injury processes have a delayed onset after the primary injury and include neuroinflammation, oxidative stress, apoptosis, metabolic abnormalities, and further injury to the BBB and axons, all of which may contribute to the progressive neurodegeneration that occurs after TBI.6, 7 Unfortunately, to date there is still a poor understanding of the complex pathogenesis of TBI, and there is no treatment that is known to improve long-term outcomes in TBI patients.6, 7
Although TBI patients often experience concurrent bone fractures, most pre-clinical studies have utilized independent ‘single-hit' injury models that do not reproduce the pathophysiologic complexities of multitrauma.3, 4, 8, 9 This has recently been recognized as a potential shortfall in the preclinical TBI field that may contribute to the failures in translating preclinical findings to the clinical setting.7 Of particular relevance is bone fracture, which is known to induce a complex post-fracture healing process that involves an extensive inflammatory response in which immune cells proliferate and infiltrate the fracture site and secrete a range of pro-inflammatory cytokines into the circulation including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6.10 Notably, each of these factors are also important in TBI and, considering the compromised BBB post-TBI, may have unprecedented access into the injured brain where they could modulate the neuroinflammatory response and ultimately TBI outcomes. Though no studies have investigated the effect of bone fracture on TBI, previous animal model studies reported increased levels of circulating inflammatory cytokines when peripheral bone fracture and TBI were combined, and similar effects occur in human multitrauma patients.8, 9, 11, 12
For these reasons, here we developed a novel mouse model of multitrauma and investigated whether a concurrent tibial fracture affected the outcomes after TBI. Adult male C57Bl/6 mice were assigned to four experimental groups: sham-TBI+sham-fracture (SHAM); sham-TBI+fracture (FX); TBI+sham-fracture (TBI); and TBI+fracture (MULTI). The methods used to induce these injuries included a closed-skull weight-drop TBI model and a closed tibial fracture model. After the assigned injuries, mice were given either a 24-hour or a 35-day recovery period. Behavioral testing and in vivo magnetic resonance imaging (MRI) were conducted 35 days after injury, and brain tissue and plasma was collected at 24 hours and 35 days after injury for post-mortem analysis.
Materials and methods
Mice
A total of 124 C57Bl/6 male mice were obtained from the Australian Animal Resource Centre (ARC, Western Australia) for use in this study. Mice were 12 weeks of age at the time of injury, were housed individually under a 12-hour light/dark cycle, and were given access to food and water ad libitum for the duration of the experiment. All procedures were approved by The Florey Institute of Neuroscience and Mental Health Animal Ethics Committee, were within the guidelines of the Australian code of practice for the care and use of animals for scientific purposes by the Australian National Health and Medical Research Council, and in compliance with the ARRIVE guidelines for how to report animal experiments.
Experimental Groups
Mice were assigned to one of four experimental injury groups: SHAM (n=26); FX (n=26); TBI (n=36); and MULTI (n=36). Twelve mice died immediately post-TBI (six TBI and six MULTI), and four mice (two TBI and two MULTI) sustained a skull fracture and were excluded from the study. As such, the MULTI and TBI groups consisted of 28 mice each. Some mice were killed at 24 hours after injury (n=15/group) for enzyme-linked immunosorbent assay (ELISA), Evans blue extravasation, edema (brain water content), and Western blot analyses. The remaining mice were given a 35-day post-injury recovery before undergoing behavioral, MRI, ELISA, and Western blot analyses.
Closed-Skull Weight-Drop Model of Traumatic Brain Injury
The weight-drop TBI and associated sham-injury procedures were based on previously described standard protocols.13 Briefly, the weight-drop device consisted of a guided- and weighted-rod (215 g) with a blunt silicone-covered impact tip (4 mm diameter). The mouse was first placed in an anesthesia induction chamber containing 4% isoflurane for 3 minutes. Once anesthetized, the mouse was placed in a nose cone that maintained the anesthetic (2% isoflurane). The head was then shaved, and a 1.5-cm longitudinal incision was made along the midline of the scalp under sterile conditions. The mouse next underwent tibial fracture or sham-fracture procedure as described below. After a total surgery/anesthesia time of 20 minutes, the mouse was removed from the nose cone, stabilized on the injury device platform, the weighted-rod was released, and the impact tip made contact between the sagittal and coronal suture. To avoid the potential confound of skull fracture, the weighted-rod was released from a distance of 2 cm, and any mice experiencing a skull fracture were excluded from the study. The rod was manually retracted immediately after the impact occurred, and the scalp incision was sutured. The TBI sham-injury procedure was identical to that described for the TBI procedure, except the weighted-rod was not released. Apnea time, loss of consciousness, and self-righting reflex time were all recorded immediately after the injury as indicators of injury outcome (Table 1).14 If apnea persisted for longer than 10 seconds, then mice were resuscitated with pure oxygen.14, 15 Both the MULTI and TBI groups had increased apnea, hindlimb withdrawal, and self-righting reflex times compared with SHAM and FX groups. All mice received 0.05 mg/kg of buprenorphine analgesic subcutaneously.
Table 1. Acute injury measures.
| SHAM | FX | TBI | MULTI | |
|---|---|---|---|---|
| Apnea | 0 | 0 | 32.5±4.1a | 25.1±3.0a |
| Hindlimb | 42.0±3.9 | 38.4±4.9 | 183.4±18.0a | 191.7±23.7a |
| Self-righting | 68.9±5.0 | 66.3±6.3 | 277.3±20.7a | 261.5±27.9a |
Abbreviations: FX, tibial fracture; MULTI, multitrauma; SHAM, sham-injuries; TBI, traumatic brain injury.
The TBI and MULTI groups experienced significantly longer apnea, unconsciousness, and self-righting reflex times (seconds) than the SHAM and FX groups. The TBI and MULTI groups did not differ from each other on any of the measures.
Greater than SHAM and FX groups, P<0.05.
Tibial Fracture
Before the assigned TBI injury was given, mice received a closed tibial fracture stabilized by intramedullary fixation as previously described.16 Isoflurane anesthesia was induced as described above; a small incision was then made inferior of the right knee, an entry point into the medullary canal of the tibia was made using a 26-G needle, and an intramedullary rod (00 insect pin, 0.3 mm diameter) was inserted inside the medullary canal. A fracture was then generated in the tibial midshaft, and a noncomminuted and transverse fracture was confirmed via X-ray. This fracture model is highly reproducible with little variability.16 After the fracture, the initial intramedullary rod was removed and a new rod was inserted that remained in situ for the remainder of the study to ensure bone-end alignment. The use of the intramedullary pin stabilizes the fracture and ensures minimal displacement during recovery.16 The incision was then sutured. Sham injury for the fracture procedure consisted of the same procedures, but no fracture was inflicted. A total anesthetic time of 20 minutes was induced in all mice before the administration of TBI procedures as described above.
Behavioral Testing
Mice underwent behavioral testing in the Y-maze, open-field, and rotarod beginning 30 days after injury. Behavioral testing was conducted over three consecutive days by an experimenter blinded to group assignment.
To assess locomotion and anxiety-like behavior, mice were tested in an open-field as previously described.14 The testing apparatus consisted of a circular open-field (100 cm diameter) enclosed by a 20-cm high wall. The mouse was placed in the center of the open-field and allowed to freely explore the arena for 5 minutes. Behavior was recorded by an overhead camera, and the total distance traveled, number of entries into the inner area of the maze (66 cm diameter), and time spent in the inner area of the maze were objectively quantified using EthoVision tracking software (Noldus, Leesburg, VA, USA).
Spatial cognition was assessed in the Y-maze as previously described.14 Y-maze testing was conducted in an apparatus consisting of three enclosed (13 cm high walls) arms of equal dimension (length=38 cm, width=8 cm) and adjoined in a Y-shape (San Diego Instruments, San Diego, CA, USA). At the distal end of each arm was an exterior visual cue. A 15-minute training trial preceded Y-maze testing. The training trial consisted of two open arms and one blocked arm (novel arm). The mouse was then placed at the distal end of one of the open arms (start arm), and allowed to freely explore both open arms. The mouse was given a 2-hour interval between the training and test trials. During the 5-minute Y-maze testing, the novel arm was unblocked, and the mouse was placed in the same start arm and allowed to freely explore all three arms. The arms and visual cues were randomized between but not within mice. Each trial was recorded by an overhead camera, and the time spent, number of entries, and time to entry into each of the arms were quantified using EthoVision tracking software (Noldus).
The rotarod was used to assess motor function as previously described.14 The apparatus consisted of a rotating barrel (diameter=3 cm) divided by walls (height=10 cm) into four equal lanes (width=5 cm; Harvard Apparatus, Holliston, TX, USA). Three trials were performed each day for two consecutive days (day 1=training, day 2=testing). For each trial, the mouse was placed on the rotating barrel, the speed was accelerated from 0.0027 to 0.27g (4 to 40 r.p.m.) at a rate of 0.00017g/8 s, and the duration of time on the rotarod that the mouse was able achieve was recorded (maximum time of 5 minutes).
Magnetic Resonance Imaging Acquisition
Once behavioral assessments were completed, in vivo MRI scanning was performed using a 4.7-T Bruker Avance III scanner with a 30-cm horizontal bore fitted with a BGA12S2 actively shielded set and running ParaVision 5.1 software (Bruker Biospec, Ettlingen, Germany).14, 15 The mouse was placed in a clear plastic box and put under isoflurane anesthesia with 3% isoflurane. Once sedated, mice were fixated supinely with a stereotactic frame. Anesthesia (2.0%) was maintained through a nose cone for the entirety of the scan and body temperature was maintained at approximately 37°C using a hot water circulation system built into the animal cradle. Moreover, a pressure-sensitive probe placed under the diaphragm of the mouse was used to monitor respiration throughout the scan.
Each scan comprises a three-plane localizer sequence followed by multislice scout images in coronal, axial, and sagittal planes to establish the correct position of the mouse brain. A T2-weighted image was obtained in the axial plane using a 2D RARE (rapid acquisition with relaxation enhancement) sequence with the following imaging parameters: repetition time=8,000 ms; RARE factor=10, effective echo time =45 ms; field of view=19.2 × 19.2 mm2; matrix size=160 × 160; number of slices=60; isotropic spatial resolution=120 × 120 × 120 μm3; and number of excitations=6.
Diffusion-weighted images were acquired in the coronal plane using a diffusion-weighted echo planar imaging sequence with the following parameters: repetition time=4,000 ms; echo time=35 ms; shots=8; field of view=15.36 × 15.36 mm2; matrix size=128 × 128; spatial resolution=120 × 120 μm2; number of slices=16; and slice thickness=500 μm. Diffusion weighting was performed with diffusion duration (δ)=4 ms, diffusion gradient separation (Δ)=11 ms and b-value=1,200 s/mm2 in 30 noncollinear directions with 5 non-diffusion images.
Magnetic Resonance Imaging Analysis
Analysis was performed as previously described.14, 15 Regions of interest were manually outlined by an experimenter who was blinded to experimental conditions on 12 consecutive coronal T2-weighted images using FSL software (Analysis Group, Oxford, UK). Regions of interest included cortex, hippocampus, lateral ventricles, and corpus callosum from each hemisphere. Volumetric analysis was performed using MATLAB (Mathworks, Natick, MA, USA). Diffusion tensor imaging (DTI) outcomes were calculated using DTIFit, part of the FMRIB Diffusion Toolbox (FDT, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT). For each mouse, the mean b0 image was calculated using MATLAB and registered to its T2-weighted image using ANTs (http://stnava.github.io/ANTs/). The calculated diffeomorphism was then used to transform the regions of interest to the diffusion space and DTI measures were calculated for each mouse.
Evans Blue Extravasation
As previously described,17, 18 Evans blue extravasation was used to investigate BBB disruption. In brief, mice were restrained and given an intravenous injection of Evans blue (200 μL of 0.5% Evans blue solution; Sigma, Sydney, Australia) into the lateral tail vein 24 hours after injury. After 30 minutes, anesthetized mice (4% isoflurane for 1 minute) were decapitated, and the injured hemisphere was dissected, weighed, and placed in a 1.5 mL tube containing 500 μL of formamide (Sigma). Tubes were transferred to a 55°C heat block and allowed to incubate for 48 hours to extract Evans blue from the tissue. A Bio-Rad Benchmark Plus Microplate Spectrophotometer (Bio-Rad Laboratories Inc., Hercules, CA, USA) was used to read the absorbencies of the Evans blue extract, and ng of Evans blue per mg of tissue was calculated.
Brain Water Content
Brain water content was used as an indicator of brain edema.18 As previously described, anesthetized mice (4% isoflurane for 1 minute) were decapitated 24 hours after injury, and the injured hemisphere was dissected. The tissue was immediately weighed (wet weight) and then dried at 60°C for 72 hours. The tissue was again weighed (dry weight), and the following formula was used to determine brain water content: water content (%)=(wet weight−dry weight)/wet weight.
Brain Tissue Collection and Preparation for Western Blotting and Enzyme-Linked Immunosorbent Assay
Anesthetized mice (4% isoflurane for 1 minute) were decapitated, and brains were removed and dissected, and the tissue was rapidly frozen in liquid nitrogen and stored at −80°C. Tissue samples were then homogenized to perform ELISA and Western blotting analysis. Brain tissue samples were added to 450 μL of radioimmunoprecipitation assay buffer with protease inhibitor. The tissue was homogenized using a hand-held electric homogenizer, and left to sit on ice for 20 minutes. The sample solution was then centrifuged at 17,000g at 4°C for 20 minutes. The protein concentration of the brain tissue lysates was quantified using a BCA Protein Assay Kit (Thermo Scientific Pierce Biotechnology, San Jose, CA, USA) and Benchmark Plus Microplate Spectrophotometer (BioRad Laboratories Inc., USA).
Brain Tissue Enzyme-Linked Immunosorbent Assay
Inflammatory cytokines IL-1β, IL-6, and TNF-α were quantified in brain tissue isolates using BD OptIEA ELISA kits (BD Biosciences, USA). All samples, standards, and controls were run in duplicate and the assay procedure conducted as per the manufacturer's instructions. At the completion of each reaction, the absorbance of each well was measured at 450 nm (using wavelength correction of 570 nm) and Microplate Manager software (Bio-Rad Laboratories Inc.) was used to calculate mean cytokine concentrations for each sample.
Western Blotting
Western blotting was used to detect expression of neutrophils, glial fibrillary acidic protein (GFAP) expression, a marker for astrogliosis, and CD68, a marker for microglia and macrophages, in brain tissue at 24 hours (n=6/group) and 35 days (n=5/group) after injury. The samples were prepared by adding 9 μL with 3 μL 4 × SDS loading dye (12.5 mL 1 mol/L Tris pH 6.8, 20 mL glycerol, 10 mL β-mercapto-ethanol, 4 g SDS, 0.01 g bromophenol blue 0.025%). Samples were then heated at 95°C for 5 minutes to denature the protein and spun down at 17,000g for 1 minute at 24°C before storage at −20°C until use. The proteins in samples were separated with SDS-polyacrylamide gel electrophoresis, and the bands of proteins were electroblotted onto polyvinyl difluoride membranes. The blots on polyvinyl difluoride were developed with anti-GFAP (1 : 10,000; Cell Signaling Technology, Beverly, MA, USA), anti-neutrophil (1 : 500; Abcam, Melbourne, Australia), anti-CD68 (1 : 250; Abcam), and loading control anti-GAPDH (1 : 1,000; Santa Cruz Biotechnology Inc., Scoresby, Australia) primary antibodies overnight at 4°C, then incubated with secondary antibodies (Dako, Sydney, Australia; Abcam) of respective animal species conjugated to HRP at room temperature for 90 minutes. The membranes were then visualized by enhanced chemiluminescent substrate kit (Amersham ECL Western blotting detection reagents, GE Healthcare, Rydalmere, Australia) and exposure to x-films. The mean intensity of the blots was quantified using Image J software (National Institutes of Health, USA).
Plasma Collection and Enzyme-Linked Immunosorbent Assay
Blood samples were collected by cardiac puncture using heparin as an anti-coagulant. Immediately after blood collection, plasma was prepared by centrifugation and stored at −80°C. Plasma IL-1β, IL-6, and TNF-α concentrations in undiluted duplicate samples were quantified using Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA) as per the manufacturer's instructions.
Statistical Analysis
All outcomes were analyzed with SPSS 21.0 software (IBM Corp, Armonk, NY, USA) using a one-way analysis of variance (ANOVA). Newman–Keuls post hoc comparisons were carried out when appropriate. Statistical significance was set at P<0.05.
Results
Multitrauma Exacerbates Neuroinflammation
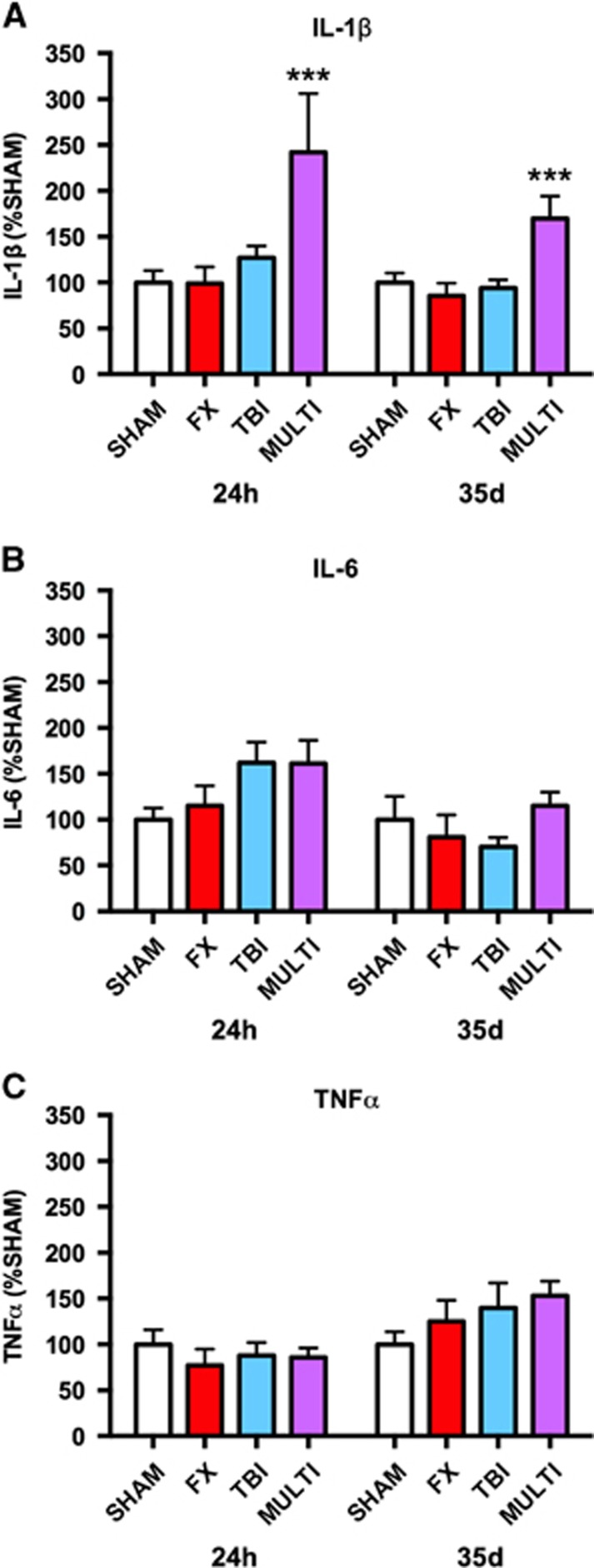
Enzyme-linked immunosorbent assay was used to investigate the brain tissue concentrations of the inflammatory cytokines IL-1β, IL-6, and TNF-α at 24 hours and 35 days after injury. One-way ANOVA identified significant injury effects in the levels of IL-1β at both 24 hours (F3,34=3.999, P<0.05; Figure 1A) and 35 days after injury (F3,39=5.921, P<0.05; Figure 1A). Post hoc analysis indicated that IL-1β levels were significantly increased in MULTI mice in comparison with SHAM, FX, and TBI groups at 24 hours (P<0.05) and 35 days after injury (P<0.01). There were no significant changes found for the levels of IL-6 and TNF-α (P>0.05; Figures 1B and 1C).
Figure 1.
Multitrauma increases brain tissue concentration of IL-1β. ELISA indicated increased brain tissue concentrations of the inflammatory cytokine IL-1β (A) in MULTI mice in comparison with SHAM, FX, and TBI groups at 24 hours and 35 days after injury. There were no significant group differences in the concentrations of IL-6 (B) and TNF-α (C). ***Greater than all other groups, P<0.05. ELISA, enzyme-linked immunosorbent assay; FX, tibial fracture; IL, interleukin; MULTI, multitrauma; SHAM, sham-injuries; TBI, traumatic brain injury; TNF, tumor necrosis factor.
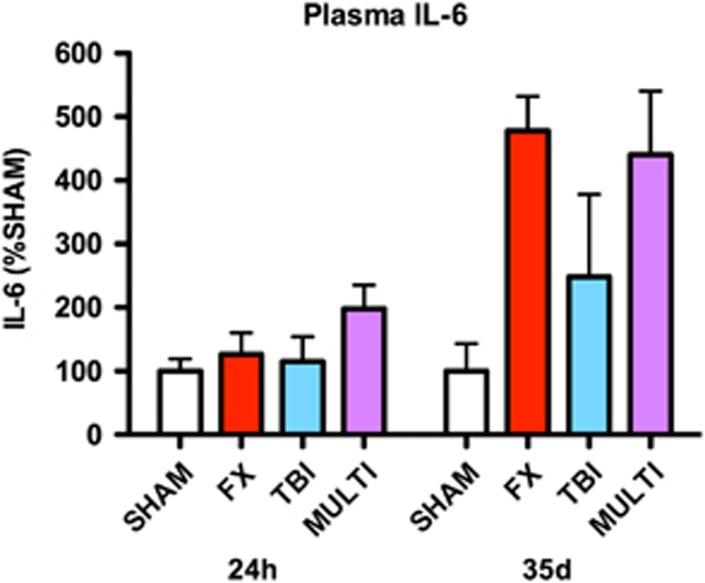
Enzyme-linked immunosorbent assay was also used to investigate plasma concentrations of IL-1β, IL-6, and TNF-α at 24 hours and 35 days after injury. Plasma IL-6 concentrations were increased twofold in the MULTI group relative to sham injured at 24 hours after injury (Figure 2); however, ANOVA failed to identify a statistically significant effect for injury (P>0.05). At 35 days after injury, IL-6 was only detectable in a proportion of the samples (Sham=2/10; FX=3/10; TBI=2/10; MULTI=7/10). Analysis of the samples with detectable concentrations of IL-6 showed over a fourfold increase in the FX and MULTI groups, and a twofold increase in the TBI, relative to sham-injured mice (Figure 2). However, once again ANOVA failed to identify a statistically significant injury effect (P>0.05). Plasma concentrations of IL-1β and TNF-α were undetectable at 24 hours and 35 days after injury.
Figure 2.
IL-6 concentration in plasma. There were no statistically significant injury effects for cytokine concentrations in plasma at 24 hours or 35 days after injury. However, IL-6 concentrations were increased twofold in the MULTI group 24 hours after injury, and over fourfold in the MULTI and FX groups at 35 days after injury, relative to SHAM. TBI, traumatic brain injury. FX, tibial fracture; IL, interleukin; MULTI, multitrauma; SHAM, sham-injuries; TBI, traumatic brain injury.
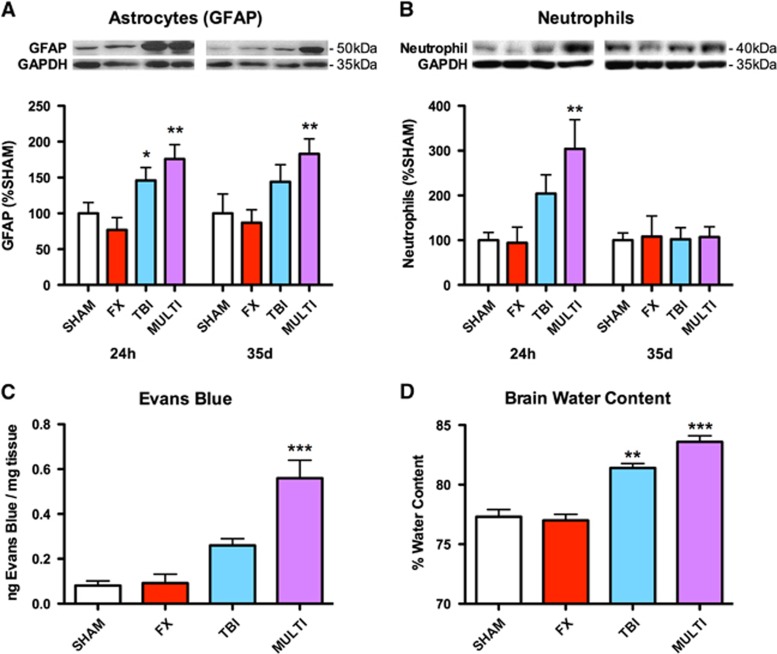
Western blotting was used to assess the expression of GFAP, a marker of astrogliosis, and neutrophils in brain tissue. One-way ANOVAs identified a significant injury effect in GFAP expression at both 24 hours (F3,19=6.440, P<0.005) and 35 days after injury (F3,15=3.834, P<0.05; Figure 3A), and a significant injury effect in neutrophil expression at 24 hours (F3,19=4.976, P<0.01; Figure 3B) but not 35 days after injury (F3,15=0.008, P>0.05; Figure 3B). Post hoc analysis revealed that MULTI mice had increased GFAP (P<0.05) and neutrophil (P<0.05) expression than SHAM and FX mice at 24 hours after injury, and increased GFAP at 35 days (P<0.05) after injury. The TBI mice only had increased GFAP expression compared with FX mice at 24 hours after injury (P<0.05). No significant effects were found for CD68 expression, a marker for microglia and macrophages, at 24 hours (F3,19 =0.125, P>0.05) or 35 days after injury (F3,15=0.333, P>0.05).
Figure 3.
Multitrauma increases GFAP and neutrophil expression, and exacerbates BBB disruption and edema. (A) Western blotting indicated increased expression of the astrogliosis marker GFAP in the brain tissue of MULTI mice in comparison with SHAM and FX groups at 24 hours and 35 days after injury. TBI mice had increased GFAP expression compared with FX mice at 24 hours after injury. (B) Western blotting indicated increased neutrophil expression in the brain tissue of MULTI mice in comparison with SHAM and FX groups at 24 hours after injury. (C) MULTI mice had increased Evans blue extravasation in brain tissue, indicating BBB damage, compared with all other groups. (D) MULTI mice had increased brain water content, indicating edema, compared with all other groups. TBI mice had increased brain water content compared with SHAM and FX groups. ***Greater than all other groups; **Greater than SHAM and FX groups; *Greater than FX group; P<0.05. BBB, blood-brain barrier; FX, tibial fracture; GFAP, glial fibrillary acidic protein; MULTI, multitrauma; SHAM, sham-injuries; TBI, traumatic brain injury.
Multitrauma Induces Blood–Brain Barrier Disruption and Edema
Evans blue extravasation in brain tissue was used to investigate BBB disruption post-injury. One-way ANOVA identified a significant injury effect in Evans blue levels at 24 hours after injury (F3,20=20.201, P<0.001; Figure 3C), with post hoc analysis revealing that MULTI mice had increased Evans blue extravasation in brain tissue compared with all other groups (P<0.01).
Brain water content was measured to assess brain edema. One-way ANOVA identified a significant injury effect in brain water content at 24 hours after injury (F3,20=42.263, P<0.001; Figure 3D). Post hoc analysis revealed that MULTI mice had increased water content compared with all other groups (P<0.05), and TBI mice had increased water content than SHAM and FX groups (P<0.05).
Multitrauma Induces Brain Damage
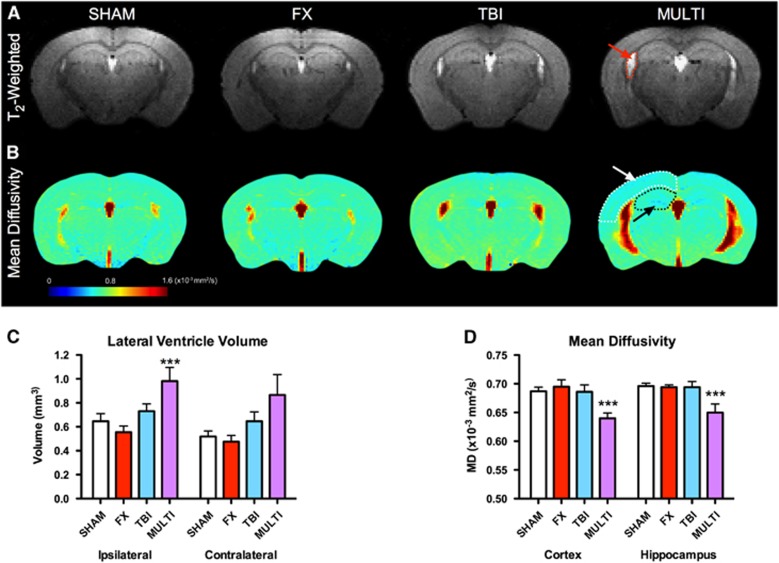
Magnetic resonance imaging was used to assess the extent of brain damage at 35 days after injury. Analysis of variance identified a significant injury effect on the volume of the ipsilateral (F3,42=5.524, P<0.05) and contralateral (F3,42=2.963, P<0.05) lateral ventricles (Figure 4A). Post hoc analysis found that the MULTI group had significantly enlarged ipsilateral ventricles in comparison with SHAM, FX, and TBI groups (P<0.05; Figure 4C). While post hoc analysis failed to identify significant between-group differences regarding the contralateral ventricle, there was a nonsignificant trend consistent with the ipsilateral data in showing larger ventricles in the MULTI mice.
Figure 4.
Multitrauma induces brain damage and diffusion abnormalities. (A) Representative T2-weighted (A) and MD; (B) images from each of the four injury groups. The arrows indicate the enlarged lateral ventricle (A, red arrow) and decreased MD in the cortex (white arrow) and hippocampus (black arrow) in the MULTI group (B). (C) The MULTI group had significantly enlarged ipsilateral ventricles in comparison with SHAM, FX, and TBI groups. (D) MULTI mice had decreased MD compared with SHAM, FX, and TBI groups in the cortex and hippocampus. ***Different from all other groups, P<0.05. FX, tibial fracture; MD, mean diffusivity; MULTI, multitrauma; SHAM, sham-injuries; TBI, traumatic brain injury.
Diffusion tensor imaging was used to investigate diffusion abnormalities at 35 days after injury. Analysis of variance identified a significant injury effect on the measure of mean diffusivity (MD) in the ipsilateral cortex (F3,42=4.658, P<0.05; Figure 4B), with post hoc analysis indicating that MULTI mice had decreased MD compared with SHAM, FX, and TBI groups (P<0.05; Figure 4D). Analysis of variance also found a significant injury effect on the measure of MD in the ipsilateral hippocampus (F3,42=3.371, P<0.05; Figure 4D). Although ANOVA revealed a significant effect for injury in the ipsilateral hippocampus, post hoc analysis failed to identify significant between-group differences.
Multitrauma Induces Behavioral and Motor Abnormalities
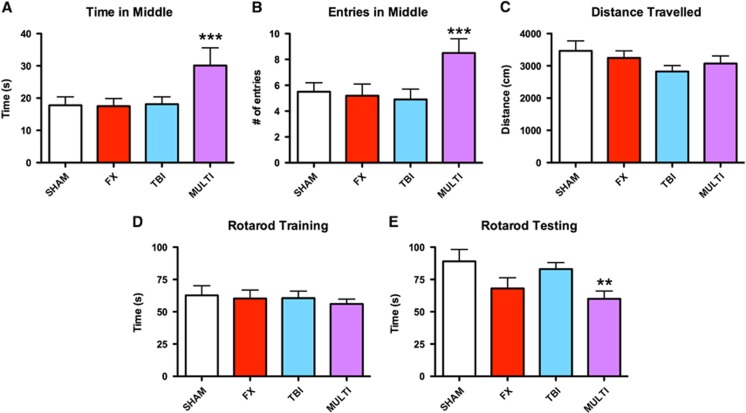
Mice were tested in the open-field to assess locomotor and anxiety-related behaviors. One-way ANOVA indicated a significant injury effect on the measures of time spent (F3,42=2.910, P<0.05; Figure 5A) and entries (F3,42=3.351, P<0.05; Figure 5B) in the center area of the open-field. Post hoc analysis revealed that the MULTI group spent significantly more time and made more entries in the center area of the open-field in comparison with all other groups (P<0.05). There were no significant differences in the distance traveled between groups, which suggested that motor ability was not a confounding factor in the other open-field outcomes (P>0.05; Figure 5C).
Figure 5.
Multitrauma induces behavioral abnormalities in the open-field and motor dysfunction on the rotarod. The MULTI group spent more time (A) and made more entries (B) in the center area of the open-field in comparison with SHAM, FX, and TBI groups. There were no differences in the distance traveled (C). There were no significant differences on the measure of duration on rotarod during training (D). However, during testing the MULTI group spent significantly less time on the rotarod (E) than both the SHAM and TBI groups. ***Greater than all other groups, P<0.05. **Less than SHAM and TBI groups; P<0.05. FX, tibial fracture; MULTI, multitrauma; SHAM, sham-injuries; TBI, traumatic brain injury.
The rotarod was used to assess motor ability at 35 days after injury. There were no significant differences on the measure of duration on the rotarod during training (P>0.05; Figure 5D). However, for rotarod testing ANOVA indicated a significant injury effect for the measure of duration of time spent on the rotarod (F3,42=3.193, P<0.05; Figure 5E). Post hoc analyses revealed that the MULTI group spent significantly less time on the rotarod than both the SHAM and TBI groups (P<0.05). There were no significant group differences on any of the Y-maze measures (P>0.05, results not shown).
Discussion
To investigate whether a concomitant peripheral bone fracture affected TBI outcomes, here we developed a novel mouse model of multitrauma that involved a closed-skull weight-drop TBI and a tibial fracture. Using this model, we found that mice administered multitrauma displayed abnormal behaviors in the open-field compared with sham-injured, TBI-only, and fracture-only groups, and had significant motor impairments on the rotarod compared with sham-injured and TBI-only groups. MRI analysis also found that multitrauma mice had enlarged lateral ventricles and DTI abnormalities in the cortex and hippocampus compared with sham-injured, TBI-only, and fracture-only groups at 35 days after injury. These changes occurred in the presence of a heightened and persistent neuroinflammatory response in the multitrauma mice, as indicated by increased expression of the pro-inflammatory cytokine IL-1β and the astrogliosis marker GFAP at 24 hours and 35 days after injury. Furthermore, the multitrauma mice also had increased neutrophil expression at 24 hours after injury compared with sham-injured and fracture-only groups, whereas the mice administered only a TBI did not significantly differ from sham-injured and fracture-only mice. Multitrauma mice also had significantly worse BBB disruption and edema compared with all other groups at 24 hours after injury. Together, these findings indicated that a concurrent tibial fracture worsened TBI outcomes, and that an exacerbated neuroinflammatory response, BBB damage, and edema may have contributed to these effects.
Nature of Behavioral Abnormalities
Open-field testing is commonly used to assess anxiety levels in rodents.14, 15 Here, the MULTI mice spent significantly more time in the inner area of the open-field, which suggested that these mice had decreased anxiety.14, 15 As this occurred in the absence of any significant differences on measures of motor function, it is unlikely that motor deficits were a confounding factor in this finding. Interestingly, previous studies have reported increased risk-taking behaviors in mice 1 month after TBI,19 which may bear relevance to our finding of mice administered multitrauma spending more time in the exposed and unsheltered area of the open-field.
The multitrauma mice were also found to spend less time on the rotarod than the sham and TBI mice, which is indicative of motor impairments.14 As the mice administered only a tibial fracture did not differ from the multitrauma mice, it is likely that the fracture component was a contributing factor to the motor deficits observed in the multitrauma group. However, the motor deficits in the multitrauma group appear to have been more severe, as the fracture group did not significantly differ from sham-injury and TBI-only mice. While motor testing was avoided acutely post-injury to avoid disruption of fracture healing, future studies might employ a more detailed assessment of motor function to provide insight into the progression and recovery of these deficits.
Why Does a Peripheral Bone Fracture Exacerbate Neuroinflammation and Brain Damage Post-Traumatic Brain Injury?
Here, we found that multitrauma in mice resulted in a heightened acute neuroinflammatory response that persisted into the chronic stages of the injury process. This was evidenced by increased neutrophil expression in multitrauma mice at 24 hours, elevated levels of the pro-inflammatory cytokine IL-1β at 24 hours and 35 days, and increased expression of GFAP, an indicator of astrogliosis, at 24 hours and 35 days after injury. IL-1β is a potent pro-inflammatory factor that is upregulated after TBI and may underlie much of the consequent inflammation. IL-1β is known to activate astrocytes and is involved in the recruitment of peripheral leukocytes, such as neutrophils, to cross the BBB and enter the brain.20, 21 In doing so, neutrophils can further damage the BBB, contribute to edema formation, increase oxidative stress, and exacerbate neuroinflammation through the further production of pro-inflammatory mediators, including IL-1β, all of which may contribute to secondary brain injury.20, 21, 22, 23 Reactive astrocytes are also capable of producing pro-inflammatory cytokines, including IL-1β, and may be linked to the accompanying increase in neutrophils at 24 hours after injury, and IL-1β levels at 24 hours and 35 days after injury.20, 21 We also found increased levels of IL-6, albeit nonsignificant, in the plasma of multitrauma mice at 24 hours and 35 days. Our lack of significant plasma findings may be due to the 24- hour post-injury timing of the analysis. Specifically, the previous studies that have reported increased levels of circulating inflammatory cytokines in combined experimental TBI and tibial fracture found that these changes peaked before 24 hours.8, 9 Nonetheless, in light of our findings of increased BBB damage, the elevated neuroinflammation in the mice given multitrauma may be related to a compromised BBB caused by TBI combined with the robust fracture-induced inflammatory response. Taken together, there are a number of factors that likely contributed to the exacerbated neuroinflammation observed in the multitrauma mice, though future research is clearly required to provide further understanding of this complex response.
Magnetic resonance imaging analysis found that the multitrauma mice had evidence of greater brain damage, as indicated by enlarged ipsilateral lateral ventricles compared with each of the other groups. Ventricular enlargement is common after TBI, is associated with worsened neurologic outcomes, and is an index of severity of brain damage.24, 25 Ventricular enlargement post-TBI may be induced by either cortical atrophy or post-traumatic hydrocephalus.24, 25 Considering that we did not detect atrophy in cortex or hippocampus on MRI, the ventricular enlargement in the MULTI mice may be due to post-traumatic hydrocephalus. Post-traumatic hydrocephalus commonly develops in the subacute stages of TBI, and is often caused by the blockage of cerebrospinal fluid circulation or absorption via blood products.24, 25 Because the MULTI mice had significantly worse BBB damage, it is reasonable to speculate that blood products may have disrupted cerebrospinal fluid circulation and resulted in post-traumatic hydrocephalus and ventricle enlargement. However, future studies are required to determine the exact mechanisms.
The DTI analysis also identified diffusion abnormalities in the brains of multitrauma mice. Specifically, multitrauma mice had a significant decrease in MD in the injured cortex and hippocampus. MD measures the ability of water to move freely in the brain and can be affected by physical barriers within structures. Previous studies have found that TBI-induced gliosis is capable of affecting DTI outcomes in a manner similar to the changes found in the multitrauma mice here.26 Given that the multitrauma mice also had increased expression of GFAP at 35 days after injury, it is possible that the potentiated astrogliosis in multitrauma mice contributed to the decreased MD in the cortex and hippocampus.21, 26 Interestingly, as the hippocampus and the cortex are involved in anxiety-related behaviors and motor function,27, 28, 29 the diffusion abnormalities and heightened neuroinflammation within these structures may have contributed to the behavioral abnormalities in mice who experienced multitrauma.30, 31, 32
Although there is evidence supporting the notion that neuroinflammation may be a contributing factor to the worsened effects found in mice given multitrauma, it is also important to consider that neuroinflammation has protective properties in the TBI setting.33 In addition, this study did not include any histologic analysis that would have provided valuable information such as the location, distribution, and phenotype of inflammatory cells and cytokines. For example, here we found no evidence of changes in microglia/macrophage levels in brain tissue at 24 hours and 35 days after injury using Western blotting, which may be related to previous findings indicating that peak microglia activation occurs 5 to 7 days after TBI.34 However, histologic analysis may have been sensitive to more subtle changes in microglia phenotype,34 and should be applied in future studies. Overall, further research is required to better understand the role and nature of the elevated inflammatory factors in brain tissue and circulation after multitrauma.
In addition, there are a number of other factors that may be modulated in the multitrauma setting, such as increased levels of growth factors and the magnitude of the inflammatory response that could affect post-injury recovery. For example, previous studies have found that multitrauma patients have elevated nerve growth factor levels in fractures,35 and it is interesting to speculate whether similar changes in growth factors might occur in the brain and affect TBI outcomes. Moreover, the magnitude of the fracture or peripheral insult may influence the consequent inflammatory response,36 and it would be of interest to examine how fractures to different bones and/or other peripheral insults affect TBI outcome. Of relevance, a previous study reported increased neuroinflammation and apoptosis in rats that received a systemic injection lipopolysaccharide immediately post-injury,37 suggesting that other inflammatory-inducing stimuli can affect TBI outcomes. Thus, future research is needed to better understand the interactive pathophysiologic effects that occur in multitrauma, and how these changes affect recovery. Finally, few studies have investigated the effect of multitrauma on TBI patient outcome, and findings from these studies have been mixed. It has been reported that multitrauma in TBI negatively impacts rehabilitation and is associated with worse long-term functional outcomes.38, 39 However, these changes have been attributed to factors such as more severe initial brain injuries in the multitrauma group, and rehabilitation complications related to the extracranial injury. Conversely, a different study reported that isolated TBI and multitrauma patients do not differ on Glasgow Outcome Scale at 6 and 12 months after injury.40 However, in this study the isolated TBI patients were significantly older than the TBI+multitrauma patients, which may have confounded these results considering that increased age was associated with worse outcomes in the same study. In light of these mixed findings, future studies are necessary to determine whether multitrauma worsens TBI-related brain damage and neurologic outcomes in patients in a manner similar to what we have found in our novel mouse model. Ideally such a study would control for the initial severity of the TBI, and serially monitor brain damage and inflammatory factors into chronic recovery.
Conclusions
We herein report a novel mouse model of multitrauma involving the combined injuries of tibial fracture and closed-skull TBI. Using this model, we discovered that mice afflicted with both a tibial fracture and TBI had worsened behavioral abnormalities and brain damage compared with mice given only a TBI, and that these effects occurred in the presence of exacerbated neuroinflammation, edema, and BBB disruption. Although future studies are required, these findings may have important implications toward better understanding the pathophysiologic complexities of multitrauma involving TBI, and in guiding therapeutic interventions.
Acknowledgments
The authors wish to acknowledge Emma Braine and Karen Griggs for their assistance with ELISA analysis.
Author Contributions
All authors contributed to the writing of the manuscript. SRS, TOB, AHK, JAH, and SJM conceptualized and designed the experiment. MS, SL, and SFS conducted ELISA, Western blotting, Evans blue extravasation, and edema analyses. DKW, SRS, and SB completed MRI analysis and interpretation. SB, MS, and SRS completed behavioral testing, analysis, and interpretation. SRS, SJM, RDB, SB, and BLG administered the injury methods.
The authors declare no conflict of interest.
Footnotes
This study was funded by a grant to SRS from the National Health and Medical Research Council (NHMRC #1062653), a fellowship to SRS from the Canadian Institute of Health Research (CIHR), and grants to SJM and BLG from La Trobe University.
References
- Al-Thani H, El-Menyar A, Abdelrahman H, Zarour A, Consunji R, Peralta R, et al. Workplace-related traumatic injuries: insights from a rapidly developing middle eastern country. J Environ Public Health. 2014;2014:430832. doi: 10.1155/2014/430832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobscha SK, Clark ME, Morasco BJ, Freeman M, Campbell R, Helfand M. Systematic review of the literature on pain in patients with polytrauma including traumatic brain injury. Pain Med. 2009;10:1200–1217. doi: 10.1111/j.1526-4637.2009.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor AJ, Mayo JA, Dougherty AL, Girard PJ, Galarneau MR. Injuries sustained in noncombat motor vehicle accidents during Operation Iraqi Freedom. Injury. 2012;43:1551–1555. doi: 10.1016/j.injury.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA, Champion HR, Copes WS, Sacco WJ. Comparison of mortality, morbidity, and severity of 59,713 head injured patients with 114,447 patients with extracranial injuries. J Trauma. 1994;37:962–968. doi: 10.1097/00005373-199412000-00016. [DOI] [PubMed] [Google Scholar]
- Gross T, Schuepp M, Attenberger C, Pargger H, Amsler F. Outcome in polytraumatized patients with and without brain injury. Acta Anaesthesiol Scand. 2012;56:1163–1174. doi: 10.1111/j.1399-6576.2012.02724.x. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegele M, Riess P, Sauerland S, Bouillon B, Hess S, McIntosh TK, et al. Characterization of a new rat model of experimental combined neurotrauma. Shock. 2005;23:476–481. doi: 10.1097/01.shk.0000159929.87737.5c. [DOI] [PubMed] [Google Scholar]
- Maegele M, Sauerland S, Bouillon B, Schafer U, Trubel H, Riess P, et al. Differential immunoresponses following experimental traumatic brain injury, bone fracture and "two-hit"-combined neurotrauma. Inflamm Res. 2007;56:318–323. doi: 10.1007/s00011-007-6141-3. [DOI] [PubMed] [Google Scholar]
- Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Sem Cell Dev Biol. 2008;19:459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Probst C, Mirzayan MJ, Mommsen P, Zeckey C, Tegeder T, Geerken L, et al. Systemic inflammatory effects of traumatic brain injury, femur fracture, and shock: an experimental murine polytrauma model. Mediat Inflamm. 2012;2012:136020. doi: 10.1155/2012/136020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton CA, Chatfield D, Brooks RA, Rushton N. Circulating levels of interleukin-6 and its soluble receptor in patients with head injury and fracture. J Bone Joint Surg. 2004;86:912–917. doi: 10.1302/0301-620x.86b6.14176. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat Protoc. 2009;4:1328–1337. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Tan XL, Wright DK, Liu SJ, Semple B, Johnston L, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is neuroprotective in experimental traumatic brain injury. J Neurotrauma. 2014;31:976–983. doi: 10.1089/neu.2013.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, Cardamone L, Liu YR, Hogan RE, Maccotta L, Wright DK, et al. Can structural or functional changes following traumatic brain injury in the rat predict epileptic outcome. Epilepsia. 2013;54:1240–1250. doi: 10.1111/epi.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindeler A, Morse A, Harry L, Godfrey C, Mikulec K, McDonald M, et al. Models of tibial fracture healing in normal and Nf1-deficient mice. J Orthop Res. 2008;26:1053–1060. doi: 10.1002/jor.20628. [DOI] [PubMed] [Google Scholar]
- Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp. 2013;73:e50062. doi: 10.3791/50062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Im Y-B, Shunmugavel A, Gilg AG, Dhindsa RK, Singh AK, et al. Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J Neuroinflammation. 2009;6:32. doi: 10.1186/1742-2094-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashaw ML, Czerniecka K, et al. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauama. 2014;31:1211–1224. doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algattas H, Huang JH. Traumatic brain injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int J Mol Sci. 2013;15:309–341. doi: 10.3390/ijms15010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Bao F, Weaver LC, Cain DP, Brown A. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. J Neuroinflammation. 2013;10:26. doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Shultz SR, Hepburn JD, Omana V, Weaver LC, Cain DP, et al. A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion injury in rats. J Neurotrauma. 2012;29:2375–2392. doi: 10.1089/neu.2012.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L, Campini R, Angelino E, Rognone F, Pastore I, Oliveri G. Posttraumatic hydrocephalus: a clinical, neuroradiologic, and neuropsychologic assessment of long-term outcome. Arch Phys Med Rehabil. 2003;84:1637–1641. doi: 10.1053/s0003-9993(03)00314-9. [DOI] [PubMed] [Google Scholar]
- Tian H-L, Xu T, Hu J, Cui Y-H, Chen H, Zhou L-F. Risk factors related to hydrocephalus after traumatic subarachnoid hemorrhage. Surg Neurol. 2008;69:241–246. doi: 10.1016/j.surneu.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe VF, Outtrim JG, Chatfield DA, Manktelow A, Hutchinson PJ, et al. Parcellating the neuroanatomical basis of impaired decision-making in traumatic brain injury. Brain. 2011;134:759–768. doi: 10.1093/brain/awq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Zou J, Verhovshek T, Chen C, Lu QB, et al. A semicircular controlled cortical impact produces long-term motor and cognitive dysfunction that correlates well with damage to both the sensorimotor cortex and hippocampus. Brain Res. 2014;1576:18–26. doi: 10.1016/j.brainres.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Piot-Grosjean O, Wahl F, Gobbo O, Stutzmann JM. Assessment of sensorimotor and cognitive deficits induced by a moderate traumatic injury in the right parietal cortex of the rat. Neurobiol Dis. 2001;8:1082–1093. doi: 10.1006/nbdi.2001.0450. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160:187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long–Evans rat: Support for an animal model of concussion. Behav Brain Res. 2011;224:326–335. doi: 10.1016/j.bbr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Bao F, Omana V, Chiu C, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma. 2012;29:281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossman MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Avila JC, Lam TI, Bingham D, Shi J, Won SJ, Kauppinen TM, et al. Microglia activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J Neuroinflammation. 2012;9:31. doi: 10.1186/1742-2094-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y-F, Li J. Serum EGF and NGF levels of patients with brain injury and limb fracture. Asian Pac J Trop Med. 2013;6:383–386. doi: 10.1016/S1995-7645(13)60043-7. [DOI] [PubMed] [Google Scholar]
- Pasquale MD, Cipolle MD, Monaco J, Simon N. Early inflammatory response correlates with the severity of injury. Crit Care Med. 1996;24:1238–1242. doi: 10.1097/00003246-199607000-00029. [DOI] [PubMed] [Google Scholar]
- Hang CH, Shi JX, Tian J, Li JS, Wu W, Yin HX. Effect of systemic LPS injection on cortical NF-kappaB activity and inflammatory response following traumatic brain injury in rats. Brain Res. 2004;1026:23–32. doi: 10.1016/j.brainres.2004.07.090. [DOI] [PubMed] [Google Scholar]
- Groswasser Z, Cohen M, Blankstein E. Polytrauma associated with traumatic brain injury: incidence, nature and impact. Brain Inj. 1990;4:161–166. doi: 10.3109/02699059009026161. [DOI] [PubMed] [Google Scholar]
- Leong BK, Mazlan M, Rahim RBA, Ganesan D. Concomitant injuries and its influence on functional outcome after traumatic brain injury. Disabil Rehabil. 2013;35:1546–1551. doi: 10.3109/09638288.2012.748832. [DOI] [PubMed] [Google Scholar]
- Kumar RG, Diamond ML, Boles JA, Berger RP, Tisherman SA, Kochanek PM, et al. Acute CSF interleukin-6 trajectories after TBI: associations with neuroinflammation, polytrauma, and outcome. Brain Behav Immun. 2015;45:253–262. doi: 10.1016/j.bbi.2014.12.021. [DOI] [PubMed] [Google Scholar]