Abstract
AIM: To elucidate the prevalence and risk factors for gallstones, primarily focusing on Helicobacter pylori (H. pylori) infection.
METHODS: A total of 10016 Chinese subjects, who had undergone physical examination, fasting 13C urea breath test and abdominal ultrasonography, had sufficient blood test data, and had finished a questionnaire, were included in this cross-sectional study. Participants (n = 1122) who had previous eradication of H. pylori were studied separately.
RESULTS: Gallstones were discovered in 9.10% of men and 8.58% of women, with no significant sex difference. Multivariate analyses displayed that age, aspartate aminotransferase, total cholesterol, H. pylori infection, hepatitis C virus (HCV) infection, and fatty liver had a significant association with gallstones (P < 0.05). Successive multiple logistic regression analysis including index of odds ratio (OR) and standardized coefficient (β) indicated that older age (OR/β = 1.056/0.055), H. pylori infection (OR/β = 1.454/0.109), HCV infection (OR/β = 1.871/0.123), and fatty liver (OR/β = 1.947/0.189) had a significant positive association with gallstones. After age stratification, H. pylori infection and fatty liver still had a significant positive association with gallstones in any age-specific groups, whereas HCV infection had a significant positive association in patients aged > 40 years. The prevalence of gallstones among H. pylori-positive, H. pylori-eradicated, and H. pylori-negative subjects was 9.47%, 9.02%, and 8.46%, respectively. The matched analysis showed that gallstones among H. pylori eradicated subjects was significantly lower compared with H. pylori-positive subjects (P < 0.05).
CONCLUSION: H. pylori infection and fatty liver have a significant positive association with gallstones. H. pylori eradication may lead to prevention of gallstones.
Keywords: Gallstones, Helicobacter pylori, Cross-sectional study
Core tip: Although the pathogenesis of gallstones remains obscure, chronic infection is already accepted as a potential risk factor. There are few large surveys analyzing background factors related to gallstones in Asia. Our study evaluated background factors associated with the presence of gallstones in a cohort of > 10000 subjects, and analysis focusing on the association between Helicobacter pylori (H. pylori) infection and gallstones in the Chinese population is the most important feature of our study. In this large survey, we found that H. pylori eradication may lead to prevention of gallstones, which should shed light on the pathophysiology of gallstones.
INTRODUCTION
Gallstones are one of the most prevalent digestive disorders requiring inpatient treatment, as well as a major public health concern worldwide[1]. The prevalence of gallstones in western countries is > 10%[2-4], but in China, they have been rarely reported. The etiology and pathogenesis of gallstones remains obscure. Gallstone formation may be associated with a complex interaction of genetic and environmental factors such as female sex, family history, and ethnicity[5-7]. Lifestyle and some other metabolic disorders also affect gallstone formation, for example, high alcohol consumption, hyperlipidemia, fatty liver, and obesity[1,8-13]. It has also been reported that internal disorders such as hepatitis C virus (HCV) infection, gallbladder polyps and several liver enzymes [aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and γ-glutamyltransferase (GTP)] display a significant association with gallstones[14-17]. In addition, the effect of the gastroduodenal environment is thought to play an important role in the presence of gallstones, and Helicobacter pylori are believed to be a mediating factor for gastric and extragastric disease. The gallbladder and bile duct may be two of the targets of chronic H. pylori infection. Therefore, we conducted a cross-sectional study to clarify the prevalence and background factors for gallstone formation and investigate the correlation between H. pylori infection and gallstones in an attempt to understand the pathogenesis of gallstones and to develop better therapeutic and preventive strategies for this disease.
MATERIALS AND METHODS
Study design and subjects
Subjects were labor union members older than 20 years and retired staff who voluntarily took part in the health examination that included abdominal ultrasonography, fasting 13C urea breath test (13C-UBT), and laboratory data at the International Health Care Center, The First Affiliated Hospital, Zhejiang University School of Medicine, from January 2010 to January 2014. All participants were informed verbally about the purpose and design of the study, and the procedures were approved by the Ethics Committee of Zhejiang University School of Medicine. Those who took proton pump inhibitors, antidiabetic drugs and anti-cholesterol drugs regularly, with a history of cholecystectomy or gastrectomy, were excluded. Participants who had H. pylori eradication previously were studied separately.
Physical examination, laboratory assessments and questionnaire
All subjects were instructed to fast overnight and peripheral venous blood samples were collected in the next morning. Laboratory tests, such as serum levels of AST, ALT, ALP, γ-GTP, total bilirubin (T-Bil), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride, total cholesterol (TC), total protein (TP), and hemoglobin concentration, were analyzed. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of the body height in meters. A questionnaire regarding gastrointestinal symptoms, medical history, lifestyle, and family history was given to all the subjects. Finally, there were questions regarding regular intake of proton pump inhibitors and antidiabetic and anti-cholesterol drugs, and history of H. pylori eradication, cholecystectomy or gastrectomy. We categorized smoking and alcohol intake into two groups: nonsmoker vs smoker (current or past smoking habit), and higher alcohol intake (often, always) vs lower alcohol intake (never, sometimes). HCV infection was defined as positivity of antibodies to HCV (anti-HCV) and without alcohol consumption. The diagnosis of H. pylori infection was based on the result of fasting 13C-UBT, and for those who had H. pylori eradication history, fasting 13C-UBT was applied at > 1 mo after completion of the standard H. pylori eradication therapy. Reference value ranges of all the tested indexes were according to the biochemical criteria of the Department of Clinical Laboratory, The First Affiliated Hospital, Zhejiang University School of Medicine.
Diagnosis of gallstones, fatty liver and gallbladder polyps
Gallstones in the gallbladder and bile duct were diagnosed by abdominal ultrasound. They were diagnosed by the presence of highly reflective echoes from the anterior surface of the stones or movement upon postural change, with or without marked posterior acoustic shadowing. Fatty liver was diagnosed by the following characteristic findings: diffuse increase in hepatic echogenicity with evident contrast between the liver and the kidney; diffuse increase in hepatic echogenicity with blurring of the intrahepatic vessels and the diaphragm; or brightness of the hepatic echogenicity with poor penetration of the posterior hepatic segments, and invisibility of the intrahepatic vessels or diaphragm. The diagnostic criterion for gallbladder polyps was an immobile echo protruding from the gallbladder wall into the lumen, without an acoustic shadow.
Statistical analysis
Statistical analyses were performed with SPSS version 17.0 (SPSS, Chicago, IL, United States). In univariate analyses, continuous data for different groups were presented as mean ± SD, and were compared using Wilcoxon’s rank-sum test. Categorical variables were compared with the Pearson’s χ2 test. Logistic regression analyses were used to evaluate the odds ratio (OR) and 95% confidence interval (CI) for gallstones using the related covariates. Standardized coefficient of each variable was calculated using multiple logistic regression analysis, which was applied again after age stratification. Cochran-Armitage test was done in order to determine the effect of H. pylori eradication on gallstone prevalence, Finally, a matched pair analysis was performed between the H. pylori-positive participants and H. pylori-eradicated subjects using McNemar’s test with matching criteria of age (± 3), TC (± 1), AST (± 1), condition of fatty liver or anti-HCV.
RESULTS
Subject characteristics
A total of 15523 subjects were enrolled in this study, and we excluded those who did not meet the inclusion criteria. All participants with a history of H. pylori eradication were evaluated separately. Thus, 10016 subjects including 4352 men and 5664 women with a mean age of 56.38 ± 14.73 years comprised the primary population (Figure 1). Gallstones were diagnosed in 882 participants (8.81%): 396 men (9.10%) and 486 women (8.58%), with a mean age of 50.50 ± 12.19 years. For the 1122 subjects with a history of H. pylori eradication, 124 were still positive for H. pylori infection and the other 998 were negative and classified as the H. pylori-eradicated group (Figure 1).
Figure 1.
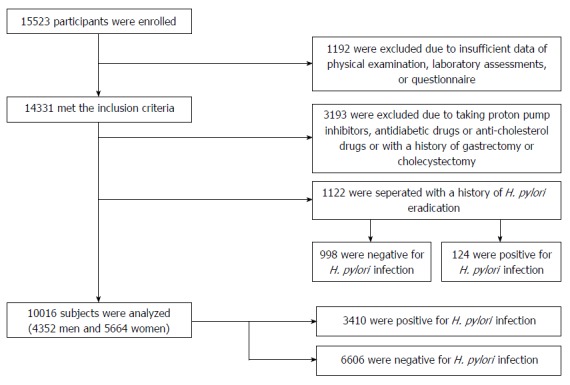
Recruitment flowchart of the study. Healthy adults (n = 15523) attended, and 10016 were analyzed in this study. Subjects who had H. pylori eradication previously (n = 1122) were analyzed separately. H. pylori: Helicobacter pylori.
Univariate and multivariate analysis evaluating background factors for gallstones
Association of the 13 continuous and seven categorized variables with the prevalence of gallstones was analyzed univariately (Table 1). H. pylori infection, anti-HCV, fatty liver, age, BMI, smoking, alcohol intake, γ-GTP, TP, ALP, AST, T-Bil, TC, triglyceride, and LDL-C showed significant association with gallstones (P < 0.05), whereas sex, ALT, HDL-C, hemoglobin and gallbladder polyps did not. Expect for sex, all factors were excluded from the performance of multivariate analysis (Table 2), and six demonstrated a significant association with the presence of gallstones. We selected these factors for multiple logistic regression and calculated OR and standardized coefficients (β) (Table 2). Older age (OR/β = 1.056/0.055), H. pylori infection (OR/β = 1.454/0.109), anti-HCV (OR/β = 1.871/0.123), and fatty liver (OR/β = 1.947/0.189) had a significant positive association with gallstones. After age stratification, only H. pylori infection, fatty liver and anti-HCV still had a significant positive association with gallstones in any age-specific groups.
Table 1.
Characteristics of the 20 variables of the 10016 subjects with or without gallstone
| Variables | Presence of gallstone (n = 882) | Absence of gallstone (n = 9134) | P value |
| Age (yr) | 56.38 ± 14.72 | 46.98 ± 11.56 | < 0.001a |
| BMI (kg/m2) | 24.82 ± 3.01 | 23.77 ± 3.20 | < 0.001a |
| ALT (U/L) | 25.58 ± 19.67 | 25.87 ± 31.52 | 0.386 |
| AST (U/L) | 24.14 ± 14.30 | 24.48 ± 24.43 | 0.001 |
| ALP (U/L) | 67.61 ± 20.02 | 63.24 ± 17.75 | < 0.001a |
| γ-GTP (U/L) | 36.52 ± 42.77 | 25.26 ± 36.02 | < 0.001a |
| TC (mmol/L) | 4.93 ± 1.07 | 4.70 ± 1.03 | < 0.001a |
| TG (mmol/L) | 1.80 ± 3.93 | 1.52 ± 1.25 | < 0.001a |
| TP (g/L) | 73.37 ± 4.39 | 71.77 ± 5.17 | < 0.001a |
| T-Bil (umol/L) | 13.63 ± 6.67 | 12.44 ± 5.79 | < 0.001a |
| HDL-Chol (mmol/L) | 1.18 ± 0.37 | 1.31 ± 2.41 | 0.091 |
| LDL-Chol (mmol/L) | 2.85 ± 6.65 | 2.60 ± 2.04 | 0.012 |
| Hb (g/L) | 144.81 ± 18.11 | 148.77 ± 18.30 | 0.721 |
| H. pylori, n (%) | < 0.001a | ||
| Positive | 323 (9.47) | 3087 (90.53) | |
| Negative | 559 (8.46) | 6047 (91.54) | |
| Alcohol intake | < 0.001a | ||
| Yes | 545 (10.88) | 4463 (89.12) | |
| No | 337 (55.89) | 4671 (44.11) | |
| Smoking | < 0.001a | ||
| Yes | 475 (13.59) | 3021 (86.41) | |
| No | 407 (6.24) | 6113 (93.76) | |
| Gender | 0.095 | ||
| Men | 396 (9.10) | 3956 (90.90) | |
| Women | 486 (8.58) | 5178 (91.42) | |
| Anti-HCV | |||
| Positive | 526 (11.98) | 3865 (88.02) | 0.001a |
| Negtive | 356 (6.33) | 5269 (93.67) | |
| Fatty liver | |||
| Presence | 572 (13.05) | 3811 (86.95) | < 0.001a |
| Absence | 310 (5.50) | 5323 (94.50) | |
| GB polyp | |||
| Presence | 402 (10.71) | 3350 (89.29) | 0.197 |
| Absence | 480 (7.67) | 5784 (92.33) | |
| Total | 882 (8.51) | 9134 (92.49) | |
Data were expressed as mean ± SD of each variable. By applying the Wilcoxon analysis, P values of the 13 continuous variables were calculated. By applying Pearson’s χ2 test, P values of the seven categorized variables were calculated.
P < 0.05, Presence vs Absence. BMI: Body mass index; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; γ-GTP: Gamma glutamyltransferase; TC: Cholesterin; TG: Triglyceride; TP: Total protein serum; T-Bil: Total bilirubin; HDL-Chol: High density lipoprotein cholesterol; LDL-Chol: Low density lipoprotein cholesterol; Hb: Hemoglobin concentration; H. Pylori: Helicobacter pylori; Anti-HCV: Antibodies to HCV; GB polyp: Gallbladder polyp.
Table 2.
Multivariate analysis and mutiple logistic analysis of the correlated variables for gallstone
| Variables |
Multivariate analysis |
Mutiple logistic analysis |
||||
| β | P value | OR (95%CI) | β | P value | OR (95%CI) | |
| Age | 0.056 | 0.009a | 1.057 (1.045-1.070) | 0.055 | < 0.001a | 1.056 (1.043-1.070) |
| Gender (female) | -1.048 | < 0.001a | 0.351 (0.265-0.770) | |||
| BMI | -0.050 | 0.105 | 0.951 (0.845-1.077) | |||
| ALP | -0.001 | 0.610 | 0.999 (0.839-1.152) | |||
| AST | 0.712 | < 0.001a | 1.048 (1.045-1.092) | |||
| γ-GT | 0.002 | 0.260 | 1.002 (1.045-1.070) | |||
| TC | 0.017 | 0.006a | 1.018 (1.006-1.029) | |||
| TG | -0.113 | 0.007a | 0.893 (0.595-0.982) | |||
| T-Bil | -0.044 | < 0.001a | 0.957 (0.856-1.081) | |||
| LDL-Chol | 0.001 | 0.946 | 1.001 (0.901-1.023) | |||
| TP | -0.108 | < 0.001a | 0.898 (0.601-0.994) | |||
| Helicobacter pylori positive | 0.588 | < 0.001a | 1.800 (1.386-2.337) | 0.109 | < 0.001a | 1.454 (1.102-2.521) |
| Anti-HCV | 1.320 | < 0.001a | 3.742 (1.426-5.217) | 0.123 | < 0.001a | 1.871 (1.441-3.681) |
| Smoking | -0.741 | < 0.001a | 0.477 (0.295-0.692) | |||
| Alcohol intake | -0.124 | 0.037 | 0.883 (0.421-0.911) | |||
| Fatty liver | 1.339 | < 0.001a | 3.814 (1.886-6.023) | 0.189 | < 0.001a | 1.947 (1.212-3.987) |
By applying logistic regression analysis, P values were calculated.
P < 0.05. β: Partial regression coefficient; SE: Standard error of partial regression coefficient.
Effect of H. pylori eradication on gallstone formation
From the multivariate analyses, we found that H. pylori infection had a significant positive association with gallstones (Tables 2 and 3); a hypothesis that H. pylori eradication might reduce the prevalence of gallstones. Therefore, we compared the above-mentioned 998 subjects who had already undergone successful H. pylori eradication therapy to 3410 participants positive for H. pylori infection and 6606 negative for H. pylori infection (Figure 1). As shown in Table 4, the prevalence of gallstones was 9.47% in the H. pylori-positive subjects (without a history of eradication therapy), 9.02% in the H. pylori-eradicated subjects, and 8.46% in the H. pylori-negative subjects (without a history of eradication therapy). There was a significant reduction in prevalence of gallstones among subjects with a history of H. pylori eradication using the Cochran-Armitage test (P < 0.0001). Using the five factors that were significantly associated with the presence of gallstones from multivariate analysis (Table 2), we compared gallstone prevalence between H. pylori-positive participants and H. pylori-eradicated subjects by matched-pair analyses. Based on age (± 3), TC (± 1), AST (± 1), fatty liver and anti-HCV, the 552 H. pylori-positive participants and 552 H. pylori-eradicated participants were matched. The prevalence of gallstones was 6.52% and 5.07%, respectively, with a significant difference by McNemar’s test (P = 0.027).
Table 3.
Positively correlated variables for gallstone formation stratified by age
| Variables |
Age (20-39 yr) |
Age (40-64 yr) |
Age (≥ 65 yr) |
|||
| OR | P value | OR | P value | OR | P value | |
| Gender (female) | 0.658 | 0.054 | 0.905 | 0.062 | 0.714 | 0.038a |
| Helicobacter pylori positive | 1.521 | 0.006a | 1.510 | 0.015a | 1.324 | < 0.001a |
| Anti-HCV | 0.988 | 0.004a | 1.598 | 0.019a | 1.563 | 0.002a |
| Fatty liver | 1.582 | 0.003a | 1.922 | < 0.001a | 1.752 | < 0.001a |
| TC | 1.025 | 0.921 | 1.520 | 0.102 | 0.914 | 0.023a |
| AST | 0.382 | 0.221 | 1.249 | 0.024 | 1.547 | < 0.001a |
P values calculated using muitiple logistic regression analysis.
P < 0.05.
Table 4.
Prevalence of gallstone in Helicobacter pylori-positive, -eradicated, and -negative subjects n (%)
| Presence of gallstones | Absence of gallstones | |
| Helicobacter pylori positive (without eradication) | 323 (9.47) | 3087 (90.53) |
| Helicobacter pylori negative after eradication | 90 (9.02) | 908 (90.98) |
| Helicobacter pylori negative (without eradication) | 559 (8.46) | 6047 (91.54) |
Cochran–Armitage test, P < 0.0001, Presence vs Absence.
DISCUSSION
The prevalence of gallstone shows regional variation with higher rates in western countries and lower rates in Asian countries. It was reported that the prevalence of gallstone was 7.9% in men and 16.6% in women in the US, and 29.5% in men and 64.1% in female American Indians[7,18]. In Asian countries, prevalence of gallstones was reported as 6.6% in Singapore, and 5.4% in Thailand[19,20]. Our study found that gallstones accounted for 9.10% of men and 8.58% of women among the 10016 Chinese subjects included (Table 1). Most of the subjects enrolled were white-collar workers or retired staff; they were relatively old, living in good conditions with little movement, and had a high-fat, high-calorie diet and many had asymptomatic gallstones, so they participated in a medical review on a regular basis. All of these factors led to an increase in the incidence of gallstones in our study compared with other regions of China. We believe that the results of health examination may be more representative of the true prevalence of gallstones in the general population of China than hospital-based studies, even autopsy studies, because hospital studies have a relatively limited patient sample and fail to show the true prevalence of gallstones in the general population.
Female gender showed no association with the formation of gallstones, whereas previous studies reported that it had a significant correlation with cholesterol stones, especially in western countries[7,10,21]. Metabolic disorders, such as obesity, diabetes mellitus, or dyslipidemia are common and accepted as risk factors for cholesterol stones[2,3,8,22,23]. Estrogen can increase the cholesterol saturation in bile, which leads to a female predominance of gallstones in western countries. However, in Asian countries such as China, a relative higher proportion of pigment stones are probably observed[24], so the effect of gender distribution may be diminished. In addition, as a result of the national policy of family planning in China since 1982, women were allowed to give birth to only one child and underwent tubal ligation after their first delivery. This led to a reduction in risk factors for gallstone formation, such as being productive and taking oral contraceptives[23,25,26]. Recently, an epidemiological survey from Taiwan showed no gender predominance in gallstone formation but a close association between use of oral contraceptives and gallstones[13]. Therefore, gallstones are mainly attributed to oral contraception rather than gender alone.
Cholesterol stones are the predominant type of gallstones[1,6] and obese people tends to have cholesterol-supersaturated bile and larger gallbladder volume, thus, dyslipidemia in obese people is a likely cause of gallstones. We discovered that high TC level appears to be related to gallstones in our multivariate analysis, but the association was not significant in multiple logistic regression analysis (Table 2). Moreover, many studies could not confirm the relationship between dyslipidemia and the presence of gallstones[13,27]. Dyslipidemia may contribute to the formation of gallstones, especially cholesterol stones, but the relationship between dyslipidemia and gallstones remains inconclusive[28]. It is reported that the mechanism and risk factors for gallstones differ among stone types. Thus, future studies should focus on the different types of gallstones.
Many reports have confirmed that BMI is an important risk factor for gallstones[7,29], but an inverse relationship between BMI and gallstones was shown in our analysis. BMI may help to establish whether a person is fat or thin, but it does not make clear a person’s fat percentage. The formation of gallstones may be more related to abdominal circumference, so other indicators, such as waist-to-hip ratio may be more meaningful[30].
It has been shown that HCV RNA can colonize gallbladder cells, impair gallbladder epithelium lipid absorption, and alter gallbladder mucosal function[31-33]. Moreover, HCV binds to apolipoprotein A1 and leads to liver steatosis and chronic hepatitis[34]. All of these factors contribute to gallstone formation. Fatty liver is related to increasing prevalence of gallstones due to insulin resistance and visceral obesity[12,13,35]. We found that HCV infection and fatty liver had a strong association with gallstones.
Age plays an important role in the prevalence of H. pylori infection, and was also a significant factor influencing the presence of gallstones in our study. In order to avoid selection bias of age, we evaluated the association between gallstones and H. pylori infection by age stratification. After age stratification, H. pylori infection and fatty liver still had a significant positive association with gallstones in age-specific groups, whereas HCV infection had a strong association in those aged > 40 years.
H. pylori can cause many gastroduodenal diseases such as atrophic gastritis, peptic ulcer, gastric carcinoma and mucosa-associated lymphoid tissue lymphoma[36,37]. Recently, it has been reported that H. pylori is also associated with extragastric diseases, such as thyroid nodules, metabolic syndrome, nonalcoholic fatty liver disease, insulin resistance and autoimmune diseases[38-41]. DNA, RNA and antigens specific for H. pylori were repeatedly detected in bile, biliary tract tissue and stone specimens[42,43]. Hence, a hypothesis that Helicobacter species are etiological agents in gallstone formation has been suggested. Our study showed a positive association between H. pylori infection and gallstones in humans by analyzing a large cohort of 10 016 adults (Tables 1 and 2). After age stratification, H. pylori infection was still a risk factor for the prevalence of gallstones (Table 3). Analysis of those who had successful H. pylori eradication also supported that a state of chronic infection promotes gallstone formation (Table 4), as was reported in a recent large-scale survey with a cohort of > 10000 subjects in Japan[44]. Accordingly, we think that H. pylori infection is a risk factor for gallstone formation in humans, although we lack details of the precise mechanism.
There were some limitations to our study. The first limitation was that the sample was not sufficiently representative. Study subjects were those who participated in health screening in the International Health Care Center, The First Affiliated Hospital of Zhejiang University School of Medicine. Most of them were white collar workers or retired staff who were older in age, in good living conditions, with a high-fat, high-calorie diet, and lack of exercise. People of lower economic status were not represented in our study, which could have led to selection bias and an increase in the incidence of gallstones. The second limitation was that a cause-and-effect relationship could not be elucidated due to the inherent limitation of a cross-sectional study. Both H. pylori infection and gallstones are common disorders worldwide, therefore, it is of importance to clarify whether H. pylori eradication can prevent gallstones. The final limitation was the lack of control for potentially confounding risk factors, such as fasting plasma glucose, oral contraceptives, and cirrhosis. We are planning a randomized prospective study to determine the effect of H. pylori eradication on gallstone formation. Most importantly, long-term follow-up of the H. pylori-positive group, H. pylori-eradicated group and H. pylori-negative group (Table 4) should verify the present conclusion that H. pylori infection increases the risk of gallstones and H. pylori eradication may lead to prevention of gallstones. Further studies are also necessary to understand thoroughly the mechanisms mediating this relationship and help to clarify the key point of disease prevention.
ACKNOWLEDGMENTS
The authors are grateful to Cheng-Fu Xu from Department of Gastroenterology, The First Affiliated Hospital, Zhejiang University School of Medicine, for statistical review, Tian-An Jiang from the Department of Ultrasound, The First Affiliated Hospital, Zhejiang University School of Medicine, for helping with the ultrasonographic examination in this study.
COMMENTS
Background
Gallstones are one of the most prevalent digestive disorders and often require surgical management. Although the pathogenesis of gallstones remains obscure, chronic infection is already accepted as a potential risk factor. Helicobacter pylori (H. pylori) is detected in bile, biliary tract tissue and stone specimens, and it has been clearly demonstrated that H. pylori promotes gallstone formation in animal and human research. In recent decades, a few large surveys have been performed, analyzing background factors related to gallstones from Europe, North America and Japan. Thus, it is necessary to conduct a large epidemiological study in China to evaluate background factors associated with the presence of gallstones, especially focusing on H. pylori infection.
Research frontiers
To date, four large-scale studies from Europe, North America and Japan analyzing background factors related to gallstones are well known: MICOL study investigating 29584 individuals (15910 men and 13674 women) from Italy found that increasing age and body mass index and a maternal family history of gallstone disease were the most consistent associations. The third NHANES survey analyzing 14238 Americans (6688 men and 7550 women) revealed that > 20 million persons had gallbladder disease in the US. Ethnic differences in gallbladder disease prevalence differed according to sex and were only partly explained by known risk factors. Swedish Twin Registry studies investigating 43141 or 58402 twin pairs in Sweden showed positive associations between BMI and the development of symptomatic gallbladder disease, high alcohol consumption was associated with a decreased risk against gallbladder disease, tobacco use had no impact on gallbladder disease. Yu Takahashi designed the research of 15551 subjects comprised of 8625 men and 6926 women, displaying that H. pylori infection is positively associated with gallstones. H. pylori eradication may lead to prevention of gallstones.
Innovations and breakthroughs
A recent study detected various Helicobacter species in bile, biliary tract tissue and stone specimens, which indicated the role of H. pylori in the formation of gallstones. There are few large surveys with a cohort of > 10000 subjects worldwide, and such a large study focusing on the relationship between H. pylori infection and gallstone formation has not been performed in China. By analysis of the large cohort of > 10000 adults, this study showed for the first time a positive association between H. pylori infection and presence of gallstones, and found that H. pylori eradication may lead to prevention of gallstones in Chinese people.
Applications
H. pylori infection had a significant positive association with gallstones in any age-specific groups. H. pylori eradication may reduce the prevalence of gallstones. Thus, positive treatment of H. pylori infection may represent a new method of treating or preventing gallstones.
Terminology
H. pylori is a Gram-negative microaerophilic microorganism that can cause many gastroduodenal diseases such as atrophic gastritis, peptic ulcer, gastric carcinoma and mucosa-associated lymphoid tissue lymphoma, as well as extragastric diseases such as thyroid nodules, nonalcoholic fatty liver disease, and autoimmune diseases.
Peer-review
The title is adequate to reveal the purpose of the study. The study was well designed and conducted. The conclusions are in accord with the results. Statistical analysis and conclusions are adequate. Limitations and future prospect are presented.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine.
Informed consent statement: All participants were informed verbally about the purpose and design of the study. Written informed consent was not required due to the observational nature of the study. The personal information of each participant was anonymized at collection or prior to analysis.
Conflict-of-interest statement: The authors declared that there is no competing interests in this study.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at xuguoqi@mail.hz.zj.cn. All participants were informed verbally about the purpose and design of the study. Written informed consent was not required due to the observational nature of the study. The personal information of each participant was anonymized at collection and anonymized prior to analysis and the risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 24, 2014
First decision: February 10, 2015
Article in press: April 28, 2015
P- Reviewer: Arismendi-Morillo G, Eren B S- Editor: Yu J L- Editor: Kerr C E- Editor: Wang CH
References
- 1.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 2.Borch K, Jönsson KA, Zdolsek JM, Halldestam I, Kullman E. Prevalence of gallstone disease in a Swedish population sample. Relations to occupation, childbirth, health status, life style, medications, and blood lipids. Scand J Gastroenterol. 1998;33:1219–1225. doi: 10.1080/00365529850172601. [DOI] [PubMed] [Google Scholar]
- 3.Moro PL, Checkley W, Gilman RH, Lescano G, Bonilla JJ, Silva B, Garcia HH. Gallstone disease in high-altitude Peruvian rural populations. Am J Gastroenterol. 1999;94:153–158. doi: 10.1111/j.1572-0241.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- 4.Salinas G, Velásquez C, Saavedra L, Ramírez E, Angulo H, Tamayo JC, Orellana A, Huivin Z, Valdivia C, Rodríguez W. Prevalence and risk factors for gallstone disease. Surg Laparosc Endosc Percutan Tech. 2004;14:250–253. doi: 10.1097/00129689-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Marschall HU, Katsika D, Rudling M, Einarsson C. The genetic background of gallstone formation: an update. Biochem Biophys Res Commun. 2010;396:58–62. doi: 10.1016/j.bbrc.2010.02.143. [DOI] [PubMed] [Google Scholar]
- 6.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157–169, vii. doi: 10.1016/j.gtc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 8.Buchner AM, Sonnenberg A. Factors influencing the prevalence of gallstones in liver disease: the beneficial and harmful influences of alcohol. Am J Gastroenterol. 2002;97:905–909. doi: 10.1111/j.1572-0241.2002.05607.x. [DOI] [PubMed] [Google Scholar]
- 9.Kratzer W, Kächele V, Mason RA, Muche R, Hay B, Wiesneth M, Hill V, Beckh K, Adler G. Gallstone prevalence in relation to smoking, alcohol, coffee consumption, and nutrition. The Ulm Gallstone Study. Scand J Gastroenterol. 1997;32:953–958. doi: 10.3109/00365529709011208. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 11.Marschall HU, Einarsson C. Gallstone disease. J Intern Med. 2007;261:529–542. doi: 10.1111/j.1365-2796.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 12.Loria P, Lonardo A, Lombardini S, Carulli L, Verrone A, Ganazzi D, Rudilosso A, D’Amico R, Bertolotti M, Carulli N. Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol. 2005;20:1176–1184. doi: 10.1111/j.1440-1746.2005.03924.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen CH, Huang MH, Yang JC, Nien CK, Etheredge GD, Yang CC, Yeh YH, Wu HS, Chou DA, Yueh SK. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol. 2006;21:1737–1743. doi: 10.1111/j.1440-1746.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang MH, Chen TH, Wang SE, Tsai YF, Su CH, Wu CW, Lui WY, Shyr YM. Biochemical predictors for absence of common bile duct stones in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2008;22:1620–1624. doi: 10.1007/s00464-007-9665-2. [DOI] [PubMed] [Google Scholar]
- 15.Chang TS, Lo SK, Shyr HY, Fang JT, Lee WC, Tai DI, Sheen IS, Lin DY, Chu CM, Liaw YF. Hepatitis C virus infection facilitates gallstone formation. J Gastroenterol Hepatol. 2005;20:1416–1421. doi: 10.1111/j.1440-1746.2005.03915.x. [DOI] [PubMed] [Google Scholar]
- 16.Acalovschi M, Buzas C, Radu C, Grigorescu M. Hepatitis C virus infection is a risk factor for gallstone disease: a prospective hospital-based study of patients with chronic viral C hepatitis. J Viral Hepat. 2009;16:860–866. doi: 10.1111/j.1365-2893.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 17.Lim SH, Kim DH, Park MJ, Kim YS, Kim CH, Yim JY, Cho KR, Kim SS, Choi SH, Kim N, et al. Is Metabolic Syndrome One of the Risk Factors for Gallbladder Polyps Found by Ultrasonography during Health Screening? Gut Liver. 2007;1:138–144. doi: 10.5009/gnl.2007.1.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everhart JE, Yeh F, Lee ET, Hill MC, Fabsitz R, Howard BV, Welty TK. Prevalence of gallbladder disease in American Indian populations: findings from the Strong Heart Study. Hepatology. 2002;35:1507–1512. doi: 10.1053/jhep.2002.33336. [DOI] [PubMed] [Google Scholar]
- 19.Hwang WS. Cholelithiasis in Singapore. I. A necropsy study. Gut. 1970;11:141–148. doi: 10.1136/gut.11.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stitnimankarn T. The necropsy incidence of gallstones in Thailand. Am J Med Sci. 1960;240:349–352. doi: 10.1097/00000441-196009000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Chapman BA, Frampton CM, Wilson IR, Chisholm RJ, Allan RB, Burt MJ. Gallstone prevalence in Christchurch: risk factors and clinical significance. N Z Med J. 2000;113:46–48. [PubMed] [Google Scholar]
- 22.Jensen KH, Jørgensen T. Incidence of gallstones in a Danish population. Gastroenterology. 1991;100:790–794. doi: 10.1016/0016-5085(91)80027-7. [DOI] [PubMed] [Google Scholar]
- 23.Moro PL, Checkley W, Gilman RH, Cabrera L, Lescano AG, Bonilla JJ, Silva B. Gallstone disease in Peruvian coastal natives and highland migrants. Gut. 2000;46:569–573. doi: 10.1136/gut.46.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho KJ, Lin XZ, Yu SC, Chen JS, Wu CZ. Cholelithiasis in Taiwan. Gallstone characteristics, surgical incidence, bile lipid composition, and role of beta-glucuronidase. Dig Dis Sci. 1995;40:1963–1973. doi: 10.1007/BF02208665. [DOI] [PubMed] [Google Scholar]
- 25.Richardson WS, Carter KM, Helm B, Garcia LA, Chambers RB, Keats BJ. Risk factors for gallstone disease in the laparoscopic era. Surg Endosc. 2002;16:450–452. doi: 10.1007/s00464-001-8306-4. [DOI] [PubMed] [Google Scholar]
- 26.Jørgensen T. Gall stones in a Danish population. Relation to weight, physical activity, smoking, coffee consumption, and diabetes mellitus. Gut. 1989;30:528–534. doi: 10.1136/gut.30.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Méndez-Sánchez N, Tanimoto MA, Cobos E, Roldán-Valadez E, Uribe M. Cholesterolosis is not associated with high cholesterol levels in patients with and without gallstone disease. J Clin Gastroenterol. 1997;25:518–521. doi: 10.1097/00004836-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Sheen IS, Liaw YF. The prevalence and incidence of cholecystolithiasis in patients with chronic liver diseases: a prospective study. Hepatology. 1989;9:538–540. doi: 10.1002/hep.1840090405. [DOI] [PubMed] [Google Scholar]
- 29.Katsika D, Tuvblad C, Einarsson C, Lichtenstein P, Marschall HU. Body mass index, alcohol, tobacco and symptomatic gallstone disease: a Swedish twin study. J Intern Med. 2007;262:581–587. doi: 10.1111/j.1365-2796.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr. 2004;80:38–44. doi: 10.1093/ajcn/80.1.38. [DOI] [PubMed] [Google Scholar]
- 31.Loriot MA, Bronowicki JP, Lagorce D, Lakehal F, Persico T, Barba G, Mergey M, Vons C, Franco D, Belghiti J, et al. Permissiveness of human biliary epithelial cells to infection by hepatitis C virus. Hepatology. 1999;29:1587–1595. doi: 10.1002/hep.510290527. [DOI] [PubMed] [Google Scholar]
- 32.Shi ST, Polyak SJ, Tu H, Taylor DR, Gretch DR, Lai MM. Hepatitis C virus NS5A colocalizes with the core protein on lipid droplets and interacts with apolipoproteins. Virology. 2002;292:198–210. doi: 10.1006/viro.2001.1225. [DOI] [PubMed] [Google Scholar]
- 33.Shoda J, Kano M, Asano T, Irimura T, Ueda T, Iwasaki R, Furukawa M, Kamiya J, Nimura Y, Todoroki T, et al. Secretory low-molecular-weight phospholipases A2 and their specific receptor in bile ducts of patients with intrahepatic calculi: factors of chronic proliferative cholangitis. Hepatology. 1999;29:1026–1036. doi: 10.1002/hep.510290440. [DOI] [PubMed] [Google Scholar]
- 34.Thomas LA, Veysey MJ, Bathgate T, King A, French G, Smeeton NC, Murphy GM, Dowling RH. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology. 2000;119:806–815. doi: 10.1053/gast.2000.16495. [DOI] [PubMed] [Google Scholar]
- 35.Fraenkel E, Takács R, Hamvas J, Lengyel G, Fehér J. [Common occurrence of non-alcoholic fatty liver disease and cholecystolithiasis] Orv Hetil. 2007;148:793–798. doi: 10.1556/OH.2007.28049. [DOI] [PubMed] [Google Scholar]
- 36.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 37.Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect. 2009;15:971–976. doi: 10.1111/j.1469-0691.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- 38.Shin DW, Kwon HT, Kang JM, Park JH, Choi HC, Park MS, Park SM, Son KY, Cho B. Association between metabolic syndrome and Helicobacter pylori infection diagnosed by histologic status and serological status. J Clin Gastroenterol. 2012;46:840–845. doi: 10.1097/MCG.0b013e3182522477. [DOI] [PubMed] [Google Scholar]
- 39.Polyzos SA, Kountouras J, Papatheodorou A, Patsiaoura K, Katsiki E, Zafeiriadou E, Zavos C, Anastasiadou K, Terpos E. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism. 2013;62:121–126. doi: 10.1016/j.metabol.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16:79–88. doi: 10.1111/j.1523-5378.2011.00822.x. [DOI] [PubMed] [Google Scholar]
- 41.Bassi V, Santinelli C, Iengo A, Romano C. Identification of a correlation between Helicobacter pylori infection and Graves’ disease. Helicobacter. 2010;15:558–562. doi: 10.1111/j.1523-5378.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 42.Farshad Sh, Alborzi A, Malek Hosseini SA, Oboodi B, Rasouli M, Japoni A, Nasiri J. Identification of Helicobacter pylori DNA in Iranian patients with gallstones. Epidemiol Infect. 2004;132:1185–1189. doi: 10.1017/s0950268804002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neri V, Margiotta M, de Francesco V, Ambrosi A, Valle ND, Fersini A, Tartaglia N, Minenna MF, Ricciardelli C, Giorgio F, et al. DNA sequences and proteic antigens of H. pylori in cholecystic bile and tissue of patients with gallstones. Aliment Pharmacol Ther. 2005;22:715–720. doi: 10.1111/j.1365-2036.2005.02644.x. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi Y, Yamamichi N, Shimamoto T, Mochizuki S, Fujishiro M, Takeuchi C, Sakaguchi Y, Niimi K, Ono S, Kodashima S, et al. Helicobacter pylori infection is positively associated with gallstones: a large-scale cross-sectional study in Japan. J Gastroenterol. 2014;49:882–889. doi: 10.1007/s00535-013-0832-z. [DOI] [PubMed] [Google Scholar]


