Abstract
Background
Declining ambulatory activity represents an important facet of disablement in Parkinson disease (PD).
Objective
The primary study aim was to compare the 2-year trajectory of ambulatory activity decline with concurrently evolving facets of disability in a small cohort of people with PD. The secondary aim was to identify baseline variables associated with ambulatory activity at 1- and 2-year follow-up assessments.
Design
This was a prospective, longitudinal cohort study.
Methods
Seventeen people with PD (Hoehn and Yahr stages 1–3) were recruited from 2 outpatient settings. Ambulatory activity data were collected at baseline and at 1- and 2-year annual assessments. Motor, mood, balance, gait, upper extremity function, quality of life, self-efficacy, and levodopa equivalent daily dose data and data on activities of daily living also were collected.
Results
Participants displayed significant 1- and 2-year declines in the amount and intensity of ambulatory activity concurrently with increasing levodopa equivalent daily dose. Worsening motor symptoms and slowing of gait were apparent only after 2 years. Concurrent changes in the remaining clinical variables were not observed. Baseline ambulatory activity and physical performance variables had the strongest relationships with 1- and 2-year mean daily steps.
Limitations
The sample was small and homogeneous.
Conclusions
Future research that combines ambulatory activity monitoring with a broader and more balanced array of measures would further illuminate the dynamic interactions among evolving facets of disablement and help determine the extent to which sustained patterns of recommended daily physical activity might slow the rate of disablement in PD.
The natural course of idiopathic Parkinson disease (PD) is one of ongoing disablement.1,2 Despite pharmacological intervention, disablement in PD commonly evolves in a multifaceted, cumulative fashion as impairments in motor and nonmotor systems, limitations in functional mobility and activities of daily living (ADL), and restrictions in the ability to participate in recreation, travel, exercise, or other physical activities.3–5 Importantly, the rate of disablement in PD may not be uniform across its various underlying components. Motor impairments with minimal activity limitations or participation restrictions are generally characteristic of disease onset.6,7 Worsening of motor impairments and increased difficulty with walking tend to appear earlier than limitations in some gait-dependent ADL tasks, including housework, dressing, and traveling in the community.3,6 Postural instability and gait impairments often mark the transition from early- to middle-stage disease, before the onset of difficulty with cognitively based ADL (eg, managing medication or money, using the telephone) in advanced disease.3,6
Declining ambulatory activity in PD has recently emerged as a growing concern8–11 because of its implications for health12–14 and its potential association with accelerating disability.7 Whether it precedes clinical impairments, evolves gradually throughout the early and middle stages of disease, or emerges more dramatically in concert with postural instability and gait impairment has not yet been determined. A better understanding of ambulatory activity decline in the context of other facets of disablement could inform clinical interventions designed to slow the rate of functional decline in PD.15
In a preliminary investigation,8 we began the process of examining ambulatory activity decline over a 1-year period in 33 people with early- to middle-stage PD. Ambulatory activity was measured directly with a step activity monitor for up to 7 days at baseline and follow-up. In addition to levodopa dosage, clinical measures of motor impairments, walking endurance, and maximal gait speed were collected at each time point. After 1 year, the participants displayed significant declines in the amount and intensity of daily ambulatory activity; concurrent changes in clinical measures were not observed. The results provided insight into physical activity and exercise behavior and suggested that the participants had difficulty meeting recommended physical activity guidelines.13,16 The findings also revealed that the amount and intensity of ambulatory activity, which characterizes many behaviors in the participation domain of the International Classification of Functioning, Disability and Health (ICF),2,17,18 may be relatively more useful than at least some clinical measures for detecting early disablement in PD.
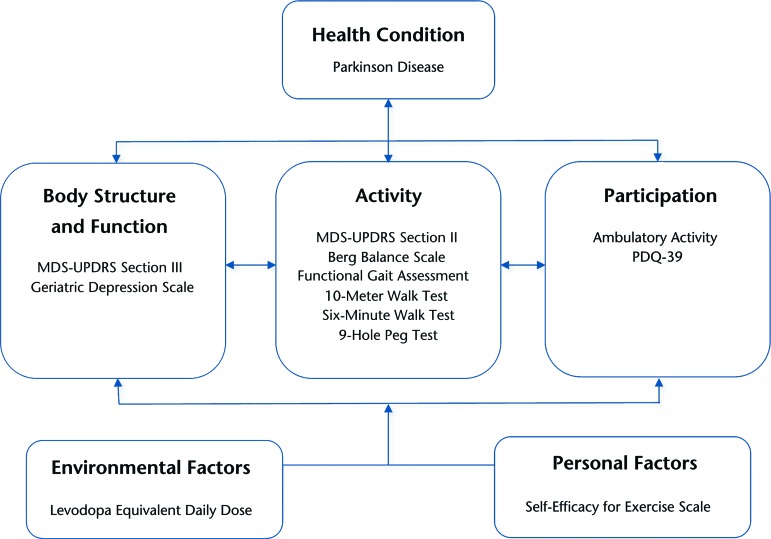
The purpose of the present study was to extend our preliminary examination of ambulatory activity decline in PD by adding a newly collected, second year of step activity data to the analysis. We also expanded our comparison of ambulatory activity decline with concurrently evolving facets of disablement by including a broader array of clinical measurement data from the baseline, 1-year, and 2-year assessments. The clinical measures collectively represented the body structure and function, activity, participation, and contextual factor domains of the ICF model (Figure). On the basis of our preliminary results,8 we hypothesized that measures reflecting the amount and intensity of daily ambulatory activity would be relatively more responsive to disablement than clinical measures. In a secondary exploratory analysis, we sought to provide a foundation for future research by identifying baseline variables that were relatively more likely to be associated with ambulatory activity at the 1- and 2-year assessments.
Figure.
Study measures categorized according to International Classification of Functioning, Disability and Health model domains. MDS-UPDRS=Movement Disorder Society–Unified Parkinson's Disease Rating Scale, PDQ-39=39-item Parkinson's Disease Questionnaire.
Method
Study Design and Sample
Participants were selected from the sample of a previously described 2-year, prospective, longitudinal cohort study19 on the basis of the availability of annual ambulatory monitoring data. Participants were recruited from outpatient movement disorder clinics and local support groups at Boston University and the University of Utah. Inclusion criteria were as follows: a diagnosis of idiopathic PD according to the criteria of the Parkinson's Disease Society Brain Bank (London, United Kingdom),20 modified Hoehn and Yahr stages 1 through 4, age ≥40 years, living in the community (not in an institution), and ability to attend assessment sessions and provide consent. Participants were excluded if they had a diagnosis of atypical parkinsonism, a classification of Hoehn and Yahr stage 5, or previous surgical management of their PD. All participants provided informed consent after initial screening.
The present study was based on a subset of people who had PD, whose baseline and 1-year ambulatory activity data we had analyzed in our preliminary study (n=33),8 and for whom ambulatory activity data from the 2-year assessment also were available (n=17). Reasons for missing ambulatory activity data at the 2-year assessment (n=16) included the limited supply of monitors, the availability of a monitor on a particular day, a participant's willingness to wear a monitor, and the presence of cognitive or integumentary impairments that might have interfered with the wearing protocol. Additional ambulatory activity monitoring details are given below.
Measures
Body structure and function domain.
Section III of the Movement Disorder Society–Unified Parkinson's Disease Rating Scale (MDS-UPDRS) was used to assess motor function.21,22 Section scores can range from 0 to 132, with higher scores indicating greater impairments related to bradykinesia, tremor, rigidity, freezing, and postural control. The reliability of the MDS-UPDRS for people with PD was previously established.23
The Geriatric Depression Scale was used to assess self-reported depressive symptoms.24 Total scores can range from 0 to 30, with higher scores indicating greater mood impairments. The Geriatric Depression Scale was previously validated for people with PD.25
Activity domain.
Section II of the MDS-UPDRS was used to assess self-reported limitations in discrete ADL tasks. Section scores can range from 0 to 52, with higher scores indicating greater activity limitations.
The Berg Balance Scale (BBS) was used to assess static and dynamic balance performance during a series of 14 discrete sitting or standing tasks.26 Total scores can range from 0 to 56, with lower scores indicating greater activity limitations. The validity and high test-retest reliability of the BBS total score were demonstrated across a variety of populations, including patients with PD.23,27
The Functional Gait Assessment (FGA) was used to assess dynamic balance performance during a series of 10 discrete walking tasks, each conducted over a short distance. Total scores can range from 0 to 30, with lower scores indicating greater activity limitations. The reliability, internal consistency, and validity of the FGA total score were established in adults who were healthy and in patients with neurological disorders.28,29
The 10-Meter Walk Test (10MWT) was used to assess gait speed during the discrete task of walking a short distance over an uncluttered, flat indoor surface. The time to walk 10 m was recorded at a participant's self-selected pace with a standardized protocol.23 Lower values indicate greater activity limitations. Gait speed is a reliable and valid indicator of gait function in people with PD.23,30
The Six-Minute Walk Test (6MWT) was used to assess sustained walking performance during the discrete task of walking a relatively long distance over an uncluttered, flat indoor surface, according to the protocol outlined by the American Thoracic Society.31 Scores are represented as distance walked in 6 minutes, with lower scores indicating greater activity limitations. The test-retest reliability of the 6MWT for people with PD is high.23
The Nine-Hole Peg Test (9HPT) was used to assess dominant upper extremity finger dexterity and movement time during the discrete task of placing into and removing from holes in a board 9 pegs as quickly as possible. Scoring is represented as the total time to complete the task, with higher scores indicating greater activity limitations. The 9HPT has high interrater reliability and good test-retest reliability in people with PD.32
Participation domain.
Natural ambulatory activity was captured at each annual assessment with a StepWatch 3 Step Activity Monitor (SAM, Orthocare Innovations, Mountlake Terrace, Washington). The SAM is the size of a pager, weighs only 38 g, and attaches at the ankle with Velcro (Velcro USA Inc, Manchester, Hew Hampshire) closures. It was validated previously for use in populations with impaired gait.8,17,33,34 Participants received a SAM at each annual assessment and were instructed to wear it during customary activity, including exercise but excluding bathing, showering, or swimming, during waking hours for 7 consecutive days. Monitors were configured to record stride counts in 1-minute intervals. The ability to detect steps was optimized according to each participant's height, typical walking speed (slow, normal, or fast), and leg motion (dynamic/fidgety, normal, or gentle/geriatric). Participants wore the SAM on the ankle of the leg with the least severe motor impairment, which was determined from scores on the lower extremity items of the MDS-UPDRS motor subsection. Oral and written instructions were provided regarding proper SAM placement and wearing schedule. Optimal accuracy was verified during the first minutes of recording by comparing monitor step counts, identified by a flashing indicator light, with visual observation. Participants returned the SAM to the researchers after 7 days.
Consistent with our preliminary investigation,8 one investigator (J.T.C.) used SAM manufacturer software to transform recorded 1-minute stride counts into step counts (step count = stride count × 2) and to calculate mean daily values for the amount and intensity of ambulatory activity. Steps, defined as the total number of steps accumulated, were used as the sole indicator of daily activity amount. Moderate-intensity minutes, defined as the mean number of minutes per day in which greater than 100 steps were recorded,13 and maximum output, defined as the mean step rate (steps per minute) during the 30 most active consecutive minutes of the day, were used to indicate daily activity intensity. Daily values were calculated for 24-hour intervals, including time spent sleeping or with the monitor off, from 12:00 am to 11:59 pm. Lower values for each parameter indicated greater participation restrictions.
The 39-item Parkinson's Disease Questionnaire is a health status instrument that is used to measure the degree of healthful, competent, and satisfying participation in daily life activities.35,36 Total scores can range from 0 to 100, with higher scores indicating greater participation restrictions. The reliability, validity, and sensitivity to change of the 39-item Parkinson's Disease Questionnaire were established in people who had PD and dwelled in the community.36
Personal factors.
The Self-Efficacy for Exercise Scale was used to capture participants' confidence in their ability to continue exercising despite barriers to exercise.37 The Self-Efficacy for Exercise Scale score is represented by an average item score from 0 (“not confident”) to 10 (“very confident”), with lower values indicating lower self-efficacy. The reliability, validity, and internal consistency (Cronbach α=.92) of the Self-Efficacy for Exercise Scale have been established.37
Environmental factors.
Drug name, dose, and frequency data were collected as ICF environmental factors at each assessment.2 The levodopa equivalent daily dose was calculated by use of an established protocol.38
Procedure
All measures were administered at baseline and at 1- and 2-year annual assessments. Participants were tested in an on-dopamine-replacement-medication state that was scheduled 0.75 to 1.5 hours after they took their dopamine replacement medications. To ensure the consistency of clinical testing procedures at the sites, we provided research personnel with a standard operating procedure manual and an instructional video that described the protocol for administering and scoring each clinical test for people with PD. Before study participants were enrolled, each evaluator rated 2 video examples of participants undergoing testing on 2 occasions separated by 1 week. We subsequently verified the intrarater and interrater reliability of the measures, with intraclass correlation coefficients (1,4) ranging from .64 to .89 across measures.
Data Analysis
All data were analyzed with the IBM SPSS statistical software program, version 21.0 (IBM Corp, Armonk, New York). Point estimators of central tendency and dispersion as well as interval estimators were calculated for demographic, clinical, and ambulatory activity parameters to describe sample characteristics. Differences among baseline, 1-year, and 2-year assessment scores were evaluated by use of a 1-way repeated-measures analysis of variance. To accommodate any violations of the sphericity assumption, we relied on the more conservative Greenhouse-Geisser F test. Degrees of freedom used for the corrected F test were not necessarily whole numbers. Post hoc pair-wise comparisons between time points were evaluated by use of the least significant difference method. P values of less than .05 were considered statistically significant. The cumulative magnitude of 1- and 2-year changes relative to the baseline was quantified for each measure by use of the Cohen d for within-subject designs.39 In a secondary analysis, we used Pearson product-moment correlations (r) to explore the strength of relationships between baseline variables and mean daily steps at 1 year and between baseline variables and mean daily steps at 2 years (α=.05).
Role of the Funding Source
This study was funded primarily by the Davis Phinney Foundation and the Parkinson Disease Foundation. Additional funding was provided by Boston University Building Interdisciplinary Research Careers in Women's Health (K12 HD043444), the National Institutes of Health (R01NS077959), the Utah Chapter of the American Parkinson Disease Association (APDA), the Greater St Louis Chapter of the APDA, and the APDA Center for Advanced PD Research at Washington University.
Results
The sample comprised 12 men and 5 women with a baseline mean age of 65.6 years (SD=9.9) and a mean duration from diagnosis of 5.1 years (SD=4.6). For 15 participants at baseline, 14 participants at 1 year, and 12 participants at 2 years, the modified Hoehn and Yahr stage was less than 3. Participants generally adhered to wearing ambulatory activity monitors for an entire week (mean days of wear at baseline, 1 year, and 2 years were 6.8 [SD=0.5], 7.0 [SD=0.6], and 6.8 [SD=0.5], respectively).
The amount and intensity of ambulatory activity declined significantly during the 2-year period (for mean daily steps: F1,81=7.3, P=.003; for moderate-intensity minutes: F1,37=10.5, P=.002; for maximum output: F1,82=16.0, P<.001). Post hoc comparisons revealed significant declines (P<.05) between baseline and the 1-year assessment, between baseline and the 2-year assessment, and between the 1-year and 2-year assessments (Table). The effect sizes for changes in ambulatory activity variables ranged from 0.23 to 0.46 at 1 year and from 0.56 to 0.59 at 2 years (Table). For the statistically significant changes observed, the rates of change at 1-year and 2-year intervals for mean daily steps, moderate-intensity minutes, maximum output, and levodopa equivalent daily dose were relatively higher than the rates of change for 10MWT and MDS-UPDRS section III values.
Table.
Baseline, 1-Year, and 2-Year Values for Study Variables, Organized by ICF Domaina
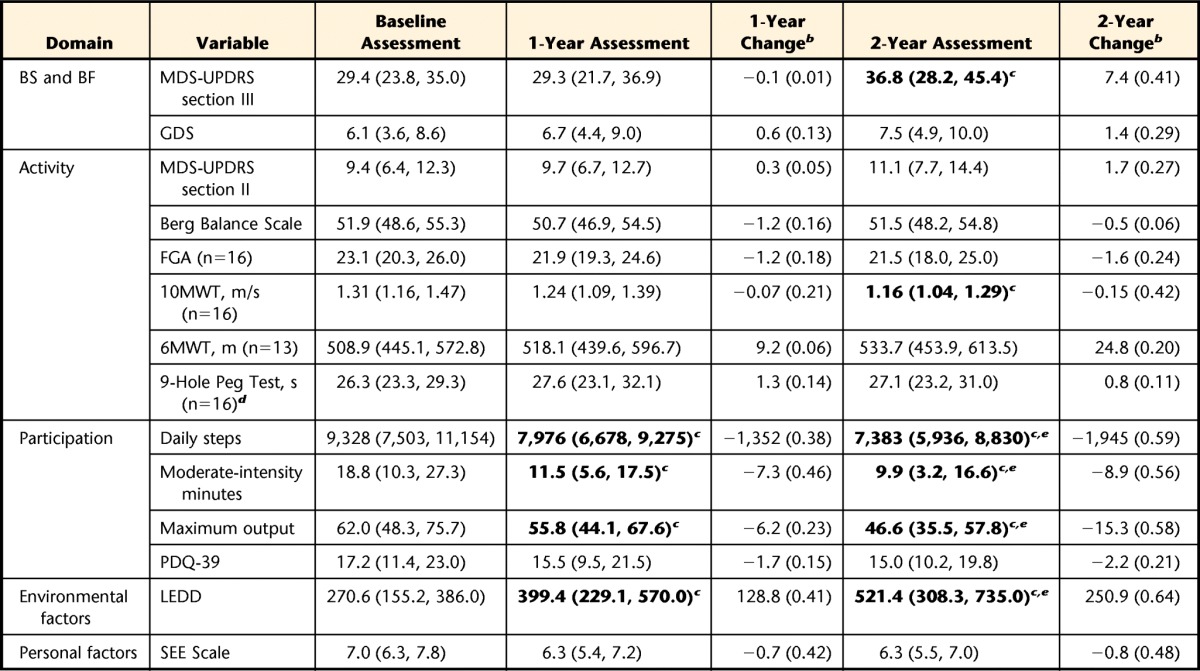
All values are reported as means (95% confidence intervals) (n=17) unless otherwise indicated. Bold type denotes a statistically significant change (P<.05). ICF=International Classification of Functioning, Disability and Health; BS and BF=body structure and function; MDS-UPDRS=Movement Disorder Society–Unified Parkinson's Disease Rating Scale (sections II and III); GDS=Geriatric Depression Scale; FGA=Functional Gait Assessment; 10MWT=10-Meter Walk Test; 6MWT=Six-Minute Walk Test; PDQ-39=39-item Parkinson's Disease Questionnaire; LEDD=levodopa equivalent daily dose; SEE=Self-Efficacy for Exercise.
b Values represent cumulative changes relative to the baseline and are reported as the mean change (Cohen d [effect size]) for within-subject designs.
c Significant change relative to the baseline.
d Values are for the dominant arm.
e Significant change relative to the 1-year assessment.
Several other significant changes occurred during the 2-year period (Table). Like ambulatory activity, therapeutic levodopa regimens evolved (F1,38=9.4), with significant increases in levodopa equivalent daily dose occurring between baseline and the 1-year assessment (P=.03), between baseline and the 2-year assessment (P=.004), and between the 1-year and 2-year assessments (P=.009). Motor impairment worsened (F1,89=6.2), but a significant increase in MDS-UPDRS section III scores relative to those at the baseline occurred only at the 2-year assessment (P=.003). Similarly, gait speed declined (F1,97=6.0), but a significant change relative to gait speed at the baseline occurred only after 2 years (P=.005). Effect sizes corresponding to statistically significant changes in clinical measures ranged from 0.41 to 0.64. None of the remaining clinical measures changed significantly at either the 1-year or the 2-year assessment (P>.05).
Baseline values for all ambulatory activity and physical performance measures (mean daily steps, moderate-intensity minutes, maximum output, BBS, FGA, 10MWT, 6MWT, and 9HPT) were significantly associated with mean daily steps at the 1- and 2-year assessments (P<.05). For each of these relationships, the mean correlation coefficient was at least .6, with the highest mean correlation occurring for baseline mean daily steps (r=.76). Age was the only factor unrelated to physical performance to have a similar mean strength of association (r=.59). The remaining baseline factors all had weaker, nonsignificant relationships with 1- and 2-year mean daily steps (mean correlation coefficients of <.5). Without exception, greater previous impairment, activity limitation, or participation restriction was associated with reduced future ambulatory activity.
Discussion
In the present study, we analyzed ambulatory activity data and related factors collected annually over 2 years from a sample of people (n=17) whose baseline and 1-year data had been included in our preliminary work (n=33).8 Our 2-year results revealed a continued pattern of decline in the amount and intensity of ambulatory activity (Table). Specifically, the mean number of accumulated steps per day (ie, steps) and the mean step rate during the 30 most active consecutive minutes of the day (ie, maximum output) declined during the second year at rates similar to those observed during the first year. In contrast, the rate of decline in the mean number of minutes per day in which at least 100 steps were recorded (ie, moderate-intensity minutes) was lower during the second year than during the first year. Nonetheless, consistent with the previous study,8 the collective cumulative 2-year decline in ambulatory activity appeared to outpace the worsening of all other impairments, activity limitations, and participation restrictions under study.
The results suggested that natural ambulatory behavior may be a particularly robust indicator of decline, especially during the earliest stages of PD, when motor impairment remains relatively mild. However, whether the rate of the observed 2-year decline in ambulatory activity would have continued in a relatively linear fashion with further disease progression remains unclear. Moreover, given that participants were enrolled in the study at various, although relatively early, points in the disease process, the collective rate of ambulatory activity decline (Table) was more likely to have reflected sample characteristics rather than population characteristics. These caveats highlight the preliminary nature of the study and lay an important foundation for future research.
The study findings may have been confounded by methodological differences between ambulatory monitoring and clinical assessment approaches. Ambulatory monitoring is relatively unobtrusive and allows people to interact naturally with their customary environment over an extended period of time. In contrast, clinical assessments are often conducted under optimal performance conditions, in which patients with PD are likely to have taken medications; walking surfaces are uncluttered, flat, and well lit; and few distractions are present. Clinical assessments may be more subject than ambulatory monitoring to patients' awareness that they are being scrutinized, which may engender beliefs about researcher or clinician expectations and thereby lead to alterations in behavior.40 Taken together, these methodological differences are important considerations when rates of disablement across the domains of the ICF model are compared.
Expanded Comparison With Clinical Measures
The ICF model portrays decrements in human function and disability as the product of dynamic interactions among various health conditions and contextual factors.1 In the present study, we sought to expand our preliminary work8 by attempting to compare the individual trajectories of representative measures from each domain of the ICF model (Figure). Consistent with previous investigations,3–5,7 our results supported the idea that individual trajectories of worsening motor, mobility, and ADL function are not parallel; they appear instead to occur at different rates and at different points in the overall disease course.
In our sample, the primary driver of disablement during the study period appeared to be the dynamic interactions of worsening motor function (ie, impairment), declining gait speed (ie, activity limitation), and diminishing ambulatory activity (ie, participation restriction). Disablement emerged despite relatively minimal disability at baseline and contextual factors that were likely to support optimal functioning (ie, sustained self-efficacy for exercise and increasing levodopa dosage [Table]). The results were consistent with a previous report of difficulty with walking as a relatively early symptom of impending disability.3
Baseline Variables Associated With Future Ambulatory Activity
The prospect of progressive inactivity in PD is of great concern because of its implications for health8,12–17 and its potential association with accelerating disability.7 In this context, baseline variables that might predict future ambulatory activity are of interest to clinicians and researchers seeking to develop effective exercise and physical activity interventions. Although many of the variables that we studied showed predictive potential, it appeared that baseline ambulatory activity was most strongly associated with ambulatory activity at the 1- and 2-year assessments. This result appeared to be consistent with the popular maxim that past behavior often predicts future behavior, especially when the behavior is well learned and routinely performed in stable contexts.41 Moreover, this result was remarkable for its simplicity, especially given the potential for many factors to influence exercise and physical activity behavior in people with PD.42
Interestingly, self-efficacy, which we previously reported to be associated with current exercise behavior in PD,42 was only weakly associated with future ambulatory activity. In our view, this result did not necessary call into question the important role of self-efficacy in exercise behavior; rather, it highlighted the potential differences between factors affecting current and future exercise behaviors and between the more specific construct of exercise behavior and the broader construct of ambulatory activity in general.43
Clinical Implications
A “secondary prevention” approach to health promotion, injury prevention, and rehabilitation intervention for people with PD is one designed to delay the onset of and to slow the rate of disablement.44 Our results suggested that people in relatively early disease stages, especially before the onset of measurable postural instability or overt gait disturbance, may benefit from interventions that specifically target public health recommendations for daily physical activity.12–14,16 Whether social, recreational, occupational, or related to exercise, recommended levels of routine physical activity provide the necessary substrate for sustaining long-term reductions in disability risk and optimal levels of health-related quality of life.45,46
Limitations and Future Directions
The present study and our related, preliminary work8 were intended to contribute to a growing body of evidence suggesting that physical activity decline is an important feature of early disablement in PD.9–11 However, the study clearly had limitations. First, our small, relatively homogeneous sample of people in relatively early disease stages limited the external validity of our findings. Second, the participants in the present study (n=17) may not have been fully representative of the original cohort (n=33). Although reasons for withdrawal were not collected, health conditions, cognitive impairment, or both may have limited at least some participants' ability to successfully wear an activity monitor at the 2-year assessment. As a result, the collective decline of the remaining participants during the second year was likely to have been a conservative estimate. Third, compared with other ICF domains, the ICF activity domain was highly represented by our measures. Finally, although ambulatory activity metrics have the potential to reveal much about ongoing and evolving ambulatory behavior, our sample was evaluated at only 2 time points separated by 1 year; therefore, our study was limited by low resolution.
Future PD research that combines ambulatory activity monitoring with a broader and more balanced array of measures across ICF domains would help to further illuminate the dynamic interactions among evolving facets of disablement, either as they occur naturally or in response to intervention. In particular, measures that capture impairments in body structure or function, participation restrictions, or personal and environmental factors are needed. The inclusion of larger, more diverse samples of people with PD would help to more fully describe the problem of physical inactivity across disease stages. Ultimately, investigators should seek to determine the extent to which—and the physiological mechanisms for how—sustained patterns of recommended daily physical activity might slow the rate of disablement in PD.
Footnotes
All authors provided concept/idea/research design. Dr Cavanaugh, Dr Ellis, and Dr Dibble provided writing. Dr Ellis, Dr Ford, Dr Foreman, and Dr Dibble provided data analysis. Dr Ellis, Dr Earhart, Dr Foreman, and Dr Dibble provided project management and fund procurement. Dr Ellis, Dr Foreman, and Dr Dibble provided participants and facilities/equipment. Dr Ellis, Dr Earhart, and Dr Foreman provided consultation (including review of manuscript before submission). The authors acknowledge the participants in this research as well as Tami DeAngelis, PT, GCS, for her assistance and persistence in educating participants, programming the monitors, and ensuring the return of the monitors.
The institutional review board at each institution approved the study protocol.
This study was funded primarily by the Davis Phinney Foundation and the Parkinson Disease Foundation. Additional funding was provided by Boston University Building Interdisciplinary Research Careers in Women's Health (K12 HD043444), the National Institutes of Health (R01NS077959), the Utah Chapter of the American Parkinson Disease Association (APDA), the Greater St Louis Chapter of the APDA, and the APDA Center for Advanced PD Research at Washington University.
References
- 1. Jette AM. Toward a common language for function, disability, and health. Phys Ther. 2006;86:726–734. [PubMed] [Google Scholar]
- 2. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 3. Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23:790–796. [DOI] [PubMed] [Google Scholar]
- 4. Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology. 2005;65:1436–1441. [DOI] [PubMed] [Google Scholar]
- 5. Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Arch Neurol. 2001;58:1611–1615. [DOI] [PubMed] [Google Scholar]
- 6. Poewe W, Mahlknecht P. The clinical progression of Parkinson's disease. Parkinsonism Relat Disord. 2009;15(suppl 4):S28–S32. [DOI] [PubMed] [Google Scholar]
- 7. Shulman LM. Understanding disability in Parkinson's disease. Mov Disord. 2010;25(suppl 1):S131–S135. [DOI] [PubMed] [Google Scholar]
- 8. Cavanaugh JT, Ellis TE, Earhart GM, et al. Capturing ambulatory activity in Parkinson's disease. J Neurol Phys Ther. 2012;36:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White DK, Wagenaar RC, Del Olmo M, Ellis T. Test-retest reliability of 24 hours of activity monitoring in individuals with Parkinson's disease in home and community. Neurorehabil Neural Repair. 2006;21:327–340. [DOI] [PubMed] [Google Scholar]
- 10. White DK, Wagenaar RC, Ellis T. Monitoring activity in individuals with Parkinson disease: a validity study. J Neurol Phys Ther. 2006;30:12–21. [DOI] [PubMed] [Google Scholar]
- 11. Skidmore FM, Mackman CA, Pav B, et al. Daily ambulatory activity levels in idiopathic Parkinson disease. J Rehabil Res Dev. 2008;45:1343–1348. [PubMed] [Google Scholar]
- 12. Tudor-Locke C, Hatano Y, Pangrazi RP, Kang M. Revisiting “how many steps are enough?” Med Sci Sports Exerc. 2008;40(7 suppl):S537–S543. [DOI] [PubMed] [Google Scholar]
- 13. Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tudor-Locke C, Washington TL, Hart TL. Expected values for steps/day in special populations. Prev Med. 2009;49:3–11. [DOI] [PubMed] [Google Scholar]
- 15. Earhart GM, Falvo MJ. Parkinson disease and exercise. Compr Physiol. 2013;3:833–848. [DOI] [PubMed] [Google Scholar]
- 16. Office of Disease Prevention and Health Promotion. Physical activity guidelines. Available at: http://www.health.gov/paguidelines/ Accessed April 10, 2015.
- 17. Cavanaugh JT, Gappmaier VO, Dibble LE, Gappmaier E. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther. 2011;35:26–33. [DOI] [PubMed] [Google Scholar]
- 18. Bowden MG, Hannold EM, Nair PM, et al. Beyond gait speed: a case report of a multidimensional approach to locomotor rehabilitation outcomes in incomplete spinal cord injury. J Neurol Phys Ther. 2008;32:129–138. [DOI] [PubMed] [Google Scholar]
- 19. Dibble LE, Cavanaugh JT, Earhart GM, et al. Charting the progression of disability in Parkinson disease: study protocol for a prospective longitudinal cohort study. BMC Neurol. 2010;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goetz CG, Poewe W, Dubois B, et al. The MDS-UPDRS: How to Apply the New UPDRS in Practice and Research Settings. Milwaukee, WI: International Parkinson and Movement Disorder Society; 2006. [Google Scholar]
- 22. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 23. Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism. Phys Ther. 2008;88:733–746. [DOI] [PubMed] [Google Scholar]
- 24. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 25. Mondolo F, Jahanshahi M, Grana A, et al. The validity of the Hospital Anxiety and Depression Scale and the Geriatric Depression Scale in Parkinson's disease. Behav Neurol. 2006;17:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berg KO, Wood-Dauphinée SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(suppl 2):S7–S11. [PubMed] [Google Scholar]
- 27. Qutubuddin AA, Pegg PO, Cifu DX, et al. Validating the Berg Balance Scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Arch Phys Med Rehabil. 2005;86:789–792. [DOI] [PubMed] [Google Scholar]
- 28. Walker ML, Austin AG, Banke GM, et al. Reference group data for the Functional Gait Assessment. Phys Ther. 2007;87:1468–1477. [DOI] [PubMed] [Google Scholar]
- 29. Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the Functional Gait Assessment. Phys Ther. 2004;4:906–918. [PubMed] [Google Scholar]
- 30. Brusse KJ, Zimdars S, Zalewski KR, Steffen TM. Testing functional performance in people with Parkinson disease. Phys Ther. 2005;85:134–141. [PubMed] [Google Scholar]
- 31. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 32. Earhart GM, Cavanaugh JT, Ellis T, et al. The 9-hole PEG test of upper extremity function: average values, test-retest reliability, and performance in people with Parkinson disease. J Neurol Phys Ther. 2011;35:157–163. [DOI] [PubMed] [Google Scholar]
- 33. Munneke M, de Jong Z, Zwinderman AH, et al. The value of a continuous ambulatory activity monitor to quantify the amount and intensity of daily activity in patients with rheumatoid arthritis. J Rheumatol. 2001;28:745–750. [PubMed] [Google Scholar]
- 34. Manns PJ, Baldwin E. Ambulatory activity of stroke survivors: measurement options for dose, intensity, and variability of activity. Stroke. 2009;40:864–867. [DOI] [PubMed] [Google Scholar]
- 35. Den Oudsten BL, Van Heck GL, De Vries J. The suitability of patient-based measures in the field of Parkinson's disease: a systematic review. Mov Disord. 2007;22:1390–1401. [DOI] [PubMed] [Google Scholar]
- 36. Jenkinson C, Fitzpatrick R, Peto V, et al. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing. 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 37. Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise Scale. Nurs Res. 2000;49:154–159. [DOI] [PubMed] [Google Scholar]
- 38. Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 39. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ouellette JA, Wood W. Habit and intention in everyday life: the multiple processes by which past behavior predicts future behavior. Psychol Bull. 1998;124:54–74. [Google Scholar]
- 42. Ellis T, Cavanaugh JT, Earhart GM, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther. 2011;91:1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manns PJ, Dunstan DW, Owen N, Healy GN. Addressing the nonexercise part of the activity continuum: a more realistic and achievable approach to activity programming for adults with mobility disability? Phys Ther. 2012;92:614–625. [DOI] [PubMed] [Google Scholar]
- 44. Ellis T, Motl RW. Physical activity behavior change in persons with neurologic disorders: overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther. 2013;37:85–90. [DOI] [PubMed] [Google Scholar]
- 45. Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada's Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2010;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spirduso WW, Cronin DL. Exercise dose-response effects on quality of life and independent living in older adults. Med Sci Sports Exerc. 2001;33(6 suppl):S598–S608. [DOI] [PubMed] [Google Scholar]