Abstract
Background
Accumulation of advanced glycation end products (AGEs) is thought to contribute to limited joint mobility in people with diabetes mellitus (DM), but the relationships among AGEs, shoulder structural changes, movement, and disability are not understood.
Objective
The purpose of this study was to determine the differences and relationships among skin intrinsic fluorescence (SIF), a proxy measure of AGEs, biceps and supraspinatus tendon thickness, upper extremity movement, and disability in groups with and without DM.
Design
This was a cross-sectional, case-control study.
Methods
Fifty-two individuals participated: 26 with type 2 DM and 26 controls matched for sex, age, and body mass index. The main outcome measures were: SIF; biceps and supraspinatus tendon thickness; 3-dimensional peak shoulder motion; and Disability of the Arm, Shoulder and Hand (DASH) questionnaire scores.
Results
Mean SIF measurements were 19% higher in the DM group compared with the control group (P<.05). Biceps tendons (mean and 95% confidence interval [CI]) (4.7 mm [4.4, 5.0] versus 3.2 mm [2.9, 3.5]) and supraspinatus tendons (6.4 mm [5.9, 6.8] versus 4.9 mm [4.4, 5.3]) were thicker and peak humerothoracic elevation (139° [135°, 146°] versus 150° [146°, 155°]) and glenohumeral external rotation (35° [26°, 46°] versus 51° [41°, 58°]) were reduced in the DM group compared with the control group (P<.05). In the DM group, SIF was correlated to biceps tendon thickness, DASH score, and shoulder motion (r=.44–.51, P<.05). The SIF score and shoulder strength explained 64% of the DASH scores (P<.01).
Limitations
Because this was a cross-sectional study design, a cause-effect relationship could not be established.
Conclusions
Accumulation of AGEs in the connective tissues of individuals with DM appears to be associated with increased tendon thickness and decreased shoulder joint mobility and upper extremity function. Physical therapists should be aware of these possible metabolic effects on structure, movement, and disability when treating people with diabetes.
Upper extremity musculoskeletal complications occur more frequently in people with diabetes mellitus (DM) compared with those without DM.1–4 Larkin et al,5 in a recent prospective study, reported that 66% of their 1,217 participants with type 1 diabetes had cheiroarthropathy, defined as the presence of any one of the following: adhesive capsulitis, carpal tunnel syndrome, flexor tenosynovitis, Dupuytren contracture, or a positive prayer sign. Prevalence of shoulder impairments is reported to be about 11% to 50% in people with DM compared with 2% to 20% in those without DM.6–9 Limited joint mobility (LJM) is a systemic problem documented in the hands, shoulders, ankles, and feet of people with DM.4,10–15 In its beginning stage, LJM at the shoulders and hands can be painless and go unnoticed but may be a precursor to the severe upper extremity impairments associated with pain and disability.13–15
The excessive accumulation of advanced glycation end products (AGEs) in the connective tissues is thought to contribute to LJM and the high incidence of musculoskeletal problems seen in DM.14 Advanced glycation end products accumulate in all people and are formed by oxidative stress and the nonenzymatic condensation of glucose and proteins.16–18 This process is greatly accelerated, however, in the presence of high levels of glucose, as seen in people with DM. Excessive levels of AGEs lead to pathological collagen cross-linking and structural changes in the tissues. Of particular interest is the excessive cross-linking in the collagen-rich musculoskeletal tissues such as tendons, ligaments, skin, and muscle.18–20 Structural changes that occur in the shoulders of individuals with diabetes as a result of excessive levels of AGEs include increased thickness in the biceps and supraspinatus tendons.14,21,22 Additionally, fibrous contractures and dense collagen matrix have been observed in the shoulder joint capsule, with adherence to the head of the humerus, rotator interval area, and coracohumeral ligament.23,24 We hypothesize that these excessive AGEs and structural changes lead to upper extremity movement impairments, pain, and disability (Fig. 1).
Figure 1.

Theoretical framework for upper extremity (UE) impairments.
The accumulation of AGEs can be estimated noninvasively, in vivo using skin intrinsic fluorescence (SIF). This method uses near-ultraviolet and blue light to excite and measure the fluorescence produced by AGEs.25–27 Estimating AGEs levels in the skin is particularly beneficial because AGEs that accumulate in the skin have an estimated half-life of 15 to 20 years and, therefore, are a good indicator of long-term glycemic exposure of collagen-rich tissues.28,29 Previous research has shown that this SIF measure is correlated to the severity of complications related to tissue-specific diabetes such as peripheral neuropathy (seen mainly in the distal lower extremities), increased arterial stiffness, and nephropathy.25,30,31 A 2014 study by Larkin et al5 showed differences in skin fluorescence, glycated hemoglobin, and functional disability between groups with and without upper extremity impairments in a sample of people with type 1 DM. However, the complete set of relationships outlined in Figure 1 among AGEs, shoulder structural changes, 3-dimensional joint motion, and upper extremity pain and disability needs to be evaluated, especially in people with type 2 DM. Such investigation should provide insight into the mechanism and management (ie, prevention or rehabilitation) of these upper extremity impairments in individuals with DM.
The purpose of this study was to determine group differences (DM versus control) and relationships among SIF (an indicator of AGEs accumulation in the skin), structural changes, and upper extremity movement impairments and disability in the group with DM (Fig. 1). We hypothesized that: (1) the SIF measure would be higher, the biceps and supraspinatus tendons would be thicker, and upper extremity movement would be reduced in the group with DM compared with the control group; (2) the SIF measure would be positively correlated to the tendon thickness and upper extremity disability and negatively correlated to shoulder movement; and (3) a significant amount of the variance of the upper extremity disability would be explained by SIF, biceps tendon thickness, movement impairments, and shoulder strength.
Method
Participants
We recruited a total of 52 participants: 26 participants with type 2 diabetes and 26 controls matched for age, body mass index (BMI), and sex who did not have diabetes (self-reported) or shoulder pain. The participants' demographic information is shown in Table 1. Both groups were recruited from the outpatient diabetes center of a large medical hospital and the Volunteers for Health at Washington University School of Medicine, St Louis, Missouri. All participants signed an institution-approved consent form prior to the clinical examination.
Table 1.
Demographic Informationa
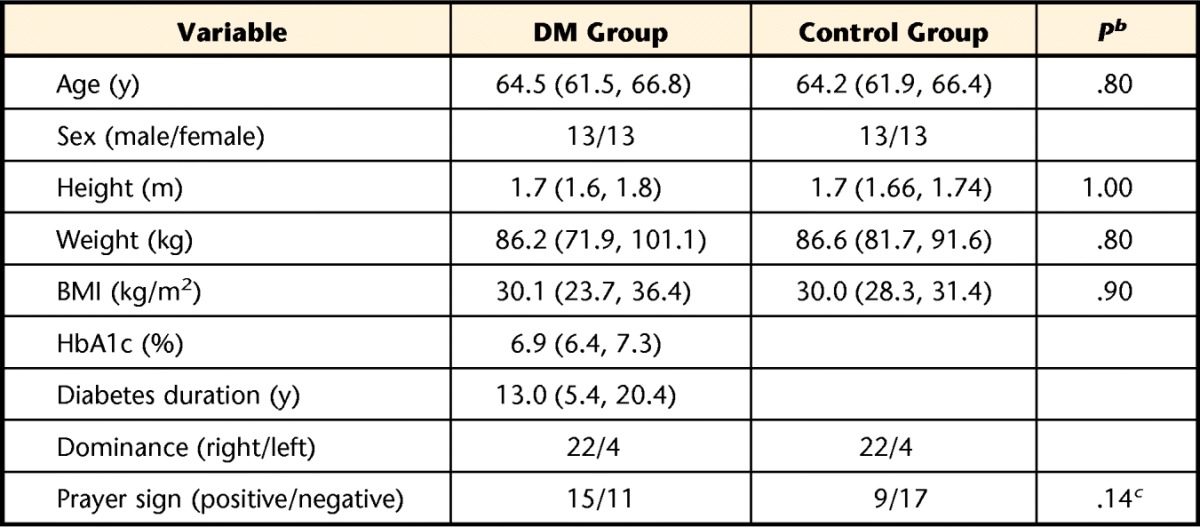
All data presented as mean (95% confidence interval) or number of participants. DM group=participants with diabetes mellitus, control group=participants without diabetes mellitus, BMI=body mass index, HbA1c=glycated hemoglobin.
b Significance determined using independent-sample Student t tests.
c Significance determined using chi-square analysis.
The intent of recruitment to meet the aims of this study was to include individuals without acute or severe shoulder problems who were attending an outpatient diabetes clinic and at high risk for developing shoulder LJM and subsequent shoulder impairments and compare them with a control group without DM or shoulder pain. Characteristics associated with systemic LJM include duration of diabetes5–8,32 and a positive prayer sign (ie, an inability to fully extend the interphalangeal joints of the hands when pressed together).32 Therefore, inclusion criteria for the group with DM were: duration of diagnosed diabetes of more than 10 years or a positive prayer sign and age between 40 and 70 years. We wanted to include the insidious development of shoulder impairments; therefore, we did not exclude individuals in the group with DM based solely on their pain levels. To eliminate other potential confounders, participants in the control group were matched for age, BMI, and sex. We also matched groups for side of hand dominance, as it may affect range of motion (ROM), strength, and possibly tendon size and would allow us to make appropriate comparisons between the DM and control groups.
In both groups, individuals were excluded if they had acute or severe shoulder problems, including a history of or current adhesive capsulitis, rotator cuff tears, recent upper extremity injury or fractures, or surgery in the upper extremity or thorax; neck pain; stroke with residual upper extremity involvement; rheumatic conditions; hypothyroid malfunctions; angina or other symptoms of myocardial ischemia; severe skin allergies in the area to be tested; known or at risk for photosensitivity reactions; or known connective tissue diseases. In addition, participants with a BMI greater than 35 kg/m2 were excluded, as the kinematic measurement errors are known to be large in people with high BMI.33 Control participants were screened by phone for shoulder pain because we wanted the group with DM to be compared with a control group without any shoulder impairments. All measurements were made by a single, trained physical therapist during a single session on the right arm using the following standardized methods for all participants.
Measurements
Skin intrinsic fluorescence.
Skin intrinsic fluorescence was measured as an indicator of AGEs (Fig. 1). Duplicate measurements of SIF were obtained from the skin on the underside of the left forearm using a SCOUT skin fluorescence spectrometer (VeraLight, Albuquerque, New Mexico). The SIF was excited with a light-emitting diode (LED) centered at 435 nm and was detected over the emission range of 470 to 655 nm. Skin reflectance was assessed with a white LED over the 435- to 655-nm spectral region. The measured skin reflectance was used to compensate for absorbance due to melanin and hemoglobin as well as individual-specific light scattering using the intrinsic fluorescence correction5 formula expressed in the equation:
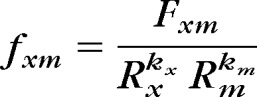 |
where the measured fluorescence (Fxm) is divided by reflectance values from the excitation and white LEDs (Rx and Rm, respectively). The reflectance values are adjusted by the dimensionless exponents, kx and km. For these analyses, the 435-nm excited fluorescence was utilized with kx set to 0.4 and km set to 0.9. The resulting intrinsic fluorescence (fxm) was integrated over the 470- to 655-nm spectral region and multiplied by 1,000 to represent SIF, reported in arbitrary units. The intraindividual skin variation in SIF assessed by the SCOUT has previously been documented in 1,185 participants with type 1 diabetes and was 4.2%.27
Tendon thickness.
Tendon thickness was measured as an indicator of structural changes occurring in the shoulder secondary to the accumulation of AGEs (Fig. 1). Ultrasound examination (Acuson XP 128/10, Siemens Medical Solutions Inc, Mountain View, California) for tendon thickness of the long head of the biceps and supraspinatus tendons was performed using a high-resolution, multifrequency (7–10 MHz) linear transducer by a single examiner. Images of the transverse view and longitudinal view were obtained for the biceps and supraspinatus tendons, respectively, as described previously.21,22 The tendon thickness was measured using the ImageJ (version 1.45s, National Institutes of Health, Bethesda, Maryland) computerized image analysis program. The maximum thickness of the biceps tendon in the transverse view was measured within the bicipital groove of the humerus. In the longitudinal view, the maximum supraspinatus tendon thickness was measured just in front of the lateral part of the humeral head, close to its insertion (anatomical neck) on the lesser tubercle. We took an additional measurement at the midpoint of the anatomical footprint (greater tubercle of the humerus) of the supraspinatus tendon to account for differences in the anatomy of the tendon among individuals. The longitudinal thickness was an average of these 2 measurements. The average of 3 thickness measurements for each tendon was used for data analyses.
Upper extremity movement.
Three-dimensional humerothoracic (humerus relative to thorax) and glenohumeral (humerus relative to scapula) joint motion was measured using the Flock of Birds electromagnetic tracking device (Ascension Technology Inc, Burlington, Vermont) and Motion Monitor software (The Motion Monitor, Innovative Sports Training Inc, Chicago, Illinois). The humerus sensor was attached to a thermoplastic cuff to reduce rotation errors and attached to the humerus using tapes. Standard methods were used to build the anatomic segments and define the motion.34,35 Measurements for 3 trials were collected during full, pain-free active ROM during scapular-plane elevation, defined as elevation in a plane 40 degrees anterior to the frontal plane. Using the same methods, the difference between the surface marker and a bone pin marker for humerus motion was 0 to 4 degrees for elevation angle and 1.7 to 2.3 degrees for axial rotation movements.33 The angles extracted for this study were the peak humerothoracic elevation and peak glenohumeral external rotation.
Shoulder flexor muscle strength.
The isometric strength of the shoulder flexor muscles was measured using a handheld, digital strain-gauge dynamometer (Microfet, Hoggan Health Industries Inc, West Jordan, Utah). Each participant was in the supine position, and standard stabilization and test positions were used.36 An average of 2 trials was used for the data analysis.
Measure of upper extremity disability.
We measured upper extremity disability using the Disability of the Arm, Shoulder and Hand (DASH) self-report questionnaire,37 which has been used previously in the diabetes population and has excellent reliability.4,7 The DASH has 30 questions, including questions on disability as well as pain. The scores were calculated for a range between 0% and 100%, where a higher number indicates more impairments.
Statistical Analyses
Study size was based on our preliminary studies of goniometric shoulder ROM differences (effect sizes=1.21–1.22)38 and previously published supraspinatus and biceps tendon thickness differences between groups with and without DM (effect size=1.8 and 1.31, respectively).21 We conservatively estimated that 26 participants per group would be required to see differences in primary variables between groups and allow multiple regression analysis in the group with DM (statistical power level=0.8, alpha=.05).
Statistical analyses of the data were performed using IBM SPSS Statistics for Windows version 22.0 (IBM Corp, Armonk, New York). Means, standard deviations, and percent changes were used to describe the variables. Differences in the demographic variables were analyzed using independent-sample Student t tests (continuous variables) and chi-square analysis (categorical variable: hand LJM, as examined by a positive prayer sign). The mean peak humerothoracic elevation and peak glenohumeral external rotation angles were converted to positive values for ease of understanding. All variables were tested for their distribution, and appropriate statistics were used. For all variables included in the a priori hypotheses, independent-sample 1-tailed Student t tests were used to examine the differences between the 2 groups. Mean and 95% confidence interval (CI) were calculated and reported. Pearson product moment correlation coefficients was used to examine relationships between SIF and tendon thickness, peak humerothoracic elevation and glenohumeral external rotation, and upper extremity pain and disability in the group with DM.
We further conducted a hierarchical multiple regression analysis to explain the variance of the DASH scores in the group with DM (n=26). The variables of interest were SIF scores, biceps tendon thickness, peak glenohumeral external rotation, and shoulder flexor muscle strength. These variables were selected a priori from the sequence of events described in Figure 1. Shoulder flexor muscle strength was added to the model because a combination of shoulder mobility and strength is necessary for adequate upper extremity function. A hierarchical multiple regression analysis was run by adding one variable at a time. The independent variable was left in the overall equation if: (1) the overall P value (for the F value) was less than .05, (2) the individual P value (based on the t-test value) was less than .10, and (3) the variable added at least 5% unique variance beyond the preceding variables. If all of these criteria were not met, the variable was removed from the equation, and the next variable was entered. Statistical significance was set at P<.05.
Role of the Funding Source
This study was supported by a grant from the Research Division of the Program in Physical Therapy, Washington University School of Medicine, St Louis, Missouri; by grant 1 R21 DK100793-01 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Dr Mueller); and by Diabetes Research and Training Center (Grant No. P30 DK020579).
Results
Differences Between Groups
The differences between groups are shown in Table 2. The mean SIF measure was higher in the DM group compared with the control group (3.1 arbitrary units [95% CI=2.5, 3.8] versus 2.6 arbitrary units [95% CI=2.4, 2.8], respectively; P=.047). The biceps tendon and supraspinatus tendon were 47% and 31% thicker (P<.001), respectively, in the DM group compared with the control group. Peak humerothoracic elevation was decreased by 11 degrees (139° [95% CI=135°, 146°] in the DM group versus 150° [95% CI=146°, 155°] in the control group; P=.002), and peak glenohumeral external rotation was decreased by 16 degrees (35° [95% CI=26°, 46°] in the DM group versus 51° [95% CI=41°, 58°] in the control group; P=.008). Shoulder flexor strength was reduced by 27% (P=.004) in the DM group compared with the control group. The mean DASH score was 19.4% (95% CI=9.8, 30.0) in the DM group, indicating that these individuals had some complaints of upper extremity disability and pain. Four control participants reported very low levels of pain and disability during their laboratory visit (mean DASH score=2.6 [95% CI=0.6, 4.9]; P<.01; Tab. 2).
Table 2.
Differences in Metabolic, Structural, and Upper Extremity Movement and Function in Between Groupsa
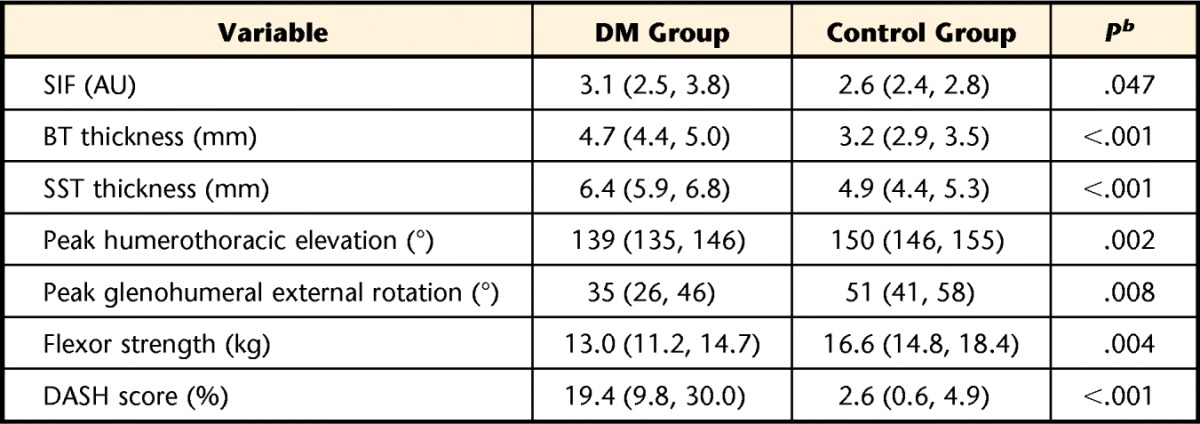
All data presented as mean (95% confidence interval). DM group=participants with diabetes mellitus, control group=participants without diabetes mellitus, SIF=skin intrinsic fluorescence, AU=arbitrary units, BT=biceps tendon, SST=supraspinatus tendon, DASH=Disability of the Arm, Shoulder and Hand questionnaire.
b Significance determined using independent-sample Student t test (one tailed) to examine group differences (P<.05).
Relationships Between SIF and Tendon Thickness, Upper Extremity Movement, and Pain and Disability in the DM Group
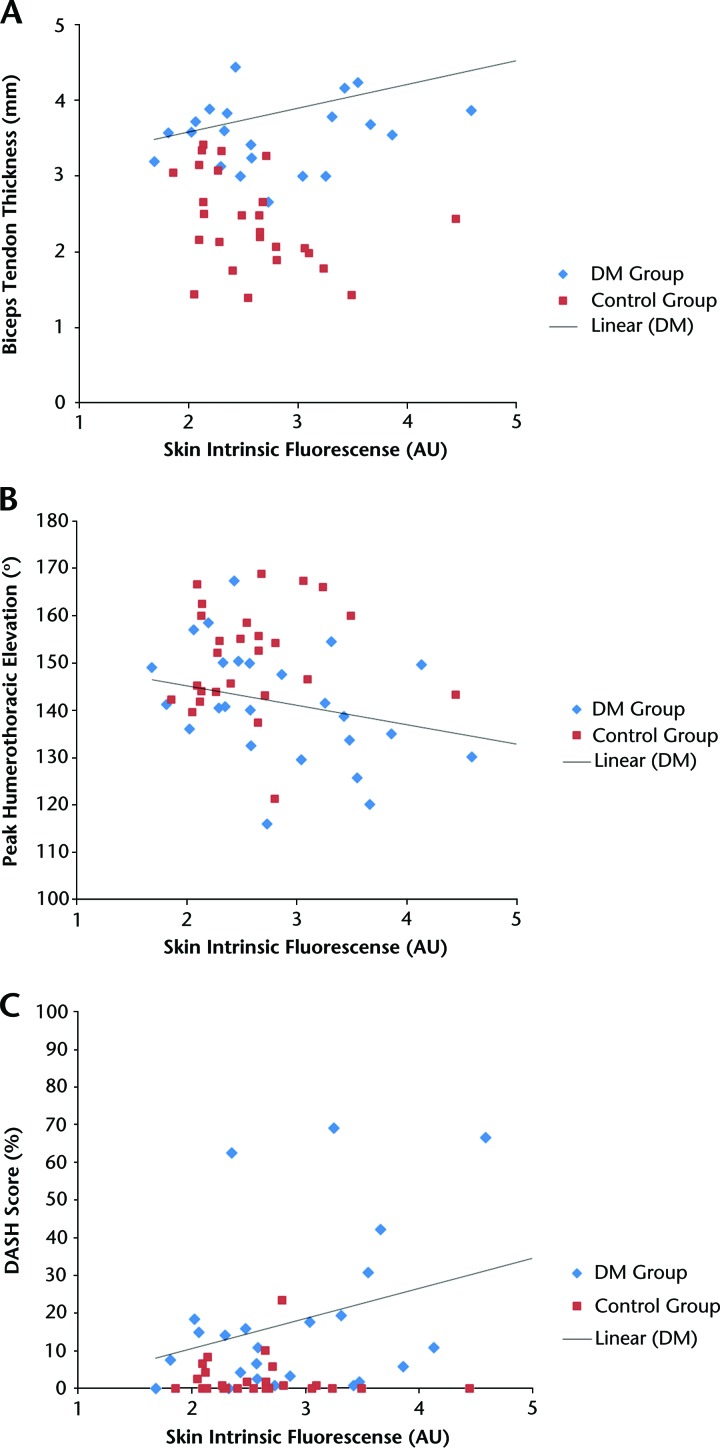
The SIF measure was correlated to biceps tendon thickness (r=.44, P=.023; Fig. 2A) but not correlated to supraspinatus tendon thickness (r=.28, P=.2). The SIF measure was negatively correlated to humerothoracic elevation (r=−.44, P=.024; Fig. 2B) but not correlated to glenohumeral external rotation (r=−.32, P=.13). The SIF measure was not related to shoulder flexor muscle strength (r=.07, P=.7). The SIF measure was correlated to the DASH scores, a measure of upper extremity disability (r=.51, P=.009; Fig. 2C). Figure 2 shows the relationship between the SIF and biceps tendon thickness, humerothoracic elevation, and DASH scores in the DM group. The control group data are shown for comparison only, and these values were not included in the correlation analyses.
Figure 2.
Correlations between skin intrinsic fluorescence and (A) biceps tendon thickness, measured in the bicipital groove (DM group: r=.44, P=.02); (B) peak humerothoracic elevation (DM group: r=−.44, P=.02); and (C) Disability of the Arm, Shoulder and Hand (DASH) questionnaire scores (DM group: r=.51, P=.009). Data points for the control group are included for comparison of distribution. Data analyzed for DM group using Pearson product moment correlation coefficients. DM=diabetes mellitus, AU=arbitrary units.
The SIF (R2=.24, P=.013) and shoulder flexor muscle strength (R2 change=.40, P<.001) explained 64% of the variance of the DASH scores in the DM group. The biceps tendon thickness and peak glenohumeral external rotation were not included in the final model because the individual contributions of these predictors were not significant, beyond the variables already entered (Tab. 3).
Table 3.
Hierarchical Regression Analysis to Predict Variance in Disability of the Arm, Shoulder and Hand (DASH) Questionnaire Scores in Participants With Diabetes (n=26)
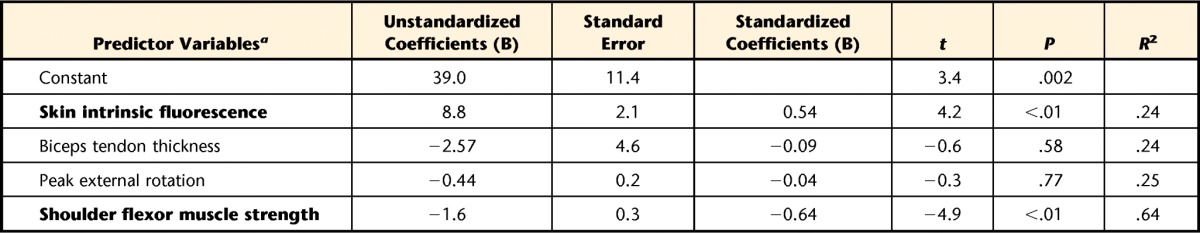
a Variables are listed in the order that they were entered in the model; bold variables indicate significant unique variance after previous variables had been entered.
Discussion
The results of this study demonstrated that the biceps and supraspinatus tendons were thicker and shoulder movements, especially humeral external rotation and muscle strength, were substantially reduced in the group with DM compared with the age-matched group without DM. The SIF, an indicator of AGEs accumulation, was related to the biceps tendon thickness and upper extremity disability and negatively correlated to the peak humerothoracic elevation. This study uniquely examined and provides insights about the relationships among a proxy measure of AGEs, structural changes, and upper extremity LJM and disability in people with type 2 DM. The results are consistent with our a priori hypotheses and the suspected deleterious effect of excessive AGEs on structure, joint movement, and pain and disability as outlined in Figure 1.
The SIF measure was approximately 19% higher in individuals with DM compared with controls in this study (Tab. 2), which is in a similar range as previous reports placing the SIF values about 17% to 33% higher in patients with DM compared with the control population.25,30,31 The recent study by Larkin et al5 indicated higher SIF values in people with type 1 DM and upper extremity musculoskeletal problems compared with those with DM who did not have musculoskeletal problems. Previous studies have used SIF to understand the relationship between accumulation of AGEs and diabetes-related complications such as coronary artery disease30,31 and polyneuropathy25 in individuals with type 1 and type 2 DM. Furthermore, the noninvasive dermal SIF may be a better marker for understanding the musculoskeletal complications in individuals with DM than blood or serum markers because the turnover of collagen is so much longer than that of red blood cells (18 years versus 3–4 months).28,29 For example, SIF has been reported to be more strongly associated with the presence of peripheral neuropathy than the mean 18-year average of the glycated hemoglobin (HbA1c).25 In our study, the SIF measures were related to biceps tendon thickness, upper extremity movement, and pain and disability but not to supraspinatus tendon thickness.
The biceps and supraspinatus tendons were considerably thicker in the group with DM compared with the age-, BMI-, and sex-matched control group (Tab. 2). Previous work in this area has shown similar results, with biceps and supraspinatus tendons thicker in groups with DM compared with control groups (4.0 mm versus 3.0–3.2 mm and 6.2–6.6 mm versus 4.9–5.2 mm, respectively).21,22
A unique contribution of our study is that the SIF measure was related to biceps tendon thickness, indicating that as the skin accumulation of AGEs increases, the tendon tends to be thicker. However, the SIF measure was not correlated to supraspinatus tendon thickness. Possible reasons for this discrepancy include: (1) SIF is an indirect measure of AGEs and, the study was underpowered to identify a relationship between the SIF measure and supraspinatus tendon thickness (a post hoc power analysis indicated 77 participants would be needed); (2) there is slightly greater measurement variability in the thickness of the supraspinatus tendon compared with the biceps tendon; and (3) other factors besides AGEs levels may uniquely affect tendon thickness in people with diabetes. Besides thickness, however, AGEs levels have been shown to be correlated to the intrinsic tendon properties of cross-link formation, distortion of the physiologic pattern of the tendon fibers, and tendon fiber sliding, all of which would affect tendon stiffness properties.39,40 Other structural changes thought to be associated with AGEs levels include fibrous contractures in the capsule and coracohumeral ligament in the shoulders of individuals with DM.23,24 In general, we hypothesize that changes in tendon thickness and other structures (ie, shoulder capsule) are primarily a result of the accumulation of AGEs that leads to LJM and movement impairments in the upper extremity (Fig. 1). Future studies need to examine in vivo extrinsic and intrinsic properties of the tendons, capsules, and connective tissues to understand better the influence of increased accumulation of AGEs on these structures.
There was substantial loss of peak glenohumeral external rotation (humerus relative to scapula) and humerothoracic (humerus relative to thorax) elevation in the DM group compared with the control group. Decreased elevation motion (about 20°) has been reported in people with DM using traditional goniometric methods of assessing ROM.13,14 A recent study in our laboratory demonstrated similar decreases of about 22 degrees and 10 degrees in the humerothoracic elevation and external rotation movement.38 We also observed decreased flexor muscle strength (10.9 kg versus 14.7 kg, respectively) in the cohort with DM and the age- and BMI-matched control group.38 High levels of AGEs accumulation in older adults have been associated with low grip strength values.41,42 Higher concentrations of AGEs in the intramuscular connective tissue may contribute to decreases in muscle function and increased disability. The SIF measure was negatively related to the peak humerothoracic elevation, indicating that as the skin AGEs accumulation increases, the movement decreases. We hypothesized that a significant portion of the upper extremity disability would be explained by SIF, biceps tendon thickness, peak glenohumeral external rotation movement, and flexor muscle strength. Sixty-four percent of the variance in the DASH scores was explained by SIF and shoulder flexor muscle strength (Tab. 3). Therefore, accumulation of AGEs and a decrease in shoulder flexor muscle strength are important correlates of adverse outcomes of upper extremity disability. Further exploration of these relationships, especially the relationship between self-reported pain and disability and AGEs, is warranted in future prospective studies.
The effect of joint movement and exercise on the development or prevention of LJM in DM is not known. Shoulder LJM may not be related to complaints of pain at the early stage. We postulate that the insidious loss of ROM and strength may hit a “threshold” and manifest as severe symptoms of pain and disability. The appropriate type and dosage of exercise prescribed may be useful in reducing LJM, strength deficits, and pain and disability. Additional research is needed to investigate the interaction of movement and metabolic complications on musculoskeletal problems in people with DM. If impairments related to functional limitations are detected early, rehabilitation and pharmaceutical (glucose lowering, AGEs inhibiting, and cross-link breaking agents) therapies may be developed to help prevent additional detrimental changes. In addition, close control of glucose level is thought to be important in minimizing the deleterious effects of diabetes on the musculoskeletal system.5
Limitations for this study are acknowledged and discussed to facilitate interpretation of the results. We assessed tendon thickness but not the intrinsic tendon qualities such as histology, stiffness, and strength. Although we evaluated tendon thickness in individuals with DM to see how it relates to movement impairments, there are other factors (eg, bone spurs, muscle stiffness, capsule stiffness) that may influence shoulder LJM. The full 3-dimensional kinematic descriptions of the humerus and scapula during reaching was beyond the scope of this article but are reported elsewhere.43 Results should not be generalized beyond the characteristics of these study groups. The DM group had relatively good control of their diabetes (mean HbA1c=6.9 [95% CI=6.4, 7.3]). Furthermore, individuals with a BMI greater than 35 kg/m2 were excluded due to documented problems obtaining their kinematic measurements.33 Therefore, this sample does not fully represent the entire population with diabetes, especially those with poor control of their diabetes or severe obesity. Additionally, control participants self-reported their diabetes status and may have had impaired glucose levels, especially those who had high BMI. Therefore, the effect sizes observed in this study may be different but likely even greater in individuals with worse glucose control or obesity compared with a control group with documented exclusion of DM. However, control participants were screened by phone to exclude those with shoulder pain to allow comparison of the group with DM to a fully nonimpaired control group. Such an exclusion criterion for the control group may have overestimated the deficits of the group with DM, but we aimed to highlight this unique group of people with DM at high risk for LJM and subsequent shoulder problems by comparing them with an impairment-free control group.
As this was a cross-sectional study design, we were not able to establish a cause-effect relationship. Also, we do not have information about the temporal relationship of the risk of diabetes and development of shoulder problems. Longitudinal studies are needed to clarify the causal relationship of these variables. A single therapist who was not blinded to group status allows the potential for measurement bias. This bias was minimized by using highly standardized measures and methods. The sample size was relatively small but, due to large effect sizes, was conservatively powered to determine differences between individuals with DM and those without DM. The sample size of participants with DM was small for regression analysis in the DM group. Additional prospective studies with larger sample sizes are necessary to confirm these findings and further clarify the relationships outlined in Figure 1.
In summary, accumulation of AGEs in the connective tissues of individuals with DM appears to be associated with increased tendon thickness and decreased shoulder joint mobility and upper extremity function. Physical therapists should be aware of these possible metabolic effects on structure, movement, and disability when treating people with diabetes. These insights can help focus future rehabilitation and pharmaceutical interventions on the mechanisms of upper extremity musculoskeletal problems in people with DM and develop targeted strategies to treat them.
Footnotes
Dr Shah, Dr Clark, Dr McGill, Dr Lang, and Dr Mueller provided concept/idea/research design. All authors provided writing. Dr Shah and Dr McGill provided data collection. Dr Shah, Dr Clark, Dr Lang, and Dr Mueller provided data analysis. Dr Shah and Dr Mueller provided project management. Dr Mueller provided fund procurement. Dr McGill provided participants. Mr Maynard and Dr Mueller provided facilities/equipment. Dr McGill and Dr Mueller provided institutional liaisons. Dr McGill, Mr Maynard, and Dr Mueller provided consultation (including review of manuscript before submission). The authors thank Victor Cheuy, Emily Martin, Lisa Simone, and Molly Burns for helping with data collection and analysis.
Parts of this study were presented in abstract form (podium) at the Combined Sections Meeting of the American Physical Therapy Association; January 4–6, 2014; Las Vegas, Nevada.
This study was a part of Dr Shah's doctoral thesis presented to the Graduate School of Arts and Sciences at the Washington University School of Medicine.
This study was supported by a grant from the Research Division of the Program in Physical Therapy, Washington University School of Medicine, St Louis, Missouri; by grant 1 R21 DK100793-01 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Dr Mueller); and by Diabetes Research and Training Center (Grant No. P30 DK020579).
References
- 1. Ramchurn N, Mashamba C, Leitch E, et al. Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med. 2009;20:718–721. [DOI] [PubMed] [Google Scholar]
- 2. Arkkila PE, Gautier JF. Musculoskeletal disorders in diabetes mellitus: an update. Best Pract Res Clin Rheumatol. 2003;17:945–970. [DOI] [PubMed] [Google Scholar]
- 3. Arkkila PE. Hand and shoulder abnormalities in diabetic patients: association with diabetes-related complications and diabetes [academic thesis]. Annales Universitatis Turkuensis 1996;244:1–136. [Google Scholar]
- 4. Laslett LL, Burnet SP, Redmond CL, McNeil JD. Predictors of shoulder pain and shoulder disability after one year in diabetic outpatients. Rheumatology (Oxford). 2008;47:1583–1586. [DOI] [PubMed] [Google Scholar]
- 5. Larkin ME, Barnie A, Braffett BH, et al. ; and the DCCT/EDIC Research Group. Musculoskeletal complications in type 1 diabetes. Diabetes Care. 2014;37:1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas SJ, McDougall C, Brown I, et al. Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. J Shoulder Elbow Surg. 2007;16:748–751. [DOI] [PubMed] [Google Scholar]
- 7. Tighe CB, Oakley WS., Jr The prevalence of a diabetic condition and adhesive capsulitis of the shoulder. South Med J. 2008;101:591–595. [DOI] [PubMed] [Google Scholar]
- 8. Cole A, Gill TK, Shanahan EM, et al. Is diabetes associated with shoulder pain or stiffness? Results from a population-based study. J Rheumatol. 2009;36:371–377. [DOI] [PubMed] [Google Scholar]
- 9. Pal B, Anderson J, Dick WC, Griffiths ID. Limitation of joint mobility and shoulder capsulitis in insulin and non-insulin dependent diabetes mellitus. Br J Rheumatol 1986;25:147–151. [DOI] [PubMed] [Google Scholar]
- 10. Molsted S, Tribler J, Snorgaard O. Musculoskeletal pain in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;96:135–140. [DOI] [PubMed] [Google Scholar]
- 11. Mueller MJ, Diamond JE, Delitto A, Sinacore DR. Insensitivity, limited joint mobility, and plantar ulcers in patients with diabetes mellitus. Phys Ther. 1989;69:453–459. [DOI] [PubMed] [Google Scholar]
- 12. Silverstein JH, Gordon G, Pollock BH, Rosenbloom AL. Long-term glycemic control influences the onset of limited joint mobility in type 1 diabetes. J Pediatr. 1998;132:944–947. [DOI] [PubMed] [Google Scholar]
- 13. Schulte L, Roberts MS, Zimmerman C, et al. A quantitative assessment of limited joint mobility in patients with diabetes: goniometric analysis of upper extremity passive range of motion. Arthritis Rheum. 1993;36:1429–1443. [DOI] [PubMed] [Google Scholar]
- 14. Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility (LJM) in elderly subjects with type II diabetes mellitus. Arch Gerontol Geriatr. 2011;53:135–410. [DOI] [PubMed] [Google Scholar]
- 15. Balci N, Balci MK, Tüzüner S. Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes mellitus: association with diabetic complications. J Diabetes Complications. 1999;13:135–140. [DOI] [PubMed] [Google Scholar]
- 16. Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992;15:1835–1843. [DOI] [PubMed] [Google Scholar]
- 17. Brik R, Berant M, Vardit P. The scleroderma-like syndrome of insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1991;7:121–128. [PubMed] [Google Scholar]
- 18. Bai PM, Phua K, Hardt T, et al. Glycation alters collagen fibril organization. Connect Tissue Res. 1992;28:1–12. [DOI] [PubMed] [Google Scholar]
- 19. Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp Diabesity Res. 2004;5:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol (1985). 2007;103:2068–2076. [DOI] [PubMed] [Google Scholar]
- 21. Akturk M, Karaahmetoglu S, Kacar M, Muftuoglu O. Thickness of the supraspinatus and biceps tendons in diabetic patients. Diabetes Care. 2002;25:408. [DOI] [PubMed] [Google Scholar]
- 22. Abate M, Schiavone C, Salini V. Sonographic evaluation of the shoulder in asymptomatic elderly subjects with diabetes. BMC Musculoskelet Disord. 2010;7;11:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bunker TD, Anthony PP. The pathology of frozen shoulder: a Dupuytren-like disease. J Bone Joint Surg Br. 1995;77:677–683. [PubMed] [Google Scholar]
- 24. Homsi C, Bordalo-Rodrigues M, Da Silva JJ, Stump XM. Ultrasound in adhesive capsulitis of the shoulder: is assessment of the coracohumeral ligament a valuable diagnostic tool? Skeletal Radiol. 2006;35:673–678. [DOI] [PubMed] [Google Scholar]
- 25. Conway BN, Aroda VR, Maynard JD, et al. Skin intrinsic fluorescence correlates with autonomic and distal symmetrical polyneuropathy in individuals with type 1 diabetes. Diabetes Care. 2011;34:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maynard JD, Rohrscheib M, Way JF, et al. Noninvasive type 2 diabetes screening: superior sensitivity to fasting plasma glucose and A1C. Diabetes Care. 2007;30:1120–1124. [DOI] [PubMed] [Google Scholar]
- 27. Cleary PA, Braffett BH, Orchard T, et al. Clinical and technical factors associated with skin intrinsic fluorescence in subjects with type 1 diabetes from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Technol Ther. 2013;15:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. [DOI] [PubMed] [Google Scholar]
- 29. Aroda VR, Conway BN, Fernandez SJ, et al. Cross-sectional evaluation of noninvasively detected skin intrinsic fluorescence and mean hemoglobin A1C in type 1 diabetes. Diabetes Technol Ther. 2013;15:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Conway BN, Aroda VR, Maynard JD, et al. Skin intrinsic fluorescence is associated with coronary artery disease in individuals with long duration of type 1 diabetes. Diabetes Care. 2012;35:2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conway BN, Edmundowicz D, Matter N, et al. Skin fluorescence correlates strongly with coronary artery calcification severity in type 1 diabetes. Diabetes Technol Ther. 2010;12:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenbloom AL, Silverstein JH, Lezotte DC, et al. Limited joint mobility in childhood diabetes indicates increased risk for microvascular disease. N Engl J Med. 1981;305:191–194. [DOI] [PubMed] [Google Scholar]
- 33. Hamming D, Braman JP, Phadke V, et al. The accuracy of measuring glenohumeral motion with a surface humeral cuff. J Biomech. 2012;45:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu G, Van der Helm FC, Veeger HE, et al. ; International Society of Biomechanics. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion? Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38:981–992. [DOI] [PubMed] [Google Scholar]
- 35. Ludewig PM, Phadke V, Braman JP, et al. Motion of the shoulder complex during multiplanar humeral elevation. J Bone Joint Surg Am. 2009;91:378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78:26–32. [DOI] [PubMed] [Google Scholar]
- 37. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Shoulder, Arm and Hand). Am J Ind Med. 1996;29:602–608. [DOI] [PubMed] [Google Scholar]
- 38. Shah KM, Clark BR, McGill JB, Mueller MJ. Upper extremity impairments, pain and disability in patients with diabetes mellitus. Physiotherapy. 2015;101:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y, Fessel G, Georgiadis M, Snedeker JG. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013;32:169–177. [DOI] [PubMed] [Google Scholar]
- 40. Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports. 2011;21:749–757. [DOI] [PubMed] [Google Scholar]
- 41. Dalal M, Ferrucci L, Sun K, et al. Elevated serum advanced glycation end products and poor grip strength in older community-dwelling women. J Gerontol A Biol Sci Med Sci. 2009;64:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Momma H, Niu K, Kobayashi Y, et al. Skin advanced glycation end product accumulation and muscle strength among adult men. Eur J Appl Physiol. 2011;111:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah KM, Clark BR, McGill JB, et al. Shoulder limited joint mobility in people with diabetes mellitus. Clin Biomech (Bristol Avon). 2015;30:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]