Abstract
Methamphetamine (METH) is a psychostimulant that causes neurologic and psychiatric abnormalities. Recent studies have suggested that its neurotoxicity may also result from its ability to compromise the blood–brain barrier (BBB). Herein, we show that METH rapidly increased the vesicular transport across endothelial cells (ECs), followed by an increase of paracellular transport. Moreover, METH triggered the release of tumor necrosis factor-alpha (TNF-α), and the blockade of this cytokine or the inhibition of nuclear factor-kappa B (NF-κB) pathway prevented endothelial dysfunction. Since astrocytes have a crucial role in modulating BBB function, we further showed that conditioned medium obtained from astrocytes previously exposed to METH had a negative impact on barrier properties also via TNF-α/NF-κB pathway. Animal studies corroborated the in vitro results. Overall, we show that METH directly interferes with EC properties or indirectly via astrocytes through the release of TNF-α and subsequent activation of NF-κB pathway culminating in barrier dysfunction.
Keywords: astrocytes, blood–brain barrier, methamphetamine, nuclear factor-kappa B, tumor necrosis factor-alpha
Introduction
Methamphetamine (METH) abuse is a serious public health problem affecting over 35 million users worldwide.1 The overall negative effects of this drug are well known, including irreversible brain damage that may cause neurologic and psychiatric anomalies.2, 3 Indeed, studies with human subjects have shown that METH users present brain structural abnormalities, which can explain behavioral problems, recall capacity, as well as memory and performance deficits observed in verbal memory tests and executive functions.3 Additionally, long-term neuronal damage is well described in humans, nonhuman primates, and rodents.4 However, despite extensive characterization of METH toxicity over the last years, many questions remain unanswered. Most hypotheses have focused on intraneuronal events such as dopamine oxidation, oxidative stress, and excitotoxicity,4 but it has been recently suggested that METH-induced neurotoxicity may also result from its ability to compromise the blood–brain barrier (BBB) function.5
The BBB is a dynamic and complex interface between the blood and the central nervous system, having a key role in brain homeostasis and protection.6 Although cerebral endothelium disturbance is commonly observed in the central nervous system (CNS) pathologies,7 neither its cause nor the effective participation of BBB in such diseases is well established. Thus, given that BBB damage is an early event in many neurologic conditions, it is not surprising the growing interest in this barrier as a therapeutic target. At the cellular level, this dynamic and specialized structure is composed by endothelial cells (ECs) associated with pericytes, basement membrane, astrocyte endfeet, microglia, and neurons, comprising the neurovascular unit.6 The brain endothelium remains the first ‘physical barrier', with lack of fenestrations, low fluid-phase endocytosis (pinocytosis), and intercellular complexes that confer low paracellular permeability and high transendothelial electrical resistance (TEER) to the BBB.6 Nevertheless, all BBB components have a unique role in its function and, among them, astrocytes are involved in the formation of the tight junction (TJ) complex, microvascular support, and in the control of water flux into the brain.8
As aforementioned, alterations in BBB function are likely involved in many neurodegenerative diseases, and drug abuse is not an exception. In fact, it was previously shown that METH compromises BBB function and its capacity to protect the brain against infection by the human immunodeficiency virus.2 However, the cellular mechanisms underlying these effects are still poorly understood. Most studies suggest that BBB alterations induced by METH result from alterations on TJ complex,5 involving oxidative stress,9 and activation of matrix metalloproteinases (MMPs).5, 10 Importantly, we have also shown that METH may trigger a neuroinflammatory response with increased production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α).11, 12 Accordingly, Lee et al13 showed that METH upregulates TNF-α gene and activates nuclear factor kappa B (NF-κB) in human brain ECs. Moreover, astrocytes modulate endothelial responses under pathologic conditions by secreting factors that cause BBB leakage.8, 14 It is noteworthy the involvement of TNF-α in BBB dysfunction,15, 16, 17 as well as in promoting the passage of circulating leukocytes into the brain.18 Despite knowing that METH may induce BBB impairment and neuroinflammation, the possible involvement of pro-inflammatory cytokines in METH-induced BBB alterations has never been investigated.
Herein, we show that METH increases BBB permeability by interfering with both paracellular and vesicular transport across ECs. Additionally, the crosstalk between astrocytes and ECs clearly shows the important role played by glial cells on barrier properties. Overall, we unravel the key role of TNF-α, via activation of NF-κB signaling, in METH-induced BBB dysfunction.
Materials and methods
Animal Studies
Animal manipulation was performed by certified researchers (Portuguese National Authority for Animal Health), and in accordance with both the European Community Council Directives (2010/63/EU) and the Portuguese law for the care and use of experimental animals (DL nº 113/2013). The present study was approved by the Foundation for Science and Technology (PTDC/SAU-FCF/098685/2008). The experiments were performed in accordance with the ARRIVE guidelines. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Primary Cultures of Rat Brain Microvascular Endothelial Cells
Primary cultures of rat brain microvascular ECs (RBMVECs) were prepared from 2-week-old Wistar rats, as previously described19 with minor modifications. Specifically, forebrains were freed of meninges, dissociated into small pieces, and digested in Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12; Biochrom AG, Berlin, Germany), containing 1 mg/mL collagenase CLS2 (Sigma-Aldrich, St. Louis, MO, USA) and 14 μg/mL DNase (Sigma-Aldrich) during 90 minutes at 37 °C. The suspension was centrifuged (1,000 × g, 8 minutes) followed by a second centrifugation of the pellet (1,000 × g, 20 minutes). Microvessels were further digested with 1 mg/mL collagenase-dispase (Roche Applied Sciences, Basel, Switzerland) and 7 μg/mL DNase (Sigma-Aldrich) in DMEM/F12 for 1 hour at 37°C, centrifuged (700 × g, 5 minutes) and seeded at high density (1.5 × 105 cells/cm2) onto tissue culture multiplates, coverslips or 12-mm Costar Transwells (Corning Life Sciences, Tewksbury, MA, USA) coated with collagen type IV/fibronectin (Sigma-Aldrich). Cultures were maintained in DMEM/F12 supplemented with 15% fetal bovine serum (GIBCO, Rockville, MD, USA), 1 ng/mL basic fibroblast growth factor (Roche Applied Sciences), 100 μg/mL heparin (Biochrom AG), 5 μg/mL gentamicin (GIBCO) and 4 μg/mL puromycin (Sigma-Aldrich) at 37°C with a humidified atmosphere of 5% CO2, for 2 days. On day 3, cells were incubated with new medium that contained all components aforementioned, except puromycin. When RBMVECs were 90% confluent, hydrocortisone (550 nmol/L; Sigma-Aldrich) was added for 1 day. Hydrocortisone was used to improve barrier proprieties of ECs, namely by the induction of TJs expression and enhancement of TEER.19
Primary Cultures of Human Brain Microvascular Endothelial Cells
Primary cultures of human brain microvascular ECs (HBMVECs) were obtained from microvessels isolated from lateral temporal cortex of adult patients undergoing surgery for the treatment of intractable epilepsy at the Neurosurgery Service, Coimbra Hospital and University Centre, as previously described.20 The present study was approved by the Hospital Ethics Committee.
Primary Cultures of Mouse Cortical Astrocytes
Astrocytes were isolated from C57BL/6J mouse pups aged P4-5. Brain cortices were isolated and incubated with digestion solution (Hank's Balanced Salt Solution (HBSS; GIBCO), 0.25% Trypsin (SAFC Biosciences Inc, Lenexa, KS, USA), 0.001% DNase (Sigma-Aldrich), 10 μL/mL Gentamicin (GIBCO) for 20 minutes at 37°C. After brief centrifugation (70 × g, 3 minutes), the pellet was incubated with the inhibitory digestion solution (HBSS with 10% fetal bovine serum), cells were dissociated and centrifuged (143 × g, 5 minutes), followed by pellet resuspension in astrocyte medium (DMEM high glucose supplemented with 10% fetal bovine serum and 1% gentamicin). Cells were plated in T-75 flasks at a density of 1.2 × 105 cells/cm2, and medium was changed after 6 hours and then every 2 days until reach confluence. Afterwards, flasks were shaken (4 hours at 37°C) to detach nonastrocytic cells, and astrocytes (adherent cells) were washed with dissociation medium [HBSS with 1 mmol/L ethylenediamine tetraacetic acid (EDTA, Sigma-Aldrich)] followed by trypsinization with 0.1% Trypsin (SAFC) in HBSS. This process was stopped by incubation with astrocyte medium followed by centrifugation (143 × g, 5 minutes). Cells were plated at different densities depending on the experiments.
Evaluation of Endothelial Cell Monolayer Integrity
Rat and human ECs were left untreated (control) or treated with different drugs as follows: METH (1 or 50 μmol/L), BAY 11-7085 (5 μmol/L BAY), lysophosphatidic acid (10 μmol/L; all from Sigma), TNF-α (0.01 μg/mL; R&D Systems, Abingdon, UK), and both anti-rat (0.01 μg/mL Ab TNF-α; PeproTech, Inc., Princeton, NJ, USA), and anti-mouse TNF-α antibodies (100 μg/mL Ab TNF-α; Upstate Biotechnology, Inc., Lake Placid, NY, USA). The flux of sodium fluorescein (Na-F, 376 Da; Sigma-Aldrich) across cell monolayer was determined as previously described.21 Moreover, TEER of monocultures was measured using a STX-2 electrode coupled to an EVOM resistance meter (World Precision Instruments, Hertfordshire, UK). Transendothelial electrical resistance readings of cell-free inserts were subtracted from the values obtained with cells, and results were expressed as % of control.
Horseradish Peroxidase Transport
Endothelial cells were left untreated (control) or exposed to METH (1 μmol/L) either alone or in the presence of BAY (5 μmol/L) for 1 hour at 37°C. Afterwards, horseradish peroxidase (HRP, 10 mg/mL; Sigma-Aldrich) transport by confluent RBMVECs was quantified during 2 hours, as previously described.21
Enzyme-Linked Immunosorbent Assay
Endothelial cells and astrocytes were left untreated (control) or exposed to METH (1 or 50 μmol/L) for different time periods as indicated in the respective graphs. The released levels of TNF-α from both cell types were quantified by ELISA Ready-SET-Go kit (eBioscience, San Diego, CA, USA), as specified in the datasheet.
Immunocytochemistry
Endothelial cells were left untreated (control) or incubated with different drugs as specified in the respective figure legends. For p65 and intracellular adhesion molecule (ICAM)-1 identification, cells were fixed with 4% paraformaldehyde (PFA; Sigma-Aldrich) for 20 minutes at room temperature (RT). Regarding claudin-5, cells were fixed with ethanol (95%)-acetic acid (5%) (v/v) for 10 minutes at −20°C,19 whereas for occludin identification, cells were pretreated with an extraction buffer (0.2% Triton X-100 in 100 mmol/L KCl, 3 mmol/L MgCl2, 1 mmol/L CaCl2, 200 mmol/L Sucrose, and 10 mmol/L HEPES, pH 7.1) before fixation with PFA. Afterwards, cells were permeabilized with 0.25% Triton X-100 (Sigma-Aldrich) and blocked with 3% bovine serum albumin (Sigma-Aldrich) during 1 hour at RT, followed by incubation with primary antibodies (rabbit anti-p65, 1:100, 2 hours at RT, Cell Signaling Technology, Inc., Danvers, MA, USA; mouse anti-claudin-5, 1:100, Invitrogen, Inchinnan Business Park, UK; rabbit anti-occludin, 1:100, Invitrogen; and rabbit anti-ICAM-1, 1:100, Santa Cruz Biotechnology Inc., Dallas, TX, USA, all during 1 hour at 37°C). Then, cells were incubated with secondary antibodies (Alexa Fluor 488 or Alexa Fluor 594, 1:200, Invitrogen) for 1 hour at RT in the dark and nuclei were stained with 4 μg/mL Hoechst 33342 (Sigma-Aldrich) for 5 minutes at RT. Finally, cells were mounted in Dako fluorescence medium (Dako North America, Carpinteria, CA, USA) and images were recorded using a LSM 710 Meta Confocal microscope (Carl Zeiss, Oberkochen, Germany). To express these results, we counted the nuclear p65-positive ECs within a total of 1,000 cells obtained from 37 to 42 visual fields acquired from 3 different slides.
Western Blot Analysis
The protocol was performed as previously described.12 Primary antibodies used were as follows: mouse anti-claudin-5 (1:200, Invitrogen), rabbit anti-occludin (1:150, Invitrogen) and rabbit anti-ICAM-1 (1:200, Santa Cruz). Secondary antibodies were as follows: alkaline phosphatase-conjugated secondary antibody anti-mouse (1:10,000) and anti-rabbit (1:20,000) (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Immunoblots were reprobed with an antibody against glyceraldehyde 3-phosphate dehydrogenase (1:500; Abcam, Cambridge, UK) to ensure equal sample loading. Bands were visualized using the enhanced chemifluorescence reagent assay on the Typhoon FLA 9000 (both from GE Healthcare), and quantification was performed using the ImageJ 1.47 software (NIH, Bethesda, MD, USA).
Astrocyte-Conditioned Medium Experiments
Astrocytes were seeded in 6-well plates at a density of 5 × 104 cells/cm2, and after 3 days cells were left untreated (control) or incubation with 50 μmol/L METH for 4 hours. Afterwards, media from untreated [CTR astrocyte-conditioned medium (ACM)] and METH-treated astrocytes (METH ACM) were centrifuged (172 × g, 5 minutes at 4°C), the supernatant was collected, and confluent RBMVECs were incubated with CTR ACM, METH ACM alone, or simultaneously with Ab TNF-α or BAY during different time periods, as indicated in the respective graphs.
Animal Treatments
Male wild-type C57BL/6J mice (3 months old; 24 to 26 g body weight; Charles River Laboratories, Barcelona, Spain) were housed under controlled environmental conditions (12 hours light:dark cycle, 24±1°C) with food and water ad libitum. Mice were divided into three different groups as follows: control group (4 × 0.9% NaCl, 2 hours apart, intraperitoneal (i.p.) injections); METH binge group (4 × 10 mg/kg METH, 2 hours apart, i.p.); METH+BAY group (mice received 20 mg/kg BAY i.p., 30 minutes before each METH administration, as previously described).22 Animals were killed 1, 2, or 24 hours after the last drug injection.
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (80 mg/kg, i.p., Sigma-Aldrich) and transcardially perfused with 10 mL of 0.05 mol/L sodium citrate in 1% PFA (pH 4.2, 37°C), followed by 20 mL of 4% PFA in 0.01 mol/L PBS, pH 7.4. Brains were removed, postfixed in 4% PFA for 24 hours at RT and transferred to 30% sucrose in 0.01 mol/L PBS, pH 7.4, for at least 24 hours at 4°C. Coronal sections (12 μm) were cut on a cryostat (Leica CM3050S, Nussloch, Germany), mounted directly onto superfrost microscope slides (Thermo Scientific, Menzel GmbH & Co KG, Braunschweig, Germany) and stored at −80°C until further use. Then, slices were rinsed in 0.01 mol/L PBS, blocked with 5% bovine serum albumin in 0.01 mol/L PBS for 1 hour at RT and incubated overnight at 4°C with rabbit anti-collagen IV (1:200; Abcam), goat anti-albumin (1:2,000; Bethyl Laboratories, Inc, Montgomery, TX, USA), and rabbit anti-TNF-α (1:100; Santa Cruz) antibodies. Afterwards, slices were incubated with Alexa Fluor 488 and Alexa Fluor 594 secondary antibodies (1:200; Invitrogen) for 1 hour at RT, followed by nuclei staining with 5 μg/mL Hoechst 33342 (Sigma-Aldrich) for 5 minutes at RT in the dark. For glial fibrillary acidic protein (GFAP) staining, slices were incubated with anti-GFAP-Cy3 conjugated antibody (1:2,000; Sigma-Aldrich). Finally, slices were mounted with Dako fluorescence medium (Dako North America) and images were recorded using a LSM 710 Meta Confocal microscope (Carl Zeiss). Quantification of collagen IV, albumin, TNF-α, and GFAP immunoreactivity was performed using the NIH ImageJ 1.47 analysis software. Specifically, a region was drawn around each striatum vessel, as well as in a close area without staining (black) to be used for background subtraction. To determine the corrected total vessel fluorescence, we used the following formula: correct total fluorescence of each vessel=(integrated intensity)−(area for the selected vessel × mean background). The results are expressed as mean of fluorescence intensity (arbitrary units) of six brain slices obtained from three different animals for each experimental group.
Statistical Analysis
Evaluation of EC monolayer integrity and western blot analysis were performed by a person blinded to treatments. Results are expressed as mean+standard error of the mean (S.E.M.). Data were analyzed using the one-way ANOVA followed by Dunnett's or Bonferroni's post hoc test, as indicated in figure legends. All statistics were calculated using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). The level of significance was P<0.05 and the ‘n' represents the total number of experiments obtained from at least three independent cell cultures or animals.
Results
Methamphetamine Impairs the Barrier Function of Endothelial Cells
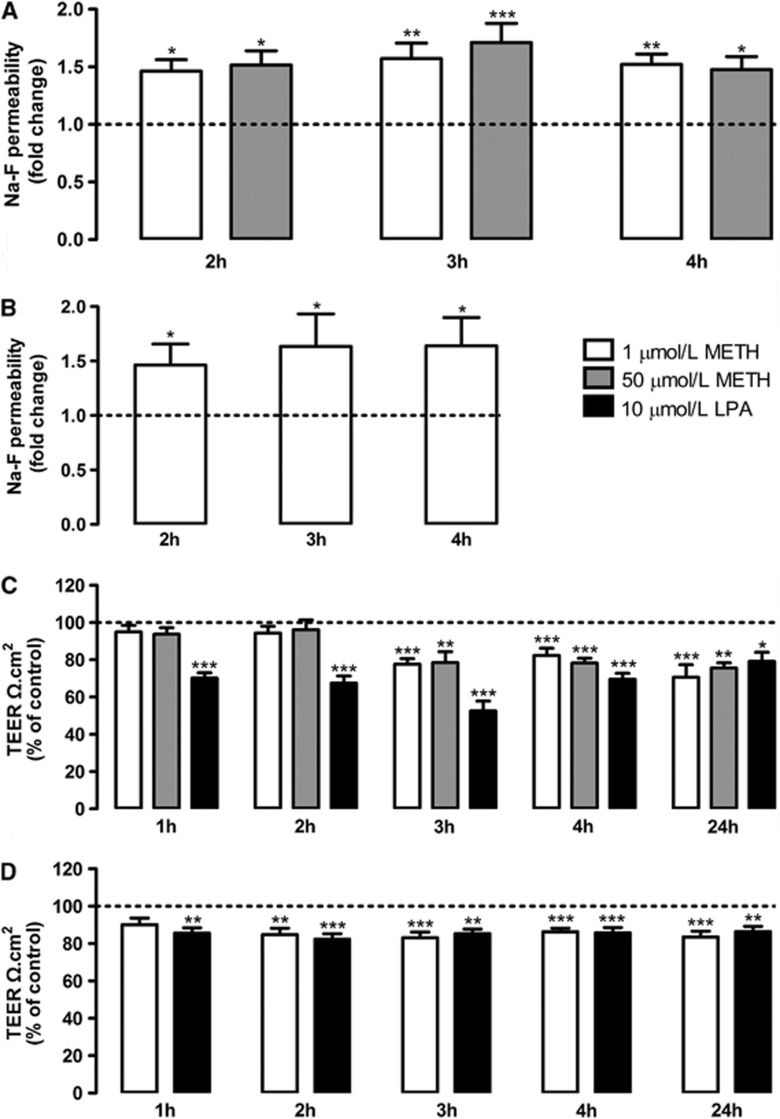
To study barrier alterations induced by METH, we used freshly prepared primary RBMVECs since this in vitro model preserves many characteristics of the intact BBB, such as expression of continuous adherens and TJs, as well as low permeability to macromolecules.6 Regarding METH concentrations used in the present study, it is important to highlight that abusers with lower dose-METH exposure present blood levels of this drug within a range of 0.1 and 11.1 μmol/L.23 However, they tend to self-administer METH in binge manner leading to higher levels. It is estimated that binge doses in a 260 mg to 1 g range may lead to 17 to 80 μmol/L of METH circulating in the blood. Thus, we decided to use concentrations that are closer to the human condition. Our results show that METH (1 and 50 μmol/L) increased the permeability to Na-F at 2, 3, and 4 hours of drug exposure (Figure 1A). Thus, we further aimed to reproduce these results in HBMVECs. Indeed, METH (1 μmol/L) increased Na-F flux (Figure 1B) across human cells. Moreover, to clarify whether BMVEC-increased permeability was due to alterations in the paracellular pathway, we further measured TEER values on both cultures. In RBMVECs, both METH concentrations led to a significant decrease in TEER at 3 hours after drug exposure (Figure 1C), which was maintained until 24 hours (Figure 1C). We used lysophosphatidic acid (10 μmol/L) as a positive control since it produces a strong and transient opening of the ionic barrier.20 Accordingly, TEER values of HBMVECs were decreased at 2 hours after drug exposure up until 24 hours (Figure 1D). Noteworthy, the concentrations of METH used in the present study neither caused EC death nor decreased cell viability (Supplementary Figure 1). These results show that METH at a low concentration does not interfere with cell viability but impairs barrier properties, proven by the increased permeability to Na-F and decreased TEER values in both rat and human BMVECs.
Figure 1.
Methamphetamine (METH) increases the permeability of brain microvascular endothelial cells (BMVECs). (A, B) Macromolecular flux across (A) rat and (B) human BMVECs was assessed using sodium fluorescein (Na-F, 376 Da) at different time points after METH exposure, n=5 to 20. (C, D) Transendothelial electrical resistance (TEER) of confluent (C) rat and (D) human BMVECs monolayers was analyzed under condition of METH or lysophosphatidic acid (LPA) exposure during different time periods, n=5 to 10. All results are shown as means+S.E.M. *P<0.05, **P<0.01, ***P<0.001 significantly different when compared with the control (dashed line) of each time point using Bonferroni's Multiple comparison test.
Methamphetamine Leads to Barrier Breakdown via Tumor Necrosis Factor-Alpha/Nuclear Factor-Kappa B Pathway
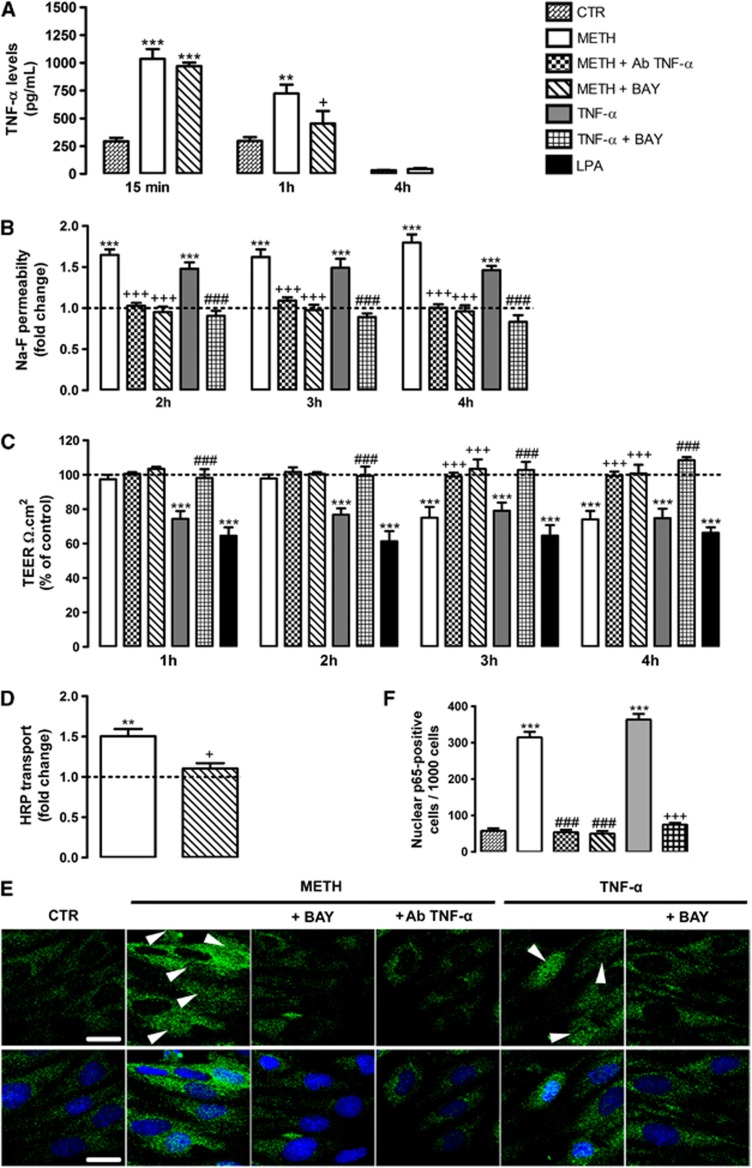
Methamphetamine is able to trigger a pronounced neuroinflammatory response with increased release of pro-inflammatory cytokines.11, 12 Moreover, TNF-α has been proposed as an important mediator of BBB breakdown.17 However, nothing is known regarding the role of TNF-α on METH-induced BBB dysfunction. Thus, we started by analyzing the effect of METH (1 μmol/L) on TNF-α protein levels released by RBMVECs, and we observed a prominent increase after 15 minutes of drug exposure (Figure 2A) that was still significantly higher than control after 1 hour, recovering to control values at 4 hours.
Figure 2.
Methamphetamine (METH) impairs barrier properties via tumor necrosis factor-alpha (TNF-α)/nuclear factor-kappa B (NF-κB) pathway. (A) METH (1 μmol/L) increased TNF-α levels after 15 minutes, which was still observed after 1 hour. The blockade of NF-κB pathway with BAY only prevented TNF-α released at 1 hour after METH. The cytokine levels are expressed as mean pg/mL+S.E.M., n=3 to 9. (B) Rat endothelial cell (RBMVEC) permeability was assessed using Na-F at different time points under the following experimental conditions: 1 μmol/L METH; METH+0.01 μg/mL Ab TNF-α; METH+5 μmol/L BAY; 0.01 μg/mL TNF-α; TNF-α+BAY, n=3 to 13. (C) Transendothelial electrical resistance (TEER) was analyzed at different time periods under the same conditions as above mentioned, n=3 to 8. (D) Horseradish peroxidase (HRP) transport across RBMVECs was evaluated in the presence of METH alone or in combination with BAY for 1 hour, n=3 to 4. The results shown are means+S.E.M. (E) Representative images showing the activation of NF-κB pathway by METH (15 minutes exposure), which was evaluated by p65 translocation into the nucleus (arrowheads). This effect was prevented by BAY, as well as by Ab TNF-α. As a positive control, TNF-α was used which effect was also prevented by BAY. p65 (green), Hoechst 33342 (blue) and scale bars=20 μm. (F) Quantification of nuclear p65-positive RBMVECs under the same experimental conditions as in (E). Cells were counted within a total of 1,000 obtained from 37 to 42 visual fields acquired from 3 different slides. **P<0.01, ***P<0.001, significantly different when compared with the control (CTR or dashed line); +P<0.05, +++P<0.001 significantly different when compared with METH; ###P<0.001 significantly different when compared with TNF-α from each time point using Bonferroni's Multiple comparison test.
To clarify the possible involvement of TNF-α on METH-induced endothelial permeability, we used a specific antibody to block the effect of this pro-inflammatory cytokine (Ab TNF-α, 0.01 μg/mL). Under such conditions, the effect of METH on Na-F permeability (Figure 2B) and TEER (Figure 2C) was prevented. Additionally, TNF-α (0.01 μg/mL) increased Na-F permeability (Figure 2B) and decreased TEER (Figure 2C), without causing EC death (Supplementary Figure 2). In fact, this pro-inflammatory cytokine has been described as an inductor of barrier opening.15, 24 Importantly, it was also documented that NF-κB is a critical signaling molecule in TNF-α-induced inflammatory response.25, 26 Thus, we further investigated the role of NF-κB in METH-induced barrier dysfunction by using an inhibitor of this pathway, BAY 11-7085.27 In more detail, BAY is an upstream inhibitor of NF-κB that prevents the activation of inhibitory κB kinase, an enzyme required for the activation of NF-κB.28 Moreover, the concentration used was based on the literature for the purpose of inhibiting NF-κB activation without causing toxicity.28 We concluded that blockade of NF-κB abrogated both METH- and TNF-α-induced barrier permeability (Figure 2B) and restored TEER to basal values (Figure 2C).
It is noteworthy that at 1 and 2 hours after METH exposure there were no alterations in TEER values (Figure 2C), although with an increase in permeability to Na-F (Figure 2B). Thus, Na-F studies give us the information regarding general permeability, whereas TEER specifically indicates alterations in paracellular transport. Taking these into consideration, we hypothesized that METH may increase EC permeability by different mechanisms. Specifically, our results show that until 2 hours there is no significant alteration in the paracellular pathway, demonstrated by unchanged TEER values (Figure 2C). Thus, we further investigated whether transcytosis occurred during this first period after METH exposure by measuring HRP flux across RBMVECs. Herein, we clearly show that METH (1 μmol/L, 1 hour) increased HRP transport, which was once again dependent on NF-κB pathway, since BAY completely prevented the effect of METH (Figure 2D). To further confirm the activation of NF-κB signaling pathway, we performed an immunocytochemical assessment of the cytoplasmic and nuclear localization of p65 NF-κB subunit (Figure 2E), followed by the quantification of nuclear p65-positive cells (Figure 2F). Interestingly, we observed p65 translocation into the nucleus to be markedly increased after METH exposure (15 minutes), which was blocked by BAY (Figures 2E and 2F). This time course is in accordance with the observed increase of TNF-α levels after 15 minutes of METH exposure (Figure 2A), which suggest that METH triggers the release of TNF-α that will activate the NF-κB pathway. To better explain this sequence of events, we showed that the first pool of TNF-α to be release (15 minutes post-METH) was independent of NF-κB pathway and so of new transcription, since BAY did not prevent this effect (Figure 2A). However, at least some of the TNF-α that is being released after 1 hour of METH exposure is already dependent of NF-κB pathway activation (Figure 2A). Moreover, we showed that TNF-α blockade with a neutralizing antibody at 15 minutes after METH prevented the translocation of p65 NF-κB subunit to the cell nuclei (Figures 2E and 2F). Additionally, to prove the direct effect of TNF-α on NF-κB activation, we showed that this cytokine promoted p65 translocation into the nucleus, which was once again prevented by blocking the NF-κB pathway with BAY (Figures 2E and 2F).
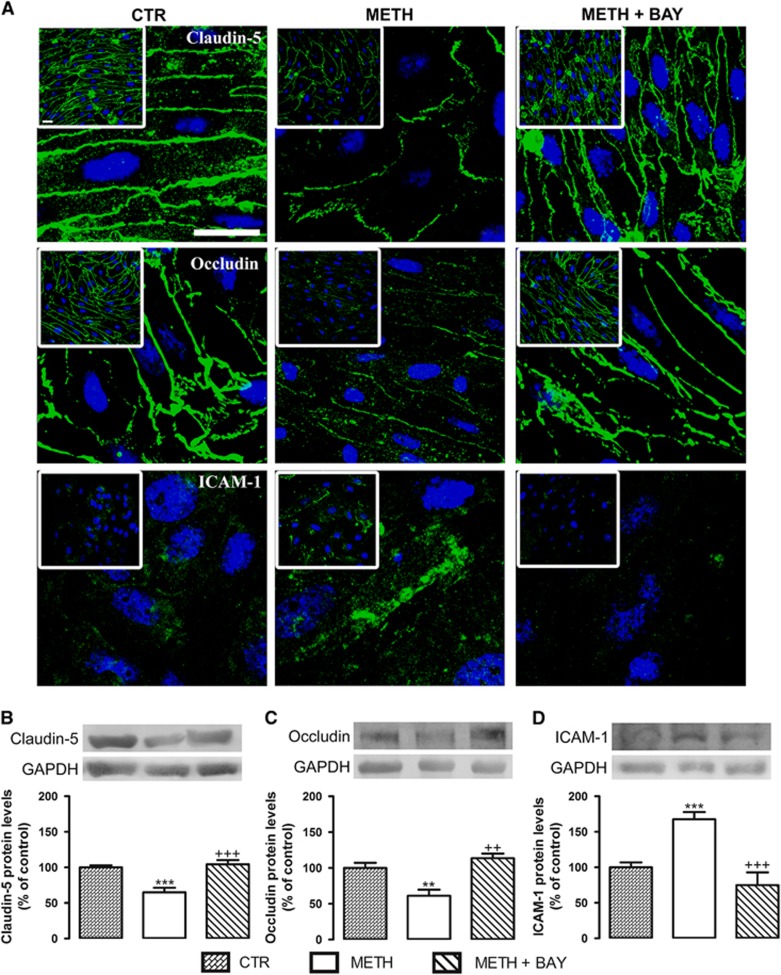
Our observations prove that just after METH exposure there is an increase of vesicular transport across RBMVECs and it is dependent on NF-κB activation. However, at 3 hours after drug incubation it was possible to observe a decrease in TEER values, pointing to a significant alteration in the paracellular pathway after this time period. Based on these conclusions, we further evaluated the expression of TJ proteins and adhesion molecules at 4 hours of drug exposure. Our results show that 1 μmol/L METH decreased claudin-5 (Figures 3A and 3B) and occludin (Figures 3A and 3C) expression, and upregulated ICAM-1 protein levels (Figures 3A and 3D). Then, to determine whether this effect of METH was also dependent on NF-κB activation, we once again blocked this pathway with BAY (5 μmol/L) showing that METH-induced alterations in TJ and adhesion proteins indeed involved the activation of NF-κB pathway.
Figure 3.
Methamphetamine (METH) decreases the expression of tight junction proteins, claudin-5 and occludin, and increases the expression of intercellular adhesion molecule 1 (ICAM-1) on rat brain microvascular endothelial cells. (A) Representative images of claudin-5, occludin, and ICAM-1 expression under different experimental conditions as follows: untreated (CTR); 1 μmol/L METH; METH+5 μmol/L BAY (4 hours of exposure). Claudin-5, occludin and ICAM-1 (all green), Hoechst 33342 (blue), and scale bar=20 μm. (B–D) Quantification of (B) claudin-5 (22 kDa), (C) occludin (65 kDa), and (D) ICAM-1 (110 kDa) protein levels under the same experimental conditions as previously specified in (A). Above the bars, representative western blot images of the different proteins, as well as the housekeeping gene (glyceraldehyde 3-phosphate dehydrogenase, GAPDH, 37 kDa), are shown. The results are expressed as mean % of the control+S.E.M., n=5 to 16 for claudin-5, n=5 to 18 for occludin, n=3 to 10 for ICAM-1. **P<0.01, ***P<0.001 significantly different when compared with the control (CTR); ++P<0.01, +++P<0.001 significantly different when compared with METH using Bonferroni's Multiple comparison test.
Taken together, these data prove that TNF-α, via activation of NF-κB signaling, is involved in METH-induced barrier impairment at both transcellular and junctional/paracellular pathways across ECs.
Role of Astrocytes on Methamphetamine-Induced Barrier Dysfunction
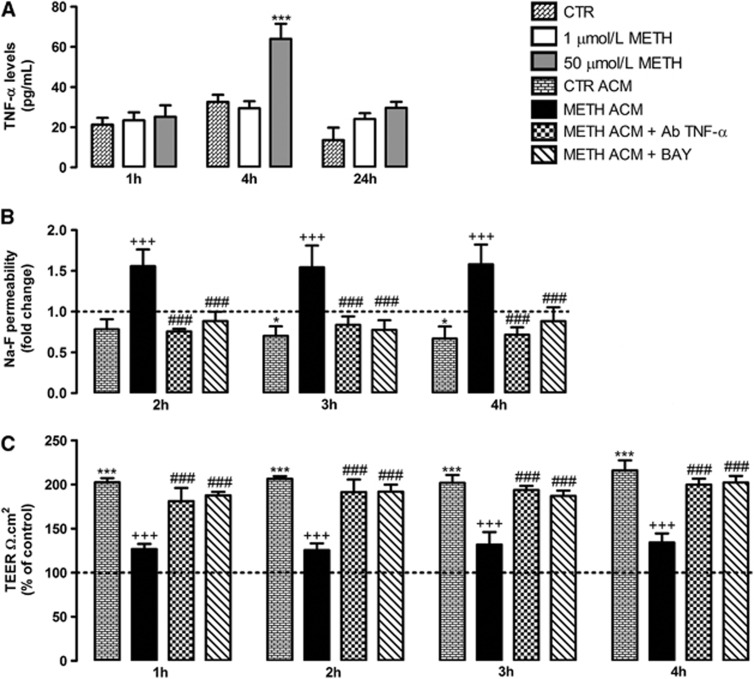
Several studies have suggested that astrocytes modulate the structure and function of cerebral endothelium.8 In fact, these cells release several factors that determine the development and maintenance of the BBB. Thus, we also aimed to clarify whether METH alters the influence of astrocytes on ECs. We first analyzed whether METH was also able to induce TNF-α release by astrocytes, and we observed an increase triggered by 50 μmol/L METH at 4 hours after exposure (Figure 4A). No significant changes were observed with a lower concentration of METH (1 μmol/L) or at other time points (1 and 24 hours; Figure 4A). Additionally, we always aim to use an in vitro model as close as possible to a human condition. Taking this into consideration, we evaluated the effect of ACM on ECs permeability and TEER. As shown in Figure 4, ACM obtained from control astrocytes (not exposed to drugs) decreased RBMVECs permeability (Figure 4B) and increased TEER (Figure 4C). This corroborates previous studies demonstrating that under normal conditions astrocytes have a beneficial role in barrier properties.8 However, when RBMVECs were exposed to ACM obtained from astrocytes exposed to 50 μmol/L METH during 4 hours (METH ACM), a significant increase in Na-F permeability (Figure 4B) and decrease in TEER (Figure 4C) were observed, when compared with CTR ACM. Importantly, from Figure 4A we know that METH ACM has increased levels of TNF-α. Thus, by using an antibody against mouse TNF-α (Ab TNF-α) we could completely prevent the increased permeability and decreased TEER induced by METH ACM at all time points analyzed (Figures 4B and 4C). Moreover, the inhibition of NF-κB pathway with BAY efficiently prevented the effects of METH ACM, similarly to the results obtained with Ab TNF-α (Figures 4B and 4C). To exclude the possibility that the remaining METH present in the ACM was responsible for the effects on RBMVECs permeability and TEER, METH concentration was determined by ELISA in METH ACM (Supplementary Figure 3). Though there are residual concentrations of both D,L-METH, the Ab TNF-α still completely prevented the BBB disruption induced by METH suggesting that TNF-α is indeed the responsible for such alterations.
Figure 4.
Tumor necrosis factor-alpha (TNF-α) released by astrocytes impairs the barrier properties of brain microvascular endothelial cells (BMVECs) via nuclear factor-kappa B (NF-κB) pathway. (A) Primary cultures of mouse cortical astrocytes were exposed to 1 or 50 μmol/L methamphetamine (METH) for 1, 4, and 24 hours. The results show that 50 μmol/L METH induces a significant increase in TNF-α levels at 4 hours. The TNF-α levels are expressed as mean pg/mL+S.E.M., n=4 to 10. (B) Macromolecular flux across primary rat BMVECs was assessed using 376 Da sodium fluorescein (Na-F) at different time points after exposure to untreated astrocyte-conditioned medium (CTR ACM), METH ACM, METH ACM+100 μg/mL Ab TNF-α, or METH ACM+5 μmol/L BAY, n=3 to 8. (C) Transendothelial electrical resistance (TEER) of confluent rat BMVEC monolayers was analyzed under the same conditions of (B), n=3 to 9. The results shown are means+S.E.M. *P<0.05, ***P<0.001 significantly different when compared with the control (CTR or dashed line); +++P<0.001 significantly different when compared with CTR ACM; ###P<0.001 significantly different when compared with METH ACM of each time point using Bonferroni's Multiple comparison test.
Overall, these results prove that METH also triggers the release of TNF-α by astrocytes that will negatively interfere with barrier function. Additionally, the activation of NF-κB signaling was once again shown to be involved in increased permeability.
Methamphetamine Induces in vivo Blood–Brain Barrier Permeability via Nuclear Factor-Kappa B Pathway
To support our in vitro results, we further used an animal model of METH-induced neurotoxicity.29 The binge administration protocol provides excellent relevance to intravenous and smoked routes of METH exposure in humans, and simulates the toxic effects of METH in nontolerant users.4 Moreover, it was recently shown that acute METH treatment causes BBB disruption.10 Herein, we used albumin staining as a marker for BBB disruption30 since this is a blood serum protein that does not cross the BBB under normal conditions. Moreover, collagen IV is one of the most expressed proteins in basal lamina and is responsible for the mechanical support of ECs.30 Thus, considering the crucial role of striatum in METH addiction and neurotoxicity,4 we further investigated the alterations in collagen IV expression and the presence of albumin in the brain parenchyma of this specific brain region. A time-course study was performed, and we concluded that METH decreased collagen IV staining just 1 hour after the last injection, being more pronounced at 2 hours, and still evident after 24 hours. These observations indicate an altered and weakened structure of microvessels (Figure 5A). Furthermore, albumin was present in the brain parenchyma after METH administration, being a clear indicator of BBB disruption. Since a huge pick of albumin immunoreactivity was observed at 2 hours after the last METH injection, the following studies were performed at this time point. Noteworthy, and in accordance with our in vitro results, the blockade of NF-κB pathway with BAY prevented the increased BBB permeability and structural alterations induced by METH (Figures 5B to 5D).
Figure 5.
Methamphetamine (METH) induces blood–brain barrier (BBB) disruption in the mice striatum via nuclear factor-kappa B (NF-κB) pathway. Mice were administered with METH (4 × 10 mg/kg, intraperitoneally (i.p.), 2 hours apart) and killed 1, 2, and 24 hours after the last injection. (A) Representative images of collagen IV (green), a marker of basement membrane that surrounds the brain vessels, and albumin (red) that is a marker of BBB disruption. It was clearly shown that METH triggered a decrease in collagen and an increase in albumin staining already 1 hour after the last METH injection, reaching a peak after 2 hours. Interestingly, the effect is still present after 24 hours of the last METH injection. (B) Representative images and (C, D) quantification of (C) albumin and (D) collagen IV immunofluorescence show that METH increases albumin extravasation and decreases collagen IV at 2 hours after the last injection, which was completely prevented by the blockade of NF-κB pathway with BAY treatment (20 mg/kg, i.p., 30 minutes before each METH injection). Total brain sections were also stained with Hoechst 33342 (blue). Scale bar=20 μm. The results shown are means+S.E.M., n=18 visual fields acquired from three different animals of each experimental condition. *P<0.05, **P<0.01, ***P<0.001 significantly different when compared with the control (CTR); +P<0.01, +++P<0.001 significantly different when compared with METH (2 hours) using Bonferroni's Multiple comparison test.
In an attempt to better clarify the effect of METH in animal BBB alterations, we also showed that this drug increases TNF-α levels in brain parenchyma (Figures 6A and 6B). Importantly, to exclude the contribution of peripheral TNF-α, we measured its levels in mouse sera and concluded that there were no alterations (Supplementary Figure 4). This suggests that increased cerebrovascular permeability was not related with peripheral TNF-α production, but instead to its production in the brain (Figures 6A and 6B), particularly around the perivascular zone. We also evaluated astrocyte activity through GFAP immunofluorescence but in general no significant alterations were found (Figure 6C). However, it is possible to observe a specific increase surrounding microvessels, which suggests an astrocytic recruitment and reorganization (Figure 6A). These observations lead us to hypothesize that METH may induce a perivascular astrogliosis31 inflammatory status. In fact, the presence of TNF-α near both the astrocytic endfeet and capillary is notorious (Figure 6A) and corroborates our in vitro results showing that both cells can produce TNF-α. Also, this cytokine's production was abrogated by the treatment with BAY, demonstrating the involvement of NF-κB pathway in TNF-α production after METH binge administration.
Figure 6.
Methamphetamine (METH) upregulates tumor necrosis factor-alpha (TNF-α) levels in the mouse striatum. Mice were administered with METH (4 × 10 mg/kg, intraperitoneally (i.p.), 2 hours apart) and killed 2 hours after the last injection. (A) Representative images of TNF-α and glial fibrillary acidic protein (GFAP) immunoreactivity showing a colocalization of the cytokine with astrocytes and vessels. (A, B) Quantification of both proteins shows a significant increase in (B) TNF-α levels that was prevented by the blockade of nuclear factor-kappa B (NF-κB) pathway with BAY, but (C) no alteration in general GFAP levels. Total brain sections were also stained with Hoechst 33342 (blue). Scale bar=20 μm. The results shown are means+S.E.M, n=18 visual fields acquired from three different animals of each experimental condition. ***P<0.001 significantly different when compared with the control (CTR); +++P<0.001 significantly different when compared with METH using Bonferroni's Multiple comparison test.
Discussion
Methamphetamine is a powerful psychostimulant drug of abuse that causes severe alterations in the CNS. In fact, the neurotoxicity triggered by this drug has been extensively studied over the last years.3 Though its impact on BBB properties was highlighted more recently,9 the intracellular mechanisms involved in METH-induced cerebral endothelium dysfunction remain unknown, as well as its effect on the crosstalk between different neurovascular unit cells. Thus, to reach our goal we first took advantage of a well-established BBB in vitro model,19 and we observed that METH increased ECs permeability at concentrations relevant to human abuse. Considering the differences between rodents and humans, we further used human ECs20 to reproduce the results obtained with rat primary cultures. As it is also known that METH can induce death of various cell types4, 12 including ECs,21 we also considered if METH-increased endothelial permeability was due to cell death. Importantly, the concentrations used in the present study did not cause EC death or decreased cell viability. This is a key issue because, in contrast to other studies, we used METH concentrations that present a most common pattern of drug use, highlighting the importance of understanding its impact on BBB function.
Multiple studies suggest that METH triggers a pronounced neuroinflammatory response,2 usually characterized by gliosis and increased levels of cytokines.11, 12 Importantly, ECs can actively participate in inflammatory events, being also an important source of pro-inflammatory cytokines.13 Since we have previously shown that TNF-α has an important role in METH-induced toxicity,11, 12 we hypothesized that this pro-inflammatory cytokine could also be involved in endothelial dysfunction induced by METH. Indeed, we proved that both brain ECs and astrocytes are an important source of TNF-α, but both cell types also showed a distinct response to METH. It is well known that METH induces different responses according to cell type, and indeed a lower concentration of the drug triggered a faster and higher release of TNF-α by ECs, when compared with astrocytes. Curiously, in ECs we also observed a fast release of TNF-α within 15 minutes after METH exposure that is independent of NF-κB activation, since BAY did not prevent its release. Instead, it seems that this first pool of TNF-α will trigger the p65 translocation into the nucleus, and so being itself a stimulator of subsequent cytokine synthesis. It is known that the brain endothelium is the first to interact with peripheral pathogens or harmful signals32, and indeed ECs contain intracellular granules called Weibel-Palade bodies that store a number of chemokines, adhesive molecules, and inflammatory cytokines.33 These may undergo exocytosis into the extracellular space within minutes of stimulation and thus allowing an immediate response of ECs33 that is independent of gene transcription.34
Additionally, we clearly showed that the inhibition of TNF-α completely prevented the increased permeability induced by METH. Accordingly, this cytokine has been shown to participate in BBB breakdown in pathologic phenomena, such as stroke and traumatic brain injury.35 Namely, rat intracerebral injection of TNF-α increased BBB permeability.17 Similar results were observed with a short acute incubation of this pro-inflammatory cytokine in a BBB in vitro model of bovine cerebral ECs and rat glial cell co-cultures.16 Moreover, to unravel the intracellular signaling that culminates in BBB impairment, we targeted the NF-κB pathway. Nuclear factor-kappa B is a potent pro-inflammatory nuclear transcription factor that is involved in the initiation and amplification of inflammatory responses. Additionally, TNF-α triggers signaling pathways that converge on the activation of transcription factor NF-κB.18, 24, 26 More recently, others have showed that TNF-α activates NF-κB in both human umbilical vein ECs26 and dermal microvascular ECs.36 Thus, NF-κB is a transcription factor that regulates the pro-inflammatory responses in ECs, including ZO-1 expression as shown in lupus condition.37 Also, Koedel et al27 showed that NF-κB inhibition had a protective effect on meningitis-associated BBB leakage. Accordingly, in the present study we concluded that TNF-α/NF-κB blockade prevented METH-induced EC dysfunction.
Knowing that increased BBB permeability under several insult conditions can occur through different mechanisms,2, 7 we further aimed to clarify what type of transport across ECs was being modulated by this TNF-α/NF-κB pathway. In fact, it was previously shown that METH increased both paracellular5, 9 and vesicular transport.21 Noteworthy, in healthy BBB endothelium nonspecific fluid-phase transcytosis is rarely observed.6 However, under several types of brain injuries the vesicular transport is increased, including in traumatic human brain edema.38 Accordingly, in this study we clearly show that just after METH exposure the transcellular permeability mediated by NF-κB is increased. Also, Tiruppathi et al39 showed that NF-κB signaling was involved in LPS-increased caveolae-mediated transendothelial albumin permeability. Besides this transcellular permeability, METH also interfered with the paracellular pathway. In fact, there was a significant decrease in the expression of occludin and claudin-5, which was prevented by NF-κB pathway blockade, similarly to the vesicular transport. Accordingly, Aveleira et al24 showed that, in primary cultures of bovine retinal ECs, TNF-α induced BBB impairment via NF-κB signaling, which was associated with decreased levels and changes in the cellular distribution of claudin-5 and ZO-1. Moreover, TNF-α/NF-κB pathway was shown to be responsible for ZO-1 downregulation and alteration in junctional localization that led to increased permeability of Caco-2 cells.40 Additionally, TNF-α was shown to induce NF-κB signaling in mouse brain ECs, and p65 overexpression repressed the claudin-5 promoter.25 Importantly, NF-κB also affects the dynamic of actin cytoskeletal assembly that is crucial for the stabilization of TJ.13 All these studies emphasize the crucial role of the TNF-α/NF-κB signaling on BBB modulation, but its link with METH use has never been identified until now.
Alterations in TJ structure and organization may also affect BBB permeability, and subsequently the movement of leukocytes and immune mediators into the brain. In fact, our group has recently showed an enhancement of lymphocyte transendothelial migration after METH.21 Thus, we hypothesized that it could also upregulate the expression of cell adhesion molecules in ECs, justifying the infiltration of leukocytes. In fact, METH increased ICAM-1 expression, which was once again prevented by the blockade of NF-κB pathway. Importantly, it was previously showed that increased levels of ICAM-1 induce ECs leakiness through alteration of cell junctions and cytoskeleton.36 Moreover, using the same concentration of TNF-α that we used, the activation of NF-κB was proven to be essential for ICAM-1 expression and consequent increase of neutrophils adhesion onto ECs.18 Herein, we clearly show that METH has a direct effect on ECs leading to significant alterations in both vesicular and paracellular transports through TNF-α/NF-κB signaling pathway.
Despite our important findings regarding the impact of METH on ECs, BBB is a complex structure. Indeed, astrocytes have a crucial role in modulating the structure and function of the cerebral endothelium, but the effect of METH on the crosstalk between brain ECs and astrocytes has never been addressed before. Kuo and Lu8 showed that ACM from human astrocytes increased TEER of HBMVECs, together with a reduction of paracellular permeability. Also, ACM induced brain endothelium properties on human umbilical vein ECs, with decreased permeability to HRP and increased expression of occludin and ZO-1.14 Accordingly, we conclude that under control conditions astrocytes release several factors that contribute to the tightness of the barrier. However, under toxic conditions astrocytes can have a negative effect on the surrounding cells, including on ECs. Specifically, Yang et al41 showed that ACM obtained from a rat cell line incubated 24 hours with bradykinin increased neuronal cell death due to reactive oxygen species, MMP-9, and heme oxygenase-1/carbon monoxide present in the medium. Also, factors released by LPS- and/or Shiga toxin 1-treated astrocytes increased transendothelial permeability in human umbilical vein ECs by reducing TJ proteins expression.14 Importantly, these authors also showed that NF-κB inhibition or TNF-α blockade inhibited the effects of ACM.14 Thus, considering that METH increases astrocytic reactivity as well as the expression of several cytokines in the mouse brain, including TNF-α,11 we hypothesize that METH could also induce endothelium dysfunction via TNF-α released by astrocytes. Indeed, we proved that barrier dysfunction could involve not only endothelial but also astrocytic TNF-α, that again culminated in increased endothelial permeability through the activation of NF-κB pathway.
To better support our in vitro results, we also investigated if METH binge administration to mice could cause BBB dysfunction. Importantly, this drug administration paradigm confers excellent significance to intravenous and smoked routes of METH exposure in humans,29 and the dose is similar to that detected in the blood of drug abusers.9, 23 Additionally, this binge protocol has been widely used in animal studies and is well characterized by striatal toxicity.42 However, little is known about the effect of this METH regimen on BBB function. Nevertheless, Urrutia et al10 showed that a similar METH binge protocol (3 × 4 mg/kg) induced BBB disruption in mouse striatum at 1, 12, and 24 hours after METH treatment, observed by extravasation of Immunoglobulin G (150 kDa). Herein, we showed that METH increased BBB permeability at 1, 2, and 24 hours after the last injection, which was evaluated by increased albumin (66 kDa) leakage and decreased expression of collagen IV. Collagen IV is the major component of the basement membrane having a decisive role in the maintenance the vessels' wall structural integrity. In fact, downregulation of this protein has been associated with pathogenesis of BBB destruction in autoimmune Encephalomyelitis43 and ischemic stroke.44 Other interesting aspect is that MMPs, particularly MMP-9, have been implicated in collagen IV degradation.43, 44 Indeed, we previously showed that METH increases the expression and activity of MMP-9, being responsible for BBB breakdown.5 Moreover, we also observed an increase of TNF-α confined to the perivascular zone, near ECs and astrocytes. Additionally, both BBB breakdown and TNF-α production were prevented by BAY, showing the involvement of NF-κB signaling in METH-induced BBB disruption. Interestingly, with our pattern of drug administration we did not detect an increase in general GFAP immunoreactivity, which is in agreement with a previous study that showed no alterations of several proteins, including GFAP, in striatal homogenates of autopsied brains obtained from chronic METH users.45 Nevertheless, it was possible to observe a specific increase of GFAP immunoreactivity around the microvessels, which indicates an astrocytic recruitment and reorganization around the capillaries highlighting a clear perivascular astrogliosis31 triggered by METH.
In conclusion, the present work shows that at concentrations relevant to human abuse, METH induces the release of TNF-α by both brain ECs and astrocytes with subsequent activation of the NF-κB pathway. This sequence of events culminates in the increase of transcellular and paracellular endothelial transport. Importantly, the blockade of TNF-α or NF-κB was able to prevent METH-induced BBB dysfunction. Moreover, these results were reproduced using rat and human cerebral ECs, as well as an animal model. So, for the first time we show that TNF-α/NF-κB signaling pathway has a central role in METH-induced brain endothelium permeability. This observation may provide an important strategy against BBB dysfunction triggered by METH and consequent brain parenchyma alterations/infections that may occur under such conditions.
Acknowledgments
We would like to acknowledge Madalena Esteves for her technical support in western blot and immunohistochemistry studies, and to Dr Flávio Reis for the technical support in mice blood collection. This work was supported by Project PTDC/SAU-FCF/098685/2008 from Foundation for Science and Technology (FCT Portugal) co-financed by COMPETE and FEDER funds, and Pest-C/SAU/UI3282/2013-2014. Also, PhD fellowships SFRH/BD/84408/2012 and SFRH/BD/85556/2012 from FCT Portugal co-financed by QREN.
Author Contributions
APS conceived the study, and together with VCS performed the animal manipulation. VCS and RAL did the experiments. FLC, IP, and MAB collaborated in the implementation of blood–brain barrier in vitro models. MR and MB provided the samples of human brain tissue. VCS, RAL, and APS analyzed the data and drafted the manuscript. CFR gave important inputs during the execution of the work. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- UNODC . World Drug Report Volume 1 Analysis. United Nations Office on Drugs and Crime: Vienna; 2007. [Google Scholar]
- Loftis JM, Janowsky A. Neuroimmune basis of methamphetamine toxicity. Int Rev Neurobiol. 2014;118:165–197. doi: 10.1016/B978-0-12-801284-0.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins T, Baptista S, Gonçalves J, Leal E, Milhazes N, Borges F, et al. Methamphetamine transiently increases the blood-brain barrier permeability in the hippocampus: role of tight junction proteins and matrix metalloproteinase-9. Brain Res. 2011;1411:28–40. doi: 10.1016/j.brainres.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Kooij G, Frenkel D, Georgopoulos S, Monsonego A, Janigro D. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia. 2012;53:45–52. doi: 10.1111/j.1528-1167.2012.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YC, Lu CH. Effect of human astrocytes on the characteristics of human brain-microvascular endothelial cells in the blood-brain barrier. Colloids Surf B Biointerfaces. 2011;86:225–231. doi: 10.1016/j.colsurfb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, Reichenbach N, et al. Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:1933–1945. doi: 10.1038/jcbfm.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia A, Rubio-Araiz A, Gutierrez-Lopez MD, ElAli A, Hermann DM, O'Shea E, et al. A study on the effect of JNK inhibitor, SP600125, on the disruption of blood-brain barrier induced by methamphetamine. Neurobiol Dis. 2013;50:49–58. doi: 10.1016/j.nbd.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Gonçalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, et al. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur J Neurosci. 2010;31:315–326. doi: 10.1111/j.1460-9568.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- Coelho-Santos V, Gonçalves J, Fontes-Ribeiro C, Silva AP. Prevention of methamphetamine-induced microglial cell death by TNF-alpha and IL-6 through activation of the JAK-STAT pathway. J Neuroinflammation. 2012;9:103. doi: 10.1186/1742-2094-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Hennig B, Yao J, Toborek M. Methamphetamine induces AP-1 and NF-kappaB binding and transactivation in human brain endothelial cells. J Neurosci Res. 2001;66:583–591. doi: 10.1002/jnr.1248. [DOI] [PubMed] [Google Scholar]
- Landoni VI, Schierloh P, de Campos Nebel M, Fernandez GC, Calatayud C, Lapponi MJ, et al. Shiga toxin 1 induces on lipopolysaccharide-treated astrocytes the release of tumor necrosis factor-alpha that alter brain-like endothelium integrity. PLoS Pathog. 2012;8:e1002632. doi: 10.1371/journal.ppat.1002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli MA, Descamps L, Dehouck MP, Cecchelli R, Joo F, Abraham CS, et al. Exposure of tumor necrosis factor-alpha to luminal membrane of bovine brain capillary endothelial cells cocultured with astrocytes induces a delayed increase of permeability and cytoplasmic stress fiber formation of actin. J Neurosci Res. 1995;41:717–726. doi: 10.1002/jnr.490410602. [DOI] [PubMed] [Google Scholar]
- Miller F, Fenart L, Landry V, Coisne C, Cecchelli R, Dehouck MP, et al. The MAP kinase pathway mediates transcytosis induced by TNF-alpha in an in vitro blood-brain barrier model. Eur J Neurosci. 2005;22:835–844. doi: 10.1111/j.1460-9568.2005.04273.x. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, et al. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther. 2007;323:488–498. doi: 10.1124/jpet.107.127035. [DOI] [PubMed] [Google Scholar]
- Tang C, Xue HL, Bai CL, Fu R. Regulation of adhesion molecules expression in TNF-alpha-stimulated brain microvascular endothelial cells by tanshinone IIA: involvement of NF-kappaB and ROS generation. Phytother Res. 2011;25:376–380. doi: 10.1002/ptr.3278. [DOI] [PubMed] [Google Scholar]
- Cardoso FL, Kittel A, Veszelka S, Palmela I, Toth A, Brites D, et al. Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS ONE. 2012;7:e35919. doi: 10.1371/journal.pone.0035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernas MJ, Cardoso FL, Daley SK, Weinand ME, Campos AR, Ferreira AJ, et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat Protoc. 2010;5:1265–1272. doi: 10.1038/nprot.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins T, Burgoyne T, Kenny BA, Hudson N, Futter CE, Ambrosio AF, et al. Methamphetamine-induced nitric oxide promotes vesicular transport in blood-brain barrier endothelial cells. Neuropharmacology. 2013;65:74–82. doi: 10.1016/j.neuropharm.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Kim SR, Park SJ, Min KH, Lee KY, Jin SM, et al. Antioxidant down-regulates interleukin-18 expression in asthma. Mol Pharmacol. 2006;70:1184–1193. doi: 10.1124/mol.106.024737. [DOI] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Ahmad N, Srivastava R, Hemmer B. TNF-alpha induced NFkappaB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine. 2012;57:269–275. doi: 10.1016/j.cyto.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Zhong X, Li X, Liu F, Tan H, Shang D. Omentin inhibits TNF-alpha-induced expression of adhesion molecules in endothelial cells via ERK/NF-kappaB pathway. Biochem Biophys Res Commun. 2012;425:401–406. doi: 10.1016/j.bbrc.2012.07.110. [DOI] [PubMed] [Google Scholar]
- Koedel U, Bayerlein I, Paul R, Sporer B, Pfister HW. Pharmacologic interference with NF-kappaB activation attenuates central nervous system complications in experimental Pneumococcal meningitis. J Infect Dis. 2000;182:1437–1445. doi: 10.1086/315877. [DOI] [PubMed] [Google Scholar]
- Prager GW, Mihaly J, Brunner PM, Koshelnick Y, Hoyer-Hansen G, Binder BR. Urokinase mediates endothelial cell survival via induction of the X-linked inhibitor of apoptosis protein. Blood. 2009;113:1383–1390. doi: 10.1182/blood-2008-06-164210. [DOI] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, et al. Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicol Teratol. 2010;32:346–355. doi: 10.1016/j.ntt.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler K, Trinkl A, Klopotowski M, Thal SC, Plesnila N, Trabold R, et al. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Saint-Laurent O, Godschalk A, Terouz S, Briels C, Larouche S, et al. Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis. 2015;74:14–24. doi: 10.1016/j.nbd.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells—conditional innate immune cells. J Hematol Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Park JY, Jou I, Park SM. Regulation of Weibel-Palade body exocytosis by alpha-synuclein in endothelial cells. J Biol Chem. 2010;285:21416–21425. doi: 10.1074/jbc.M110.103499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–308. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Tobinick E, Kim NM, Reyzin G, Rodriguez-Romanacce H, DePuy V. Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS drugs. 2012;26:1051–1070. doi: 10.1007/s40263-012-0013-2. [DOI] [PubMed] [Google Scholar]
- Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol. 2007;127:762–774. doi: 10.1038/sj.jid.5700670. [DOI] [PubMed] [Google Scholar]
- Jacob A, Hack B, Chen P, Quigg RJ, Alexander JJ. C5a/CD88 signaling alters blood-brain barrier integrity in lupus through nuclear factor-kappaB. J Neurochem. 2011;119:1041–1051. doi: 10.1111/j.1471-4159.2011.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castejon OJ. Increased vesicular and vacuolar transendothelial transport in traumatic human brain oedema. A review. Folia Neuropathol. 2013;51:93–102. doi: 10.5114/fn.2013.35951. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Shimizu J, Miyawaki-Shimizu K, Vogel SM, Bair AM, Minshall RD, et al. Role of NF-kappaB-dependent caveolin-1 expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J Biol Chem. 2008;283:4210–4218. doi: 10.1074/jbc.M703153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Yang CM, Hsieh HL, Lin CC, Shih RH, Chi PL, Cheng SE, et al. Multiple factors from bradykinin-challenged astrocytes contribute to the neuronal apoptosis: involvement of astroglial ROS, MMP-9, and HO-1/CO system. Mol Neurobiol. 2013;47:1020–1033. doi: 10.1007/s12035-013-8402-1. [DOI] [PubMed] [Google Scholar]
- Beauvais G, Atwell K, Jayanthi S, Ladenheim B, Cadet JL. Involvement of dopamine receptors in binge methamphetamine-induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS ONE. 2011;6:e28946. doi: 10.1371/journal.pone.0028946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kan QC, Xu Y, Zhang GX, Zhu L. Inhibitory effect of matrine on blood-brain barrier disruption for the treatment of experimental autoimmune encephalomyelitis. Mediators Inflamm. 2013;2013:736085. doi: 10.1155/2013/736085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- Tong J, Fitzmaurice P, Furukawa Y, Schmunk GA, Wickham DJ, Ang LC, et al. Is brain gliosis a characteristic of chronic methamphetamine use in the human. Neurobiol Dis. 2014;67:107–118. doi: 10.1016/j.nbd.2014.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.