Abstract
AIM: To examine the association between circulating 25-hydroxyvitamin D [25(OH)D] levels and colorectal adenoma in a case-control study and a meta-analysis.
METHODS: We conducted a matched case-control study (112 cases and 112 matched controls) and combined 15 studies, including our study, in a meta-analysis. The study-specific odds ratios (ORs) and 95% confidence intervals (CIs) were pooled using a random-effects model. In total, 5454 colorectal adenomas and 6656 controls were included in the meta-analysis.
RESULTS: In a meta-analysis including 14 previous studies and our study, we observed a significant inverse association between circulating 25(OH)D levels and colorectal adenoma (OR = 0.68; 95%CI: 0.54-0.82) when comparing the highest category with the lowest category. Stratification by adenoma location (proximal or distal adenoma) showed similar estimates. When we stratified by study region, the ORs (95%CIs) were 0.70 (0.52-0.88) in the US and 0.66 (0.34-0.97) in Asia.
CONCLUSION: These data suggest an inverse association between circulating 25(OH)D levels and colorectal adenoma in both Western and Asian populations.
Keywords: 25-hydroxyvitamin D levels, Colorectal adenoma, Cancer prevention
Core tip: Growing evidence from epidemiologic studies suggests a preventive effect of vitamin D against colorectal cancer. Colorectal adenoma is considered to be a precursor lesion of colorectal cancer. We conducted a case-control study in Korean adults and also calculated a summary estimate through a meta-analysis to examine the association between circulating 25-hydroxyvitamin D[25(OH)D] levels and colorectal adenoma. We found an inverse association between circulating 25(OH)D levels and colorectal adenoma, and this association was consistent for Asian populations.
INTRODUCTION
Vitamin D is synthesized in human skin that is exposed to ultraviolet light and is also obtained through supplements and several food sources. Because the vitamin D from food does not adequately reflect the vitamin D status of an individual, circulating concentrations of 25-hydroxyvitamin D [25(OH)D] may be a suitable measurement of vitamin D status. However, 1,25(OH)2D may not be a good indicator of vitamin D because it is tightly regulated by various factors[1].
Growing evidence suggests that low vitamin D levels are prevalent worldwide[2,3], partly because of an insufficient supply of vitamin D from natural food sources[4], a shift to sedentary lifestyles, and a lack of outdoor activities. Garland et al[5] suggested that exposure to solar radiation could be a protective factor for colon cancer in an ecologic study, and several epidemiologic studies found a reduction in colorectal cancer with better vitamin D status[6,7]. 1,25-dihydroxyvitamin D [1,25(OH)2D], the active form of vitamin D, exerts its inhibitory effects on tumors in the normal and neoplastic colonic epithelium by affecting cell growth regulation, cell cycle regulation, and apoptosis and by interacting with proto-oncogenes and tumor suppressor genes[8].
The incidence rate of colorectal cancer has increased in some Asian countries, which have undergone dramatic lifestyle changes. For example, the colorectal cancer incidence has steadily increased in Korea by 4.7% annually from 1999 to 2010[9]. Colorectal cancer often develops from a colorectal adenoma in a process known as the adenoma-carcinoma sequence.
A considerable proportion of the East Asian population has low vitamin D status. The prevalence of < 20 ng/mL vitamin D levels was 56.0% in Korean adults of the Korean National Health and Nutrition Examination Survey 2008[10], 69.2% in the middle-aged or elderly Chinese population in a cross-sectional study[11], and 35.3% in postmenopausal Japanese women[12]. Despite the increase in colorectal cancer incidence and the low vitamin D status in Asian populations, only a few Asian studies on vitamin D and colorectal adenoma have been conducted. One Japanese study found a lower prevalence of colorectal adenoma with high vitamin D status during the winter season[13], and another Japanese study observed an inverse, but nonlinear, association[14]. One Korean case-control study performed an analysis among 143 age- and gender-matched case and control pairs and found an inverse association[15]. Although we reported a potential inverse association between circulating vitamin D levels and colorectal adenoma in a previous meta-analysis[16], a limited number of Asian studies did not allow us to examine whether this association observed was applied to Asian populations.
To determine whether circulating vitamin D levels are inversely associated with colorectal adenoma, we analyzed the association between colorectal adenoma and 25(OH)D levels among a matched case-control study of Korean adults, and we conducted a meta-analysis of 15 studies, including 14 previous published studies and our study.
MATERIALS AND METHODS
Case control study
Study population: We conducted a case-control study among 45- to 71-year-old men and women who underwent colonoscopy at a university hospital in Daegu, city of Korea, from August 2011 to September 2012. The size, subtype and number of colorectal adenomas were determined through colonoscopy and pathological examination. Polyps were classified as adenomatous, hyperplastic, or other nonadenomatous. Only adenomatous polyps were included as cases. We included both first and recurrent adenomas (4.7%). To minimize the influence of fasting status or sex, we performed 1:1 matching by fasting status and sex. As a result, a total of 112 cases and 112 matched-controls were included. This study was approved by the Institutional Review Board of Daegu Catholic University Medical Center. Written informed consent was obtained from all participants.
Measurement of circulating vitamin D levels: Participants provided blood samples between January and February 2013. Blood samples were centrifuged and sent on ice to the Neodin Medical Institute (Seoul, South Korea). Concentrations of serum 25(OH)D were measured using the DiaSorin radioimmunoassay (RIA) method at Neodin Medical Institute. The intra-assay coefficient of variation (CV) was less than 2%. All laboratory technicians were blinded to the case status.
Assessment of lifestyle factors: We asked participants for information about their demographic characteristics, lifestyle factors, and family history of colorectal cancer. Dietary intake was assessed using a validated food frequency questionnaire (FFQ)[17]. The height and weight of participants were directly measured, and the body mass index (BMI) was calculated by dividing the weight in kilograms by the square of the height in meters. Participants were asked about the age at which they started and/or quit drinking and about the amount and frequency of alcohol consumption, such as rice wine (makgeolli), wine, beer, and liquor. Questions regarding cigarette smoking habits included queries about whether the participant smoked more than 20 packs of cigarettes, the age of smoking initiation and cessation, the amount of cigarettes smoked per day during regular smoking, and the total duration of regular cigarette smoking. The total pack years of smoking was calculated based on the total duration of regular cigarette smoking and the amount of cigarettes smoked per day during regular smoking. The metabolic equivalent of task (MET)-hours per week was calculated for physical activity.
Statistical analysis: The characteristics of the participants were compared between cases and controls using the means and standard deviations (SDs) for continuous variables or using the frequencies and percentages for categorical variables. The differences between continuous variables were analyzed by paired t-tests, and those between categorical variables were analyzed using the Mantel-Haenszel test. We used conditional logistic regression analysis to obtain the odds ratios (ORs) and 95% confidence intervals (CIs) of colorectal adenoma according to the quartile of the 25(OH)D levels. A test for trends was performed by including the median of each 25(OH)D quartile as a continuous variable. We adjusted for age (years, continuous), BMI (kg/m2, 18.5-<23, 23-<25, > 25), alcohol drinking (men: nondrinker, past drinker, ≤ 1/mo, 2-4/mo, 2-3/wk, ≥ 4/wk, women: nondrinker, ever drinker), smoking status (men: never, 0-<20, 20-<30, > 30 pack-years of smoking; women: never, ever smoker), folate intake (mcg/d, continuous), and menopausal status and hormone replacement use for women only (premenopausal, postmenopausal without hormone replacement therapy, postmenopausal with hormone replacement therapy, postmenopausal with nonresponse about hormone replacement therapy). We examined whether the associations differed by adenoma calcium intake (median, < 412.5, ≥ 412.5 mg/d). We used the likelihood ratio test (LRT) to test the null hypothesis that there was no interaction due to the potential effect factors of colorectal adenoma. All P values were two-sided, and P < 0.05 was considered to be statistically significant. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina).
Meta-analysis
Selection of studies: We searched the PubMed database for studies published through February 25, 2015. We used the following terms for a PubMed search restricted to articles reported in English-language journals: (“Vitamin D” OR “Calcifediol” OR “circulating 25(OH)vitamin D” OR “25-hydroxylvitamin D” OR “25-hydroxyvitamin D” OR “25(OH)D”) AND (“colorectal adenoma” OR “adenomas” OR “adenomatous” OR “CRA”). We also searched the Web of Science database using the term (25 hydroxyvitamin D and colorectal adenoma) in a search query of the topic field. In total, 203 articles were identified in the PubMed database and 55 articles in Web of Science. The title and abstract of each selected paper were examined in detail to determine whether the article was relevant. We also manually searched the bibliographies of the retrieved articles. The major criteria were as follows: (1) serum or plasma 25(OH)D was assayed as the factor of interest; (2) the outcome of interest was colorectal adenoma or adenoma recurrence; (3) the relative risk (RR) and 95%CIs were reported; and (4) articles were published as full-text manuscripts. If studies were duplicated[18-21], the study with the larger sample size[19] or a pooled analysis with another study[20] was included. Eligibility criteria were assessed by Choi YJ, and selected manuscripts were checked by an independent author (Lee JE). Two authors (Choi YJ and Lee JE) independently assessed the quality of each study using the Newcastle-Ottawa Scale[22]. Score differences greater than 1 between the two authors were resolved by consensus. We identified fifteen studies[13-15,19,20,23-31] that examined serum or plasma 25(OH)D levels and first colorectal adenoma or adenoma recurrence (Figure 1). We excluded study where the units of the 25(OH)D levels were not available[31]. The following data were extracted from the selected articles: the first author, published year, study region, sex, study design, endpoint, type of endoscopy, study dates (follow-up duration), number of cases and controls, mean or median of 25(OH)D, 25(OH)D levels comparing the highest category with the lowest category, OR (95%CI), and adjusted covariates. This meta-analysis was performed according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines[32].
Figure 1.
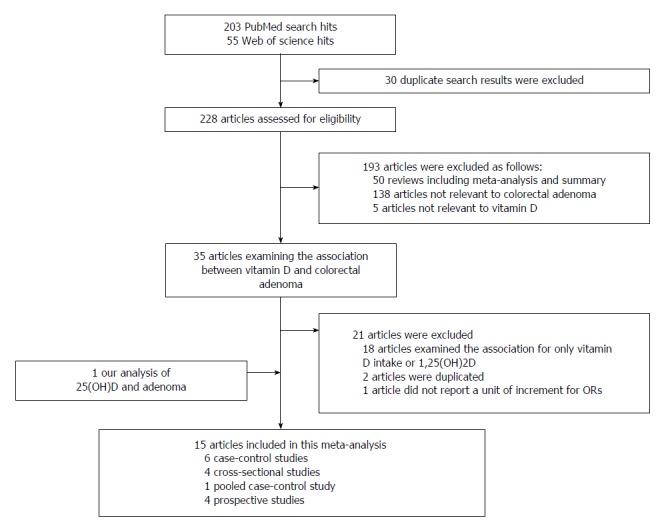
Flow chart of study selection process.
Statistical analysis: For a meta-analysis of the association between 25(OH)D levels and colorectal adenoma, including the association found in our case-control study, we computed the summary RR and 95%CIs using a random-effects model[33]. The RR of each study was extracted from the most fully adjusted models if available. Estimates of the studies were weighted by the inverse of their variance. In the main analysis, we compared the highest category with the lowest category of circulating 25(OH)D levels. For a study that reported only a dose-response relationship[26], we calculated the OR (95%CI) of a mean difference in 25(OH)D levels between the highest and the lowest categories in the other studies for categorical comparison. We also constructed a dose-response model. If the RR per standard unit of increase was not presented in the studies, we converted the categorical RR to a dose-dependent RR using the method suggested by Greenland et al[34] and Orsini et al[35]. For this analysis, we assigned the midpoint of the upper and lower levels in each category. If the highest or the lowest boundary was not reported, we assumed that the interval in the highest or the lowest category had the same amplitude as the adjacent category. We calculated the RRs and 95%CIs for 10 ng/mL increments in the 25(OH)D levels.
We performed subgroup analyses and meta-regression analyses to assess potential sources of heterogeneity due to sex, calcium intake (high or low), geographic location (United States or Asia), or adenoma location (proximal or distal).
The between-study heterogeneity was evaluated using a Q test[33]. We evaluated for a potential publication bias using a funnel plot and Egger linear regression test[36]. All meta-analyses were performed using STATA 11 statistical software (StataCorp, College Station, TX, United States). All P values were two-sided, and P < 0.05 was considered to be statistically significant.
RESULTS
Case-control study
The characteristics according to colorectal adenoma prevalence are presented in Table 1. The mean age was 60.3 (SD = 5.3) years for the cases and 59.7 (SD = 5.4) years for the controls. The waist circumference and BMI were higher among the cases than among the controls. The cases consumed a higher amount of alcohol than did the controls. The mean 25(OH)D levels were 15.7 (SD = 5.8) ng/mL for the cases and 16.7 (SD = 5.6) ng/mL for the controls. Overall, 81.3% of the cases and 73.2% of the controls had 25(OH)D levels < 20 ng/mL. In our study, 76.3% of the men and 78.2% of the women had 25(OH)D levels < 20 ng/mL.
Table 1.
Characteristics of patients according to adenoma case, n (%)
| Adenoma (n = 112) | No adenoma (n = 112) | P value | |
| Age (yr), mean ± SD | 60.3 ± 5.3 | 59.5 ± 5.4 | 0.37 |
| Men | 57 (50.9) | 57 (50.9) | Matched |
| 25(OH)D (ng/mL), mean ± SD | 15.7 ± 5.8 | 16.8 ± 5.6 | 0.16 |
| 25(OH)D < 20 ng/mL | 91 (81.3) | 82 (73.2) | 0.12 |
| Education | 0.54 | ||
| Elementary school graduate | 16 (14.3) | 10 (9.1) | |
| Middle school graduate | 25 (22.3) | 31 (28.2) | |
| High school graduate | 50 (44.6) | 46 (41.8) | |
| College graduate or above | 21 (18.8) | 23 (20.9) | |
| Waist circumference (cm), mean ± SD | 87.0 ± 7.8 | 84.6 ± 6.7 | 0.02 |
| BMI (kg/m2) | 0.10 | ||
| 18.5 < BMI < 23 | 33 (29.5) | 36 (32.1) | |
| 23 ≤ BMI < 25 | 32 (28.6) | 45 (40.2) | |
| 25 ≤ BMI | 47 (41.9) | 31 (27.7) | |
| Family history | 0.53 | ||
| Yes | 6 (5.4) | 4 (3.6) | |
| No | 106 (94.6) | 108 (96.4) | |
| Smoking status | 0.29 | ||
| Non smoker | 56 (50.9) | 64 (57.7) | |
| Past smoker | 37 (33.6) | 31 (27.9) | |
| Current smoker | 17 (15.5) | 16 (14.4) | |
| Alcohol drinking status | 0.002 | ||
| Never drinker | 41 (36.6) | 57 (50.9) | |
| Past drinker | 6 (5.4) | 6 (5.4) | |
| Current drinker | 65 (58.0) | 49 (43.8) | |
| Physical activity (MET-hr/wk), mean ± SD | 29.0 ± 32.0 | 24.3 ± 25.2 | 0.22 |
| Supplement use | 0.08 | ||
| Yes | 42 (37.5) | 56 (50.0) | |
| No | 70 (62.5) | 56 (50.0) | |
| Red meat intake | 0.34 | ||
| ≤ 1/mo | 11 (10.1) | 10 (8.9) | |
| 2-4/mo | 77 (70.6) | 88 (78.6) | |
| ≥ 2/wk | 21 (19.3) | 14 (12.5) | |
| Energy intake (kcal/d), mean ± SD | 1688.8 ± 581.9 | 1692.4 ± 488.4 | 0.96 |
P values were calculated using the Mantel-Haenszel test for categorical variables and the paired t test on loge-transformed variables. BMI: Body mass index.
We found no association between the 25(OH)D levels and colorectal adenoma for men, but we found a suggestive inverse trend for women (Table 2); the multivariate ORs and 95%CIs compared to the bottom quartile of 25(OH)D levels were 0.89 (0.26-3.10) for the 2nd quartile, 0.54 (0.13-2.16) for the 3rd quartile, and 0.22 (0.04-1.15) for the 4th quartile (P for trend = 0.05). When we stratified our data by adenoma location (proximal or distal), the ORs (95%CIs) were 0.55 (0.22-1.42) for distal adenomas and 0.59 (0.23-1.54) for proximal adenomas. Stratification by calcium intake showed 0.71 (0.19-2.58) for low calcium intake (less than median levels) and 0.36 (0.10-1.30) for high calcium intake (median or greater levels) of ORs (95%CIs) comparing the 4th quartile with the other three lower quartiles, but the difference was not statistically significant (P for interaction = 0.36).
Table 2.
Odds ratios and 95% confidence intervals for adenoma according to quartile of 25-hydroxyvitamin D level
|
Quartile of serum 25(OH) D levels |
P for trend | ||||
| Quartile1 | Quartile2 | Quartile3 | Quartile4 | ||
| All patients | |||||
| Median (ng/mL) | 10.33 | 14.25 | 17.91 | 24.21 | |
| Case/control | 37/28 | 26/28 | 28/28 | 21/28 | |
| Model 1 | 1.00 | 0.71 (0.35-1.45) | 0.73 (0.33-1.61) | 0.52 (0.23-1.20) | 0.15 |
| Model 2 | 1.00 | 0.72 (0.31-1.66) | 0.73 (0.29-1.82) | 0.49 (0.19-1.27) | 0.16 |
| Men | |||||
| Median (ng/mL) | 11.08 | 14.6 | 17.65 | 22.66 | |
| Case/control | 15/14 | 13/14 | 17/15 | 12/14 | |
| Model 1 | 1.00 | 0.95 (0.35-2.57) | 1.10 (0.29-4.18) | 0.80 (0.25-2.60) | 0.74 |
| Model 2 | 1.00 | 1.31 (0.36-4.82) | 1.26 (0.23-6.87) | 1.80 (0.40-8.12) | 0.46 |
| Women | |||||
| Median (ng/mL) | 9.48 | 13.53 | 18.4 | 25.42 | |
| Case/control | 16/14 | 22/14 | 8/14 | 9/13 | |
| Model 1 | 1.00 | 1.31 (0.53-3.24) | 0.51 (0.18-1.51) | 0.65 (0.20-2.14) | 0.16 |
| Model 2 | 1.00 | 0.89 (0.26-3.10) | 0.54 (0.13-2.16) | 0.22 (0.04-1.15) | 0.05 |
Model 1 was adjusted for age (continuous). Model 2 was adjusted for the following covariates; men: age (years, continuous), body mass index (BMI) (kg/m2, 18.5- < 23, 23- < 25, > 25), alcohol drinking (nondrinker, past drinker, ≤ 1/mo, 2-4/mo, 2-3/wk, ≥ 4/wk), smoking status (never, 0- < 20, 20- < 30, > 30 pack-years of smoking), and folate intake (mcg/d, continuous); women: age (years, continuous), BMI (kg/m2, 18.5- < 23, 23- < 25, > 25), alcohol drinking (nondrinker, ever drinker), smoking status (never, ever smoker), folate intake (mcg/d, continuous), and menopausal status and hormone replacement use (premenopausal, postmenopausal without hormone replacement therapy, postmenopausal with hormone replacement therapy, postmenopausal with nonresponse about hormone replacement therapy use).
Meta-analysis
A total of 15 articles reporting 5454 colorectal adenomas and 6656 controls were included in the meta-analysis (Table 3). The 25(OH)D levels were measured at baseline during clinical trials of colorectal adenoma recurrence[20,26]. As a result, we included 6 case-control studies, 4 cross-sectional studies, 1 pooled case-control study, and 4 prospective studies (two used a clinical trial design[20,26]). Ten studies were conducted in the United States, 1 in Austria, 2 in Japan, and 2 in Korea. Nine studies used the first adenoma as the endpoint, and two prospective studies from clinical trials considered adenoma recurrence as the endpoint. The other two studies[25], including our study, included a small proportion of participants who had recurrence. The endpoint of the study was not clear in one article[30]. Adenomatous polyps were determined through colonoscopy except in four studies; two studies used both colonoscopy and sigmoidoscopy[23,25], and the other two studies used only sigmoidoscopy[24,27]. Two studies conducted pooled analyses; the pooled analysis including three colonoscopy-based case-control studies (the Cancer Prevention Research Unit (CPRU), the Markers of Adenomatous Polyps (MAP) studies in North Carolina and the MAPII study in South Carolina)[28] and a pooled analysis of two randomized clinical trials (the Wheat Bran Fiber Trial and the Ursodeoxycholic Acid Trial)[20]. Out of 15 studies, 7 studies adjusted for the month of blood draw[14,19,25,27,29,30,37], one study matched the case and control by date of blood draw[23], and two Korean studies[15], including our case-control study, collected blood samples only in the winter.
Table 3.
Included studies of circulating levels of 25-hydroxyvitamin D and colorectal adenoma
| First author, year | Country (sex) | Study design | study dates | Endpoint | Type of endoscopy | 25(OH)D, mean or median | No. of cases/controls | 25(OH)D levels in the highest vs lowest categories | OR (95%Cl) |
| Platz, 2000 | United States (W) | Prospective study | 1989-1996 | First adenoma | Sigmoidoscopy or colonoscopy | 26.4 in cases and 26.8 ng/mL in controls, mean | 326/326 | 38.0 ng/mL vs 16.3 ng/mL, median | 1.00 (ref), 0.64, 0.58, 1.04 (0.66-1.66) |
| Levine, 2001 | United States (M, W) | Case-control study | 1991-1993 | First adenoma | Sigmoidoscopy | 25.6 in cases and 26.9 ng/mL in controls, mean | 473/506 | 34.3-115 ng/mL vs 1-15.2 ng/mL, range | 1.00 (ref), 0.99, 0.86, 0.74 (0.51-1.09) |
| Peters, 2001 | United States (M, W) | Case-control study | 1994-1996 | First (61%) or recurrent adenoma | Colonoscopy (86.2%) or sigmoidoscopy | 24.7 in cases and 26.5 ng/mL in controls, median | 236/218 | 33.7-67.2 ng/mL vs 5.3-19.4 ng/mL, range | 1.0(ref), 0.40, 0.67, 0.47, 0.43(0.23-0.81) |
| Grau, 2003 | United States (M, W) | Prospective study | 1992-1996 | Recurrent adenoma | Colonoscopy | 29.1 ng/mL, median | 376/422 | - | 0.99 (0.91-1.07) OR for serum vitD levels per 12 (1SD) units increase |
| Peters, 2004 | United States (M, W) | Prospective study | 1993-1999 | First advanced adenoma | Sigmoidoscopy | 27.0 in cases and 28.3 ng/mL in controls, mean | 394/397 | - | 0.87 (0.75-1.01) OR for serum vitD levels per 10 units increase. |
| Miller, 2007 | United States (M, W) | Cross-sectional study | 1998-2000 | First adenoma | Colonoscopy | 27.5 in cases and 31.4 ng/mL in controls, mean | 111/238 | > 33.8 ng/mL vs < 20.8 ng/mL, range | 1.00 (ref), 0.74, 0.51 (0.27-0.98) |
| Takahashi, 2010 | Japan (M) | Case-control study | 1997-2004 | First adenoma | Colonoscopy | 26.2 in cases and 26.1 ng/mL in controls, mean | 656/648 | ≥ 30 ng/mL vs < 22 ng/ml, range | 1.00 (ref), 1.21, 1.21, 1.25 (0.85-1.84) |
| Fedirko, 2010 | United States (M, W) | Pooled case-control study | 1991-2002 | First adenoma | Colonoscopy | 24.5 in cases and 25.5 ng/mL in controls, mean | 616/770 | 1.00 (ref), 0.77, 0.85, 0.59 (0.41-0.84) | |
| Adams, 2011 | United States (M, W) | Cross-sectional study | 1998- 2003 | Fitst or recurrent adenoma | Colonoscopy | 23.1 in cases and 24.9 ng/mL in controls, mean | 149/ 225 | > 28.9 ng/mL vs ≤ 20.5 ng/ml, range | 1.00 (ref), 0.97, 0.71 (0.38-1.30) |
| Ashktorab, 2011 | United States (M, W) | Case-control study | Colonoscopy | 41.2 in cases and 41.4 ng/mL in controls, mean | 93/187 | > 57.7 ng/mL vs < 29.5 ng/mL, range | 1.00 (ref), 1.4, 1.9, 0.6 (0.3-1.4) | ||
| Hong, 2012 | Korea (M, W) | Case-control study | 2009-2010 | First adenoma | Colonoscopy | 20.0 in cases and 25.0 ng/mL in controls, mean | 143/ 143 | ≥ 23.9 ng/mL vs < 14.3 ng/mL, range | 1.00 (ref), 0.87, 0.40, 0.38 (0.18-0.80) |
| Yamaji, 2012 | Japan (M,W) | Cross-sectional study | 2004-2005 | First adenoma | Colonoscopy | 737/ 703 | 32 ng/mL vs 16 ng/mL, median | 1.00 (ref), 0.86, 0.91, 1.03, 0.64 (0.45-0.92) | |
| Jacobs, 2013 | United States (M, W) | Prospective study | 1990-1999 | Recurrent adenoma | Colonoscopy | 942/ 1132 | > 30 ng/mL vs < 20 ng/mL, range | 1.00 (ref), 0.91, 0.95 (0.73-1.24) | |
| Aigner, 2014 | Austria (W) | Cross-sectional study | 2010-2013 | First adenoma | Colonoscopy | 22.8 ng/mL in women | 90/629 | 0.976 (0.954-0.999) for 1 ng/mL increment | |
| Choi, 2015 (our study) | Korea (M, W) | Case-control study | 2011-2012 | First or recurrent(5%) adenoma | Colonoscopy | 15.7 ng/mL in cases and 16.6 in controls | 112/112 | 23.4 ng/mL vs 10.0 ng/mL, mean | 1.00 (ref), 0.72, 0.73, 0.49 (0.19-1.27) |
When we conducted a meta-analysis of all the studies, we found an inverse association between the 25(OH)D levels and colorectal adenoma; the combined RR (95%CI) was 0.68 (0.54-0.82) when comparing the highest category with the lowest category (Figure 2). Because we found heterogeneity across studies (P < 0.001), we omitted one study at a time to examine whether one study influenced the overall results. When we excluded two studies[13,26] from the analysis, the heterogeneity decreased, but the results were similar to those from the analysis where we included all the studies. When we calculated a dose-response relationship, the combined RR (95%CI) was 0.93 (0.89-0.97) for a 10 ng/mL increment in 25(OH)D levels. When we limited our meta-analysis to the studies that included only the first colorectal adenoma, the combined RR (95%CI) was 0.65 (0.52-0.78) when comparing the highest category with the lowest category. We investigated the association between 25(OH)D levels and colorectal adenoma according to sex, study region, calcium intake, or adenoma site (Table 4). Stratification by study region showed that the ORs (95%CIs) were 0.70 (0.52-0.88) in the US and 0.66 (0.34-0.97) in Asia. The associations did not vary by these factors.
Figure 2.
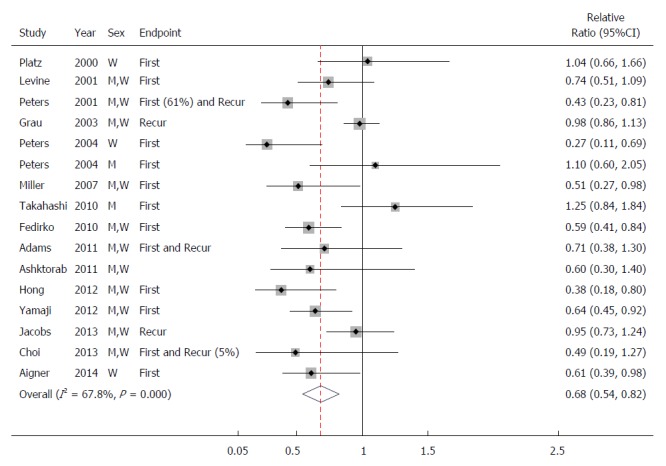
Study-specific and combined odd ratios and 95%CIs of colorectal adenoma comparing the highest category with the lowest category of circulating 25-hydroxyvitamin D levels. First: First adenoma as an endpoint; Recur: Recurrent adenoma as an endpoint.
Table 4.
Combined relative risk (RR)s and 95% confidence intervals of colorectal adenoma for the associations by sex, calcium intake, study region, and adenoma site
| Studies, n | Ref. | Combined RR (95%CI) comparing the highest vs the lowest categories | P for difference | |
| Sex | 0.30 | |||
| Men | 6 | [13-15,20,27] and our study | 0.78 (0.51-1.06) | |
| Women | 7 | [14,15,20,23,27,37] and our study | 0.58 (0.33-0.83) | |
| Calcium intake | 0.48 | |||
| Low | 7 | [14,19,24-26,28] and our study | 0.73 (0.46-1.01) | |
| High | 7 | [14,19,24-26,28] and our study | 0.61 (0.40-0.82) | |
| Study region | 0.88 | |||
| United States | 10 | [19,21,23-30] | 0.70 (0.52-0.88) | |
| Asia | 4 | [13-15] and our study | 0.66 (0.35-0.98) | |
| Site | 0.50 | |||
| Distal adenoma | 10 | [13-15,21,23-25,27,28] and our study | 0.67 (0.53-0.81) | |
| Proximal adenoma | 8 | [13-15,21,25,28,37] and our study | 0.61 (0.44-0.79) |
DISCUSSION
In a case-control study of Korean adults, we found an inverse association between circulating serum 25(OH)D levels and colorectal adenoma in women but not in men. When we combined estimates from case-control, cross-sectional or prospective studies in a meta-analysis, higher circulating vitamin D levels were associated with a lower prevalence of colorectal adenoma. The associations were similar across proximal and distal sites. Notably, our meta-analysis showed a significant inverse association between 25(OH)D levels and colorectal adenoma in Asian populations.
We found an inverse association only among women in our case-control study. We cannot rule out the possibility that our finding could be attributed to chance or potential residual confounding factors among men; however, the stronger association among women compared with that in men in our meta-analysis and the stronger, or only significant for women, association in some studies[21,27] warrant further studies.
Although we found an inverse association between circulating vitamin D levels and colorectal adenoma in a previous meta-analysis[16], a limited number of Asian studies did not allow us to explore whether high vitamin D was associated with a lower prevalence of colorectal adenoma in Asian populations along with Western populations. Because more studies have been published and we added our study, we examined the potential benefit of vitamin D against colorectal neoplasia in Asian populations. It needs further prospective studies.
Because vitamin D and calcium are metabolically interrelated[38], we examined whether the association between 25(OH)D levels and colorectal adenoma varied by calcium intake in the meta-analysis. Although we found a stronger association among those with high calcium intake than those with low intake, the difference was not statistically significant.
Experimental studies have shown that 1,25(OH)2D inhibits cellular proliferation, induces differentiation and apoptosis, and inhibits angiogenesis[39,40]. In an in vitro study, colon tumor tissues expressed a lower vitamin D receptor level than did normal tissues, and the tumor with a higher receptor level was more responsive to 1,25(OH)2D[41]. Additionally, the administration of 1,25(OH)2D or vitamin D analogues induced the expression of genes involved in cell differentiation[42,43]. In an in vivo study, vitamin D treatment in Wistar rats reduced the apoptosis in colon tumors[44].
Our study has several strengths and limitations. We collected blood samples only during the winter season; therefore, individual seasonal variations should not contribute to our findings. We performed a comprehensive meta-analysis to combine existing evidence and found an inverse association between 25(OH)D levels and colorectal adenoma. The limitations of our case-control study include the small sample size, the single measurement of 25(OH)D levels, and the possibility of the presence of residual confounding factors. Also, because our study participants provide blood samples after colonoscopy, vitamin D levels could have been changed if participants altered their lifestyle such as outdoor activities and dairy food intake. Especially, if vitamin D levels in participants with adenoma increased, the association would have been attenuated toward no association. However, an inverse association observed in a meta-analysis may suggest that an inverse association in women in our case-control study may not be a seriously biased result. We cannot rule out the possibility that no association in men could be partly due to the limitations of retrospective nature in our study.
In summary, the results from our case-control study and meta-analysis showed that circulating 25(OH)D levels are inversely associated with the prevalence of colorectal adenoma in both Western and Asian populations.
COMMENTS
Background
Low sunlight exposure, partly due to sedentary lifestyles and lack of outdoor activities, has been suggested to contribute to colorectal cancer development, and the role of vitamin D in colorectal cancer prevention has drawn increasing attention.
Research frontiers
The authors conducted a case-control study in a Korean population and calculated a summary estimate through a meta-analysis to examine the association between circulating 25-hydroxyvitamin D [25(OH)D] levels and colorectal adenoma.
Innovations and breakthroughs
This study suggest an inverse association between circulating 25(OH)D levels and colorectal adenoma in both Western and Asian populations.
Applications
This study suggests that vitamin D may exhibit a protective effect against the early stages of colorectal neoplasia.
Terminology
25(OH)D is hydroxylated in the kidney to form 1,25-dihydroxyvitamin D. 1,25(OH)2D, a biologically active form of vitamin D, is responsible for most biologic functions. Circulating concentration of 25(OH)D is known to be a good reflection of exposure to sunlight and dietary intake of vitamin D.
Peer-review
This is a well written manuscript in writing and structure. The authors performed a case-control study among Korean adults determining the association between colorectal adenoma and 25(OH)D levels, moreover they systematically summarized the studies that have been performed previously.
Footnotes
Supported by Grants from Basic Science Research Program through the National Research Foundation of Korea, which is funded by the Korean Government, No. NRF-2011-0011028; and the Sookmyung Women’s University Research Grants, No. 1-1503-0168.
Conflict-of-interest statement: There is no conflict of interest to declare.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 2, 2015
First decision: April 13, 2015
Article in press: June 10, 2015
P- Reviewer: Bordonaro M, Zhou X S- Editor: Yu J L- Editor: O’Neill M E- Editor: Liu XM
References
- 1.Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington: DC; 1997. pp. 250–287. [PubMed] [Google Scholar]
- 2.Arabi A, El Rassi R, El-Hajj Fuleihan G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat Rev Endocrinol. 2010;6:550–561. doi: 10.1038/nrendo.2010.146. [DOI] [PubMed] [Google Scholar]
- 3.Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 4.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 5.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 6.Lee JE, Li H, Chan AT, Hollis BW, Lee IM, Stampfer MJ, Wu K, Giovannucci E, Ma J. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res (Phila) 2011;4:735–743. doi: 10.1158/1940-6207.CAPR-10-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E. Epidemiology of vitamin D and colorectal cancer: casual or causal link? J Steroid Biochem Mol Biol. 2010;121:349–354. doi: 10.1016/j.jsbmb.2010.03.085. [DOI] [PubMed] [Google Scholar]
- 8.Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952:73–87. doi: 10.1111/j.1749-6632.2001.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 9.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, Lim J, Kye S, Joung H. Association between vitamin D status and metabolic syndrome risk among Korean population: based on the Korean National Health and Nutrition Examination Survey IV-2, 2008. Diabetes Res Clin Pract. 2012;96:230–236. doi: 10.1016/j.diabres.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, Li X, Yang X, Chen Y, Lin X. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Tsugawa N, Saito T, Ishikawa M, Tsuchiya Y, Hyodo K, Maruyama K, Oshiki R, Kobayashi R, Nashimoto M, et al. Vitamin D status, bone mass, and bone metabolism in home-dwelling postmenopausal Japanese women: Yokogoshi Study. Bone. 2008;42:271–277. doi: 10.1016/j.bone.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi R, Mizoue T, Otake T, Fukumoto J, Tajima O, Tabata S, Abe H, Ohnaka K, Kono S. Circulating vitamin D and colorectal adenomas in Japanese men. Cancer Sci. 2010;101:1695–1700. doi: 10.1111/j.1349-7006.2010.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaji T, Iwasaki M, Sasazuki S, Sakamoto H, Yoshida T, Tsugane S. Association between plasma 25-hydroxyvitamin D and colorectal adenoma according to dietary calcium intake and vitamin D receptor polymorphism. Am J Epidemiol. 2012;175:236–244. doi: 10.1093/aje/kwr295. [DOI] [PubMed] [Google Scholar]
- 15.Hong SN, Kim JH, Choe WH, Lee SY, Seol DC, Moon HW, Hur M, Yun YM, Sung IK, Park HS, et al. Circulating vitamin D and colorectal adenoma in asymptomatic average-risk individuals who underwent first screening colonoscopy: a case-control study. Dig Dis Sci. 2012;57:753–763. doi: 10.1007/s10620-011-1926-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee JE. Circulating levels of vitamin D, vitamin D receptor polymorphisms, and colorectal adenoma: a meta-analysis. Nutr Res Pract. 2011;5:464–470. doi: 10.4162/nrp.2011.5.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 18.Miller EA, Keku TO, Satia JA, Martin CF, Galanko JA, Sandler RS. Calcium, vitamin D, and apoptosis in the rectal epithelium. Cancer Epidemiol Biomarkers Prev. 2005;14:525–528. doi: 10.1158/1055-9965.EPI-04-0466. [DOI] [PubMed] [Google Scholar]
- 19.Miller EA, Keku TO, Satia JA, Martin CF, Galanko JA, Sandler RS. Calcium, dietary, and lifestyle factors in the prevention of colorectal adenomas. Cancer. 2007;109:510–517. doi: 10.1002/cncr.22453. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs ET, Hibler EA, Lance P, Sardo CL, Jurutka PW. Association between circulating concentrations of 25(OH)D and colorectal adenoma: a pooled analysis. Int J Cancer. 2013;133:2980–2988. doi: 10.1002/ijc.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs ET, Alberts DS, Benuzillo J, Hollis BW, Thompson PA, Martínez ME. Serum 25(OH)D levels, dietary intake of vitamin D, and colorectal adenoma recurrence. J Steroid Biochem Mol Biol. 2007;103:752–756. doi: 10.1016/j.jsbmb.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-aottawa Scle (NOS) for assessing the quality of nonrandomised sutdies in meta-analysis, Dept of Epidemiology and Community Medicine, University of Ottawa: Ottawa, Canada. (accessed 10 February 2011) Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23.Platz EA, Hankinson SE, Hollis BW, Colditz GA, Hunter DJ, Speizer FE, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and adenomatous polyps of the distal colorectum. Cancer Epidemiol Biomarkers Prev. 2000;9:1059–1065. [PubMed] [Google Scholar]
- 24.Levine AJ, Harper JM, Ervin CM, Chen YH, Harmon E, Xue S, Lee ER, Frankel HD, Haile RW. Serum 25-hydroxyvitamin D, dietary calcium intake, and distal colorectal adenoma risk. Nutr Cancer. 2001;39:35–41. doi: 10.1207/S15327914nc391_5. [DOI] [PubMed] [Google Scholar]
- 25.Peters U, McGlynn KA, Chatterjee N, Gunter E, Garcia-Closas M, Rothman N, Sinha R. Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10:1267–1274. [PubMed] [Google Scholar]
- 26.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, Heber D. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 27.Peters U, Hayes RB, Chatterjee N, Shao W, Schoen RE, Pinsky P, Hollis BW, McGlynn KA. Circulating vitamin D metabolites, polymorphism in vitamin D receptor, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2004;13:546–552. [PubMed] [Google Scholar]
- 28.Fedirko V, Bostick RM, Goodman M, Flanders WD, Gross MD. Blood 25-hydroxyvitamin D3 concentrations and incident sporadic colorectal adenoma risk: a pooled case-control study. Am J Epidemiol. 2010;172:489–500. doi: 10.1093/aje/kwq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams SV, Newcomb PA, Burnett-Hartman AN, White E, Mandelson MT, Potter JD. Circulating 25-hydroxyvitamin-D and risk of colorectal adenomas and hyperplastic polyps. Nutr Cancer. 2011;63:319–326. doi: 10.1080/01635581.2011.535960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashktorab H, Nguza B, Fatemi M, Nouraie M, Smoot DT, Schäffer AA, Kupfer SS, Camargo CA, Brim H. Case-control study of vitamin D, dickkopf homolog 1 (DKK1) gene methylation, VDR gene polymorphism and the risk of colon adenoma in African Americans. PLoS One. 2011;6:e25314. doi: 10.1371/journal.pone.0025314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng XE, Lipka S, Li T, Shahzad G, Levine E, Vlacancich R, Takeshige U, Mustacchia P. The relationship of vitamin D status, smoking, and colorectal adenoma: a retrospective study in an ethnically diverse community. J Steroid Biochem Mol Biol. 2013;136:280–283. doi: 10.1016/j.jsbmb.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 35.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. STATA J. 2006;6:40. [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aigner E, Stadlmayr A, Huber-Schönauer U, Zwerina J, Husar-Memmer E, Niederseer D, Trauner M, Heuberger A, Hohla F, Schett G, et al. Gender- and site-specific differences of colorectal neoplasia relate to vitamin D. Aliment Pharmacol Ther. 2014;40:1341–1348. doi: 10.1111/apt.12981. [DOI] [PubMed] [Google Scholar]
- 38.Heaney RP. Vitamin D and calcium interactions: functional outcomes. Am J Clin Nutr. 2008;88:541S–544S. doi: 10.1093/ajcn/88.2.541S. [DOI] [PubMed] [Google Scholar]
- 39.Holt PR, Arber N, Halmos B, Forde K, Kissileff H, McGlynn KA, Moss SF, Kurihara N, Fan K, Yang K, et al. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev. 2002;11:113–119. [PubMed] [Google Scholar]
- 40.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 41.Shabahang M, Buras RR, Davoodi F, Schumaker LM, Nauta RJ, Evans SR. 1,25-Dihydroxyvitamin D3 receptor as a marker of human colon carcinoma cell line differentiation and growth inhibition. Cancer Res. 1993;53:3712–3718. [PubMed] [Google Scholar]
- 42.Chen A, Davis BH, Bissonnette M, Scaglione-Sewell B, Brasitus TA. 1,25-Dihydroxyvitamin D(3) stimulates activator protein-1-dependent Caco-2 cell differentiation. J Biol Chem. 1999;274:35505–35513. doi: 10.1074/jbc.274.50.35505. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Garcia NI, Palmer HG, Garcia M, Gonzalez-Martin A, del Rio M, Barettino D, Volpert O, Muñoz A, Jimenez B. 1alpha,25-Dihydroxyvitamin D3 regulates the expression of Id1 and Id2 genes and the angiogenic phenotype of human colon carcinoma cells. Oncogene. 2005;24:6533–6544. doi: 10.1038/sj.onc.1208801. [DOI] [PubMed] [Google Scholar]
- 44.Iseki K, Tatsuta M, Uehara H, Iishi H, Yano H, Sakai N, Ishiguro S. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer. 1999;81:730–733. doi: 10.1002/(sici)1097-0215(19990531)81:5<730::aid-ijc11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]


