Abstract
AIM: To comprehensively review and quantitatively summarize results from intervention studies that examined the effects of intact cereal dietary fiber on parameters of bowel function.
METHODS: A systematic literature search was conducted using PubMed and EMBASE. Supplementary literature searches included screening reference lists from relevant studies and reviews. Eligible outcomes were stool wet and dry weight, percentage water in stools, stool frequency and consistency, and total transit time. Weighted regression analyses generated mean change (± SD) in these measures per g/d of dietary fiber.
RESULTS: Sixty-five intervention studies among generally healthy populations were identified. A quantitative examination of the effects of non-wheat sources of intact cereal dietary fibers was not possible due to an insufficient number of studies. Weighted regression analyses demonstrated that each extra g/d of wheat fiber increased total stool weight by 3.7 ± 0.09 g/d (P < 0.0001; 95%CI: 3.50-3.84), dry stool weight by 0.75 ± 0.03 g/d (P < 0.0001; 95%CI: 0.69-0.82), and stool frequency by 0.004 ± 0.002 times/d (P = 0.0346; 95%CI: 0.0003-0.0078). Transit time decreased by 0.78 ± 0.13 h per additional g/d (P < 0.0001; 95%CI: 0.53-1.04) of wheat fiber among those with an initial transit time greater than 48 h.
CONCLUSION: Wheat dietary fiber, and predominately wheat bran dietary fiber, improves measures of bowel function.
Keywords: Comprehensive review, Dietary fiber, Wheat bran, Cereal, Bowel function
Core tip: This comprehensive review evaluates available data on the effects of intact cereal dietary fiber on bowel function and provides a quantitative summary of the effect of intact wheat fiber on bowel function using weighted regression analysis. Insufficient observations were available from non-wheat cereals for quantitative analysis. Results found an increase in total stool weight of 3.7 ± 0.09 g per gram intact wheat fiber. Transit time decreased by approximately 45 min per gram intact wheat fiber when initial transit time was greater than 48 h. Therefore, intact wheat dietary fiber, predominantly from wheat bran, improves bowel function.
INTRODUCTION
Composition, consistency, frequency, and weight of bowel movements are key indicators of intestinal and digestive health[1]. Abnormalities in these factors serve as diagnostic criteria for prevalent gastrointestinal disorders such as functional constipation[2,3]. According to the most widely accepted criteria (Rome III)[2], characteristics of functional constipation include defecation associated with straining, hard stools, a sensation of incomplete evacuation or anorectal obstruction, manual maneuvering to facilitate defecation, and less than three stools per week. Normal, healthy bowel function, on the other hand, is characterized by soft, regularly shaped stool that is easy to pass, and bowel movements occurring twice per day to three times per week, depending on the individual[4]. Functional constipation is a heterogeneous and common disorder that affects apparently healthy populations[5]. Reports of prevalence vary widely, depending on definition, demographic factors, and sampling[6-9], but could be as high as 27%[3].
Constipation and digestive discomfort have multiple etiologies[3], including certain medications, abuse of laxatives, hormonal disorders and inadequate dietary fiber intakes. Suboptimal dietary fiber consumption is increasingly a global concern, as average intakes are well below recommendations across many countries[10,11]. This creates considerable clinical and public health opportunities to identify strategies that will increase dietary fiber intakes to improve bowel function and help prevent digestive disorders. In addition, increasing dietary fiber consumption offers a safer and cost-effective alternative to laxatives for preventing or alleviating symptoms of constipation[5].
Dietary fiber is naturally present in different food groups, including cereals, vegetables, fruits, beans, and peas. This review provides an overview of intervention studies examining intact cereal dietary fibers (ICDF), which are derived from any part of the cereal plant, including the kernel, hull, or stalk and are minimally processed, although some degree of processing may be required to obtain the fiber-rich portion of the kernel (e.g., milling of bran) or to improve food functionality or safety (e.g., pearling, grinding, or bleaching). In contrast, fibers that are extracted, isolated, or made by chemical or enzymatic means, such as the synthesis of fibers from endosperm starch or the enzymatic hydrolysis of long chain fibers into oligosaccharides are not ICDF and are not included in this analysis. Cereal bran, the hard outer layer of a grain kernel, is a highly concentrated source of dietary fiber: per 100 g, wheat bran contains 43 g fiber, rice bran contains 21 g fiber, and oat bran contains 15 g fiber[12].
Although a large body of literature supports a role of ICDF, predominately wheat bran fiber[5,13], in promoting normal, healthy bowel function through increasing stool weight, past reviews were conducted more than two decades ago[14,15]. Since that time, a number of intervention studies have been published. In addition, less is known about the effects of wheat fiber on other measures of bowel function or the effectiveness of other ICDF such as those from oat, barley, rice, corn, and sorghum. Therefore, the purpose of the present study was to review, evaluate, and quantitatively summarize results from published intervention studies that examined the effects of ICDF on parameters for healthy bowel function, including stool wet weight, stool dry weight, percentage water in stool, stool frequency, intestinal transit time, and stool consistency. Although the heterogeneity of included studies does not allow for a meta-analytical approach, a quantitative estimate using weighted regression analysis on indicated parameters is provided on the pooled results of available studies.
MATERIALS AND METHODS
Literature search and study selection
A comprehensive literature search using PubMed and EMBASE was performed to identify intervention studies in human populations through 6 October 2012 (PubMed) and 18 October 2012 (EMBASE) with no lower date limit. The full search string used in each database is available in the Online Data Supplement (Appendix 1). A combination of free text terms, with different spellings and designed to capture relevant cereals and grains, fiber or bran, and relevant bowel function outcomes (e.g., stool, transit, volume, and bulk), was used. Supplementary literature searches involved examining the reference lists of all relevant studies and pertinent reviews to identify articles not captured in the initial search. The search flow is illustrated in Figure 1. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[16]. The PRISMA checklist is available in the Online Data Supplement (Appendix 2).
Figure 1.
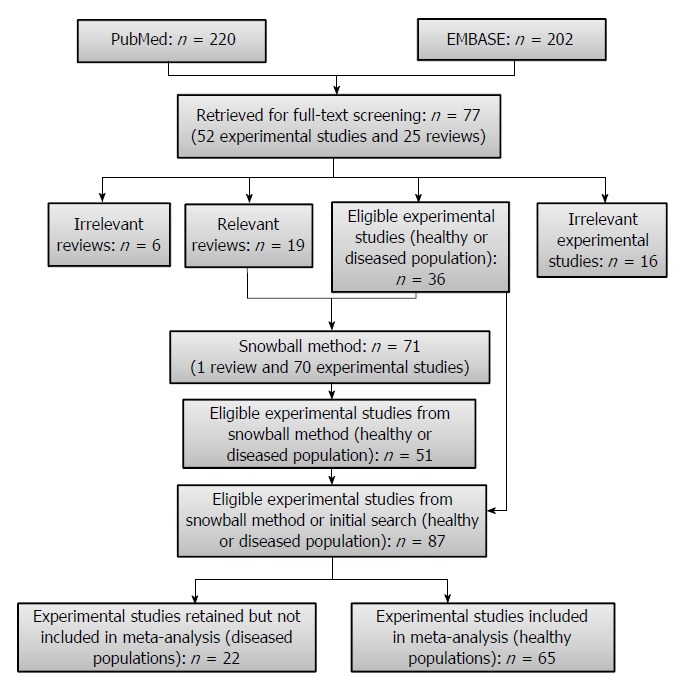
Literature search flow diagram.
Interventions were considered eligible if the following criteria were met: (1) the study was performed with an ICDF; (2) the study was conducted in a human population aged > 1 year; (3) a relevant outcome measurement of bowel function, including total stool weight, stool dry weight, percentage stool water, number of bowel movements per day, consistency of stools, or transit time, was examined; and (4) the publication was written in the English language. Study populations with underlying gastrointestinal disorders, such as constipation, diarrhea, irritable bowel syndrome, diverticular disease, or ulcerative colitis, were eligible for inclusion in the search strategy and data extraction, but were not included in the present quantitative analyses. Both controlled and uncontrolled trials were included in this systematic review. Two independent reviewers (de Vries J and Verbeke K) screened the titles and abstracts for relevance to the systematic review to ensure quality-control. Potentially eligible articles were reviewed jointly to resolve any discrepancies.
Data extraction and quality assessment
The following general study information was extracted using FileMaker Pro software: first author; hypothesis; sex; sample size; study design; duration; physiological characteristics of participants; details of the intervention; details of the control group; background diet (including fiber content); fiber intervention; total fiber intake (background diet fiber plus fiber intervention); dose of food or ingredient in intervention; measured outcome parameter; method used to measure outcome parameter; and description of any adverse events. Outcome data for total stool weight (g/d), stool dry weight (g/d), percentage water in stools, number of bowel movements per day, consistency of stools, and transit time (h) were extracted, and included baseline and trial end values, change-from-baseline values, statistical significance of change values, and differences in the trial end value between the intervention and control arm. Lowest effective dose was identified by visual inspection of the data as reported in the individual studies.
Study quality was assessed through assignment of scores according to two appraisal systems: (1) criteria developed by the FSANZ for the review of publications that are considered to support submitted health claims (0-15 points)[17]; and (2) criteria for human intervention studies as described by Welch et al[18] (0-20 points).
Statistical analysis
The included publications report on intervention studies with a diversity of study designs. Therefore, a meta-analytical approach according to PRISMA criteria was not feasible. Instead, the potential effect of ICDF on bowel function parameters was quantitatively estimated by a weighted regression on the results of those ICDF that had more than 5 observations per parameter. A weighted regression by sample size was chosen because not all publications reported SD on their results.
Means, standard deviations, and 95%CI for ICDF dose (g/d) and bowel function parameters were generated. Weighted regression analyses, in which data from each published study were weighted by the number of subjects used in the study, was performed using SAS version 9.2 (Cary, North Carolina, United States). The regression analysis was not forced through zero because the intercept was different from zero (3.06 ± 1.52 g; P < 0.0439; 95%CI: 0.08-6.03). Stool consistency was an eligible outcome, but due to the diversity of both the methods used to estimate stool consistency and the qualitative reporting of results, weighted regression analysis was not possible. For the analysis of total transit time, a multivariable weighted regression analysis was performed to account for differences in the relationship with the intervention fiber amount that depended upon the initial transit time. Comparisons of the effects of wheat fiber vs other ICDFs on bowel function parameters were not feasible due to a limited number of studies examining other ICDFs.
Data used for the control group differed according to the type of study. For placebo-controlled trials, data from the control arm was used. The control in these studies was most often white wheat, a usual diet, or a gelatin capsule. In some cases, a positive control, such as a laxative, cellulose, wheat bran (if another type of ICDF was examined), or another cereal was used. For uncontrolled trials, the baseline values of the intervention group were used as the control. Some studies conducted a dose-response intervention, in which case the lowest dose was considered the control.
RESULTS
Study characteristics
A flow diagram of the literature search is shown in Figure 1. The literature search included both healthy and diseased populations until the final stage, at which time the studies conducted in healthy populations, were separated from studies conducted in diseased populations. The search yielded 220 references in PubMed and 202 references in EMBASE, of which 77 articles were retained for full-text screening and reference list review. The 77 articles included both original experimental research publications (n = 52) and reviews (n = 25). Thirty-six of the experimental studies were deemed eligible and 19 of the review articles were deemed relevant for screening of reference lists (snowball method). Overall, screening of reference lists from all relevant review articles and eligible experimental studies resulted in 71 additional articles (1 review and 70 experimental studies) that subsequently underwent full-text screening. Fifty-one of the 71 articles were eligible for inclusion. Therefore, the 51 eligible experimental studies identified by the snowball method and the 36 eligible experimental studies identified in the initial search resulted in a combined total of 87 experimental studies, 65 of which were conducted in generally healthy populations and therefore included in the quantitative analyses. From the 65 studies, 87 study arms examined the effect of ICDF on total fecal weight, 47 on dry fecal weight, 36 on percentage fecal water, 43 on stool frequency/bowel movements, and 57 on transit time.
Primary characteristics, including the first author, publication year, sex distribution of study population, type of study design, and the specific ICDF evaluated, of the 65 interventions are provided in the Online Data Supplement (Appendix 3)[11,19-82]. Fifty-seven percent of the studies were placebo-controlled, 32% were randomized, and 6% were single- or double-blinded. Wheat fiber, and primarily wheat bran fiber (90% of wheat fiber studies), was the most common dietary fiber provided in the intervention with 75 observations in 65 intervention studies. Only 13 of the observations were ICDF from other sources, including corn (n = 4), barley (n = 3), rye (n = 2), oat (n = 1), rice (n = 1), and sorghum (n = 1). Most publications, also the more recent ones, provide insufficient details for an adequate description of the dietary fiber sources used.
Stool bulking, stool frequency, and transit time
Table 1 shows the number of comparisons for different ICDFs and different bowel function outcomes. It also presents the level of fiber provided across the interventions. Table 2 presents the mean ± SD and 95%CI effects, plus ranges from the individual studies, of the fiber intervention on total stool weight (g/d), dry stool weight (g/d), percentage water in stool (%), and stool frequency (times/d), as well as the average fiber intakes provided in the interventions for each of these outcomes for wheat, barley, and corn. Table 2 also shows results from the weighted regression analysis of wheat fiber (per g/d), compared to control, on change in total and dry stool weight, stool frequency (number of defecations/day), and transit time (h). The mean effects and weighted change on bowel function parameters among interventions that used ICDF from barley and corn were not estimated given the limited number of observations (< 5 observations were available for each). The data of oat, rice, rye, and sorghum ICDF on these parameters from the individual studies are listed in the supplemental information (Appendix 4).
Table 1.
Summary of comparisons for different intact cereal dietary fibers and bowel function outcomes
|
Source of intact fiber |
|||||||
| Wheat | Barley | Corn | Oat | Rice | Rye | Sorghum | |
| Total stool weight | |||||||
| Observations1, n | 75 | 3 | 4 | 1 | 2 | 2 | 1 |
| Fiber intervention (g/d), mean ± SD or range2 | 15.2 ± 8.3 | 10.2, 23 | 6.0, 42 | 14.3 | 17.1, 20.7 | 13, 20.6 | 2.5 |
| Dry stool weight | |||||||
| Observations, n | 40 | 1 | 3 | 1 | 1 | 1 | - |
| Fiber intervention (g/d), mean ± SD or range2 | 14.7 ± 8.5 | 21 | 6, 42 | 14.3 | 20.7 | 20.6 | - |
| Fecal water | |||||||
| Observations, n | 30 | 3 | 2 | - | 1 | - | - |
| Level of fiber interv. (g/d), mean ± SD or range2 | 16.0 ± 7.4 | 10.2, 23 | 15, 42 | - | 20.7 | - | - |
| Stool frequency | |||||||
| Observations, n | 34 | 2 | 2 | - | 2 | 2 | 1 |
| Fiber intervention (g/d), mean ± SD or range2 | 13.6 ± 6.4 | 21, 23 | 15, 42 | - | 17.1, 20.7 | 20.6, 36.4 | 2.5 |
| Transit time | |||||||
| Observations, n | 52 | - | - | 1 | 2 | 1 | 1 |
| Fiber intervention (g/d), mean ± SD or range2 | 14.8 ± 8,6 | - | - | 2.7 | 17.1, 20.7 | 20.6 | 2.5 |
May include > 1 observation from studies examining > 1 dose of intact cereal dietary fiber;
Fiber intakes are shown as mean ± SD of all observations if > 5 observations were available, the range of values from individual studies if 2-4 observations were available, and a single estimate if only one observation was available.
Table 2.
Fiber intakes and effects on total stool weight, dry stool weight, percentage water in stool, stool frequency, and transit time
|
Source of intact cereal dietary fiber |
|||
| Wheat | Barley | Corn | |
| Total stool weight | |||
| Observations, n | 75 | 3 | 4 |
| Fiber (g/d), mean ± SD or range | 15.2 ± 8.3 | 10.2-23 | 6.0-42 |
| Total effect (g/d), mean ± SD or range | 65.4 ± 37.8 | 49.6-65 | 1.2-96.3 |
| Average fecal bulking index, Δ in g/d stool weight per g/d fiber | 4.7 ± 2.7 | 3.6 ± 2.4 | 2.1 ± 1.5 |
| Fecal bulking index by regression, Δ in g/d stool weight per g/d fiber | 3.67 ± 0.09b | - | - |
| (3.50-3.84) | |||
| Dry stool weight | |||
| Observations, n | 40 | 1 | 3 |
| Fiber (g/d), mean ± SD or range | 14.7 ± 8.5 | - | 6-42 |
| Total effect (g/d), mean ± SD or range | 14.4 ± 9.4 | - | 4.8-31 |
| Fecal bulking index by regression, Δ in g/d stool weight per g/d fiber | 0.75 ± 0.03b | - | 0.7-0.9 |
| (0.69-0.82) | |||
| Fecal water | |||
| Observations, n | 30 | 3 | 2 |
| Fiber (g/d), mean ± SD or range | 16.0 ± 7.4 | 10.2-23 | - |
| Total effect by regression (∆% water), mean ± SD or range | 1.5 ± 2.1 | -1.8-10 | - |
| Stool frequency | |||
| Observations, n | 34 | 2 | 2 |
| Fiber (g/d), mean ± SD or range | 13.6 ± 6.4 | - | - |
| Total effect (times/d), mean ± SD or range | 0.34 ± 0.23 | - | - |
| Frequency index by regression, Δ in times/d per g/d fiber | 0.004 ± 0.002a | - | - |
| (0.003-0.078) | |||
| Transit time | |||
| Observations, n | 52 | 0 | 0 |
| Fiber (g/d), mean ± SD | 14.8 ± 8,4 | - | - |
| Δ in h per g/d fiber by regression (those with initial transit time between 24-48 h) | 0.78 ± 0.13b | - | - |
| (0.53-1.04) | |||
| Δ in h per g/d fiber by regression (those with initial transit time between 48-96 h) | -0.75 ± 0.04b | - | - |
| (-0.84- -0.67) | |||
P < 0.05,
P < 0.01 vs control.
Among the studies included in the quantitative analysis (wheat fiber studies), mean fiber intakes ranged from 13.6 ± 6.4 g/d among studies that examined stool frequency to 16.0 ± 7.4 g/d among studies that investigated percentage water in stool. On average, the wheat fiber intervention increased total stool weight by 65.4 ± 37.8 g/d, dry stool weight by 14.4 ± 9.4 g/d, percentage water in stool by 1.5% ± 2.1%, and stool frequency by 0.34 ± 0.23 bowel movements per day.
The weighted changes per g/d of wheat fiber intake were as follows: an increase of 3.7 ± 0.09 g/d (P < 0.0001; 95%CI: 3.50-3.84) for total stool weight; an increase of 0.75 ± 0.03 g/d (P < 0.0001; 95%CI: 0.69-0.82) for dry stool weight. Weighted regression analysis of the results of all studies did not reveal an effect of the fiber intervention on transit time. Upon stratification by baseline transit time, an increase of 0.78 ± 0.13 h/g (P < 0.0001; 95%CI: 0.53-1.04) of wheat fiber was observed among those with an initial transit time of 24-48 h, and a decrease of 0.75 ± 0.04 h/g [P < 0.0001; 95%CI: (-0.84) - (-0.67)] of wheat fiber was observed among those with an initial transit time of 48-96 h. Individual study data on the change in the bowel function parameters per gram of wheat fiber intake are shown in Figure 2 for total stool weight, Figure 3 for dry stool weight, Figure 4 for percentage water in stool, and Figure 5 for stool frequency. The lowest effective dose of wheat fiber that significantly increased fecal output, as reported in one of the included individual intervention studies, was 5.7 g/d (P < 0.05)[53].
Figure 2.
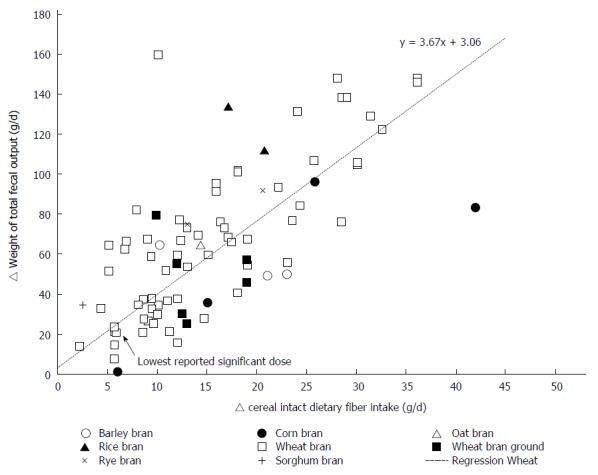
The delta weight of total fecal output (g/d) related to the amount of intact cereal dietary fiber intervention (g/d) in healthy individuals.
Figure 3.
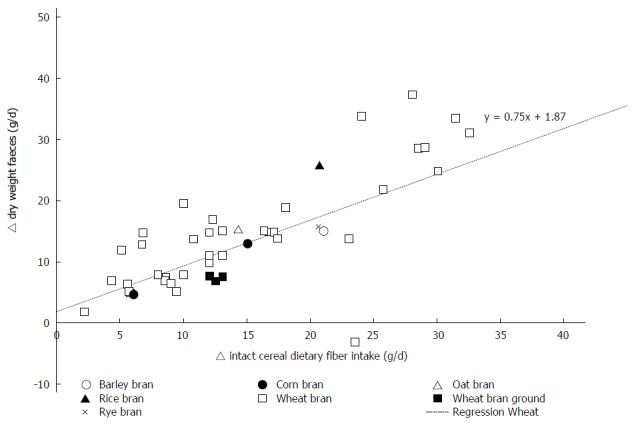
The delta weight of dry fecal output (g/d) related to the amount of intact cereal dietary fiber intervention (g/d) in healthy individuals.
Figure 4.
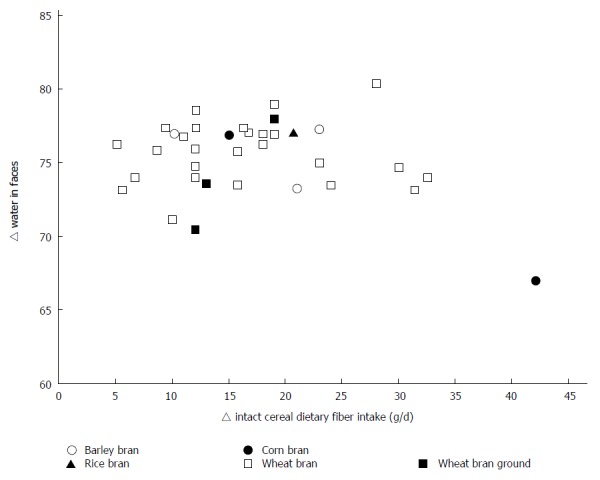
The % fecal water related to the amount of intact cereal dietary fiber intervention (g/d) in healthy individuals.
Figure 5.
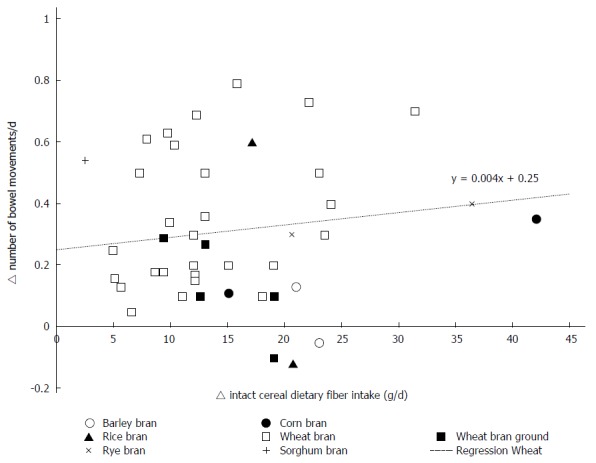
The delta number of bowel movements related to the amount of intact cereal dietary fiber intervention (g/d) in healthy individuals.
DISCUSSION
The present review provides the most comprehensive evaluation to date on the effects of ICDF on multiple measures of bowel function. Wheat fiber, and primarily wheat bran fiber, was found to improve measures of bowel function, including total stool weight, dry stool weight, and stool frequency, as well as intestinal transit time among those with an initial transit time greater than 48 h.
Wheat bran fiber is the most extensively studied cereal fiber for measures related to bowel function[5,13], with the first study dating back more than 90 years[82]. Leading nutrition and health authorities, including the US Institute of Medicine[83], Health Canada[84], and the European Food Standards Agency (EFSA)[85], have concluded that wheat bran fiber increases stool bulking and shortens intestinal transit time. In 2010, EFSA provided a Scientific Opinion[85], wherein an unequivocal cause and effect relationship between the consumption of wheat bran fiber and an increase in stool bulk and intestinal transit time was concluded and two health claims in relation to these intestinal functions were passed. A health claim was also approved by the Canadian Food Inspection Agency after the agency concluded that wheat bran promotes laxation and regularity[84]. Furthermore, wheat bran is considered the benchmark against which other fibers are compared for their effects on regularity[84]. Compared to wheat bran fiber, less is known concerning the effects of other sources of ICDF on bowel function, largely because far fewer studies have been conducted on other ICDF.
Given the heterogeneity in study designs utilized in the individual studies, a weighted regression, rather than a traditional meta-analysis, was considered to be a superior method to examine the effect of wheat fiber on stool parameters. The average fecal bulking index, reported in Table 2, provides an indicative estimate of effect of ICDF from wheat (75 observations), barley (3 observations) and corn (4 observations) on total fecal bulking. These results indicate that wheat bran might have the best properties to increase total fecal bulk.
Different sources of dietary fiber are not equal in their functionality and effects on bowel function, as evidenced by Health Canada using wheat bran as the gold standard fiber[84]. Cummings[14] evaluated nearly 100 interventions, published from 1932 to 1991, on dietary fiber and fecal weight, and compared the effectiveness of different sources of fiber. Among 41 interventions that examined wheat fiber-which consisted largely of wheat bran-the mean increase in fecal weight per g/d of wheat fiber was 5.4 g. The mean increases in fecal weight per g/d of other sources of fiber were smaller in magnitude: fruit and vegetables (4.7 g), gums and mucilages (3.7 g), cellulose (3.5 g), oats (3.4 g), corn (3.3 g), legumes (2.2 g), and pectin (1.2 g). Of note, findings for the other cereal sources of ICDF were from fewer studies. Nevertheless, based on the available evidence, wheat fiber was the most effective source of fiber for increasing fecal weight.
The varying effects of different dietary fibers on fecal bulking are likely related to different underlying mechanisms of action. The effects of wheat bran fiber on stool weight are largely attributable to its high resistance to fermentation by colonic bacteria, combined with its water binding capacity (1 g of fiber binds about 3 g of water), therefore contributing to a stronger effect on increasing stool bulking compared to more easily fermented ICDF, such as those from oats and barley[14,86,87]. The resulting increased volume of fecal mass stimulates colonic movement, thereby helping to reduce transit time and increase stool frequency[14].
Since the review on fiber and bowel function conducted by Cummings[14] more than 40 interventions have been published, 30 of which were in healthy populations and therefore included in the present evaluation. The heterogeneity of the included studies did not allow for a meta-analytical approach according to PRISMA requirements. Therefore, a weighted regression analysis by sample size was conducted as an alternative approach to achieve a quantitative estimate. Similar to the findings by Cummings[14], an increase in fecal weight was observed in the current analysis. The smaller estimated increase in fecal weight, compared to the earlier review (3.7 g/d vs 5.4 g/d)[14], is likely due to the weighted regression method applied in the present analysis, in which the regression equation was not forced through zero, thus influencing the slope of the regression line. Based on a visual inspection of a funnel plot on the total stool weight data (Appendix 5), publication bias is unlikely the cause of the positive intercept of the regression. Furthermore a greater number of placebo-controlled trials were included in the present analysis.
In addition, changes in other bowel function parameters, such as stool frequency, transit time, dry stool weight, and percentage water in stools were also quantitatively evaluated; studies on stool composition were too heterogeneous to allow for a quantitative approach. Provision of wheat fiber showed beneficial effects on dry stool weight and stool frequency, as well as on intestinal transit time among those with an initial transit time greater than 48 h. This arbitrary level of 48 h was used because normal stool frequency was considered to be between 1 to 2 bowel movements per day. When transit time is already optimal, i.e., between 24 and 48 h, additional dietary fiber would not be expected to alter transit[88]. Adding dietary fiber that is resistant to fermentation does not increase the overall percentage of water as the amount of water bound by the fiber is similar to the average water content of fecal samples (about 75%).
Different methodologies were used in the different studies to determine transit time. First, several markers, including indigestible dye, radio-opaque markers, poly-ethylene glycol, and chromium sesquioxide, were used to estimate transit time. Secondly, transit time was calculated in different ways based on the recovery of the markers in the feces. Cummings et al[32] demonstrated that the mean transit time method with a single dose estimate was approximately 15% lower compared to an estimate with the 80% method. Wrick et al[51] examined the use of radio-opaque pellets, poly-ethylene glycol and chromium sesquioxide as markers to estimate transit time and concluded that there was no significant difference in transit time estimates between marker types. It remains possible that the different methods may yield different estimates of transit time. However, an analysis stratified by the type of methodology to estimate transit time would have lowered the power of the analysis. We concluded that a weighted regression analysis on all available data, categorized according to initial transit time with a cut off point of 48 h, provided the best estimate on the effects of ICDF on transit time.
Inadequate dietary fiber intake is increasingly a global concern, as average intakes are well below recommendations across many countries[10,11]. The International Life Sciences Institute Europe Dietary Carbohydrates Task Force summarized sex-specific dietary fiber consumption across nine European countries, in addition to the United States and Japan[10]. The resulting report found that average dietary fiber intakes were below the lower end of the World Health Organization recommendation (25-40 g/d)[89], with only a few exceptions. These findings have potentially serious health consequences beyond impaired bowel function[60,83]. Inadequate dietary fiber intakes have been associated with increased risk for type 2 diabetes, cardiovascular disease, certain cancers, weight gain, diverticular disease, obesity, and constipation[60,83]. Given the high content of dietary fiber in wheat bran (43 g compared to 21 g in rice bran and 15 g in oat bran, per 100 g[12]), wheat bran can play an important role in helping individuals increase overall dietary fiber intakes. Increasing wheat bran intake is a relatively simple dietary strategy to improve bowel function.
A notable strength of this research is the large volume of studies evaluated (n = 65), highlighting its comprehensive and inclusive nature on ICDF and bowel function. A number of parameters of bowel function that had not been quantitatively evaluated previously were examined, which is a substantial contribution to the literature. In addition, this review includes 20 years of research since the last review by Cummings[14]. Several limitations should also be considered. Due to the exhaustive and inclusive nature of this review, a large number of included interventions were uncontrolled trials, and most studies were not randomized. Therefore, observed changes in parameters for healthy bowel function cannot be fully attributed to the intervention, as the placebo effect remains possible[90]. In addition, proper quantitative evaluations of the effects of other ICDF were not feasible due to the limited available data. Future studies that examine other ICDF will provide valuable contributions to this line of research.
In summary, the current comprehensive review of interventions with ICDF on bowel function is spanning more than 90 years of research in healthy individuals. The results of the 65 included publications indicate that wheat fiber promotes healthy bowel function through improvements in total stool weight, dry stool weight, intestinal transit time, and stool frequency. Based on the large volume of available evidence, incorporating wheat fiber, primarily wheat bran fiber, into the diet can positively affect bowel function. As wheat was the only cereal for which a quantitative estimate of its effect was possible, more research on the effects of other cereals is warranted.
COMMENTS
Background
Composition, consistency, frequency, and weight of bowel movements are key indicators of intestinal and digestive health. Infrequent bowel movements and low stool weights are very common in many countries.
Research frontiers
The effects of intact cereal dietary fibers on bowel function have not been systematically reviewed previously. Constipation and irregular bowel movements are highly prevalent in the general population but can often be averted through simple, realistic, and inexpensive changes in dietary practices such as increasing dietary fiber intakes.
Innovations and breakthroughs
This systematic review provides the first quantitative estimate of the effect of wheat fiber on multiple measures of bowel function based on the results of 90 years of research.
Applications
Findings from this comprehensive review may help gastroenterologists choose relatively inexpensive solutions in the prevention of constipation.
Peer-review
This is a useful review on the impact of specific dietary cereal fibers on bowel functions.
Footnotes
Supported by Funding from Kellogg Company, Battle Creek, MI, United States.
Conflict-of-interest statement: Dr. de Vries J has received fees for systematically reviewing the literature reported in this publication from Kellogg Company, Battle Creek, MI, USA. Dr. Miller PE received fees while working at Exponent, Inc from Kellogg Company, Battle Creek, MI, USA, to support the writing process of the manuscript. Professor Verbeke K, PhD, is co-beneficiary of the WK Kellogg Chair in Cereal Sciences and Nutrition (Kellogg Company, Battle Creek, MI, USA).
Data sharing statement: No additional data are publically available. Interested readers can contact the first author.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 30, 2015
First decision: March 10, 2015
Article in press: June 10, 2015
P- Reviewer: Klurfeld DM S- Editor: Wang JL L- Editor: A E- Editor: Liu XM
References
- 1.Grabitske HA, Slavin JL. Laxation and the like: assessing digestive health. Nutr Today. 2008;43:193–198. [Google Scholar]
- 2.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 4.National Digestive Diseases Information Clearinghouse, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Digestive Diseases A-Z List of Topics and Titles. Updated 4 December 2013. Available from: http://digestive.niddk.nih.gov/ddiseases/a-z.aspx. (cited 10 December 2013)
- 5.O’Sullivan K. The superior benefits of wheat bran fibre in digestive health. European Gastroenterol Hepatology Review. 2012;8:90–93. [Google Scholar]
- 6.Stewart ML, Nikhanj SD, Timm DA, Thomas W, Slavin JL. Evaluation of the effect of four fibers on laxation, gastrointestinal tolerance and serum markers in healthy humans. Ann Nutr Metab. 2010;56:91–98. doi: 10.1159/000275962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald ER, Di Lorenzo C, Cipriani L, Colborn DK, Burgers R, Wald A. Bowel habits and toilet training in a diverse population of children. J Pediatr Gastroenterol Nutr. 2009;48:294–298. doi: 10.1097/mpg.0b013e31817efbf7. [DOI] [PubMed] [Google Scholar]
- 8.Pare P, Ferrazzi S, Thompson WG, Irvine EJ, Rance L. An epidemiological survey of constipation in canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 9.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 10.Gray J. Dietary fibre. Definition, analysis, physiology and health. ILSI Europe Dietary Fibre Concise Monograph Series. Available from: http://www.ilsi.org/Europe/Publications/C2006Diet_FibEng.pdf.
- 11.Stephen AM, Wiggins HS, Englyst HN, Cole TJ, Wayman BJ, Cummings JH. The effect of age, sex and level of intake of dietary fibre from wheat on large-bowel function in thirty healthy subjects. Br J Nutr. 1986;56:349–361. doi: 10.1079/bjn19860116. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 26. Software v.1.3.1. Nutrient Data Laboratory Home Page, 2011. Available from: http://ndb.nal.usda.gov/ndb/search/list.
- 13.Stevenson L, Phillips F, O’Sullivan K, Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int J Food Sci Nutr. 2012;63:1001–1013. doi: 10.3109/09637486.2012.687366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings JH. The effect of dietary fiber on fecal weight and composition. 2nd ed. In: Spiller GS, editor. CRC Handbook on dietary fiber in nutrition. Boca Raton: FL; 1993. pp. 263–349. [Google Scholar]
- 15.Müller-Lissner SA. Effect of wheat bran on weight of stool and gastrointestinal transit time: a meta analysis. Br Med J (Clin Res Ed) 1988;296:615–617. doi: 10.1136/bmj.296.6622.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food Standards Australia New Zealand. Guidance on Substantiation of Food-Health Relationships - Version 1.0. Australia: Guidance on Substantiation of Food-Health; 2012. [Google Scholar]
- 18.Welch RW, Antoine JM, Berta JL, Bub A, de Vries J, Guarner F, Hasselwander O, Hendriks H, Jäkel M, Koletzko BV, et al. Guidelines for the design, conduct and reporting of human intervention studies to evaluate the health benefits of foods. Br J Nutr. 2011;106 Suppl 2:S3–S15. doi: 10.1017/S0007114511003606. [DOI] [PubMed] [Google Scholar]
- 19.Cowgill GR, Anderson WE. Laxative effects of wheat bran and “washed bran” in healthy men. JAMA. 1932;98:1866–1875. [Google Scholar]
- 20.Williams RD, Olmsted WH. The effect of cellulose, hemicellulose and lignin on the weight of the stool: a contribution to the study of laxation in man. J Nutr. 1936;11:433–449. [Google Scholar]
- 21.Hoppert CA, Clark AJ. Bran muffins and normal laxation. J Am Diet Assoc. 1942;18:524. [Google Scholar]
- 22.Hoppert CA, Clark AJ. Digestibility and effect on laxation of crude fiber and cellulose in certain common foods. J Am Diet Assoc. 1945;21:157–160. [Google Scholar]
- 23.MCCANCE RA, PRIOR KM, WIDDOWSON EM. A radiological study of the rate of passage of brown and white bread through the digestive tract of man. Br J Nutr. 1953;7:98–104. doi: 10.1079/bjn19530013. [DOI] [PubMed] [Google Scholar]
- 24.Eastwood MA, Kirkpatrick JR, Mitchell WD, Bone A, Hamilton T. Effects of dietary supplements of wheat bran and cellulose on faeces and bowel function. Br Med J. 1973;4:392–394. doi: 10.1136/bmj.4.5889.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payler DK. Food fibre and bowel behaviour. Lancet. 1973;1:1394. doi: 10.1016/s0140-6736(73)91727-3. [DOI] [PubMed] [Google Scholar]
- 26.Payler DK, Pomare EW, Heaton KW, Harvey RF. The effect of wheat bran on intestinal transit. Gut. 1975;16:209–213. doi: 10.1136/gut.16.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins DJ, Hill MS, Cummings JH. Effect of wheat fiber on blood lipids, fecal steroid excretion and serum iron. Am J Clin Nutr. 1975;28:1408–1411. doi: 10.1093/ajcn/28.12.1408. [DOI] [PubMed] [Google Scholar]
- 28.Baird IM, Walters RL, Davies PS, Hill MJ, Drasar BS, Southgate DA. The effects of two dietary fiber supplements on gastrointestinal transit, stool weight and frequency, and bacterial flora, and fecal bile acids in normal subjects. Metabolism. 1977;26:117–128. doi: 10.1016/0026-0495(77)90047-6. [DOI] [PubMed] [Google Scholar]
- 29.Southgate DA, Branch WJ, Hill MJ, Drasar BS, Walters RL, Davies PS, Baird IM. Metabolic responses to dietary supplements of bran. Metabolism. 1976;25:1129–1135. doi: 10.1016/0026-0495(76)90020-2. [DOI] [PubMed] [Google Scholar]
- 30.Walters RL, Baird IM, Davies PS, Hill MJ, Drasar BS, Southgate DA, Green J, Morgan B. Effects of two types of dietary fibre on faecal steroid and lipid excretion. Br Med J. 1975;2:536–538. doi: 10.1136/bmj.2.5970.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings JH, Hill MJ, Jenkins DJ, Pearson JR, Wiggins HS. Changes in fecal composition and colonic function due to cereal fiber. Am J Clin Nutr. 1976;29:1468–1473. doi: 10.1093/ajcn/29.12.1468. [DOI] [PubMed] [Google Scholar]
- 32.Cummings JH, Jenkins DJ, Wiggins HS. Measurement of the mean transit time of dietary residue through the human gut. Gut. 1976;17:210–218. doi: 10.1136/gut.17.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drasar BS, Jenkins DJ, Cummings JH. The influence of a diet rich in wheat fibre on the human faecal flora. J Med Microbiol. 1976;9:423–431. doi: 10.1099/00222615-9-4-423. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs H-M S, Floch MH. The effect of dietary fiber supplementation in man. II. Alteration in fecal physiology and bacterial flora. Am J Clin Nutr. 1976;29:1443–1447. doi: 10.1093/ajcn/29.12.1443. [DOI] [PubMed] [Google Scholar]
- 35.Reinhold JG, Faradji B, Abadi P, Ismail-Beigi F. Decreased absorption of calcium, magnesium, zinc and phosphorus by humans due to increased fiber and phosphorus consumption as wheat bread. J Nutr. 1976;106:493–503. doi: 10.1093/jn/106.4.493. [DOI] [PubMed] [Google Scholar]
- 36.Wyman JB, Heaton KW, Manning AP, Wicks AC. The effect on intestinal transit and the feces of raw and cooked bran in different doses. Am J Clin Nutr. 1976;29:1474–1479. doi: 10.1093/ajcn/29.12.1474. [DOI] [PubMed] [Google Scholar]
- 37.Kay RM, Truswell AS. The effect of wheat fibre on plasma lipids and faecal steroid excretion in man. Br J Nutr. 1977;37:227–235. doi: 10.1079/bjn19770024. [DOI] [PubMed] [Google Scholar]
- 38.Winreich J, Pedersen O, Dinesen K. Role of bran in normals. Serum levels of cholesterols, triglyceride, calcium and total 3 alpha-hydroxycholanic acid, and intestinal transit time. Acta Med Scand. 1977;202:125–130. [PubMed] [Google Scholar]
- 39.Brodribb AJ, Groves C. Effect of bran particle size on stool weight. Gut. 1978;19:60–63. doi: 10.1136/gut.19.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings JH, Branch W, Jenkins DJ, Southgate DA, Houston H, James WP. Colonic response to dietary fibre from carrot, cabbage, apple, bran. Lancet. 1978;1:5–9. doi: 10.1016/s0140-6736(78)90357-4. [DOI] [PubMed] [Google Scholar]
- 41.Cummings JH, Hill MJ, Jivraj T, Houston H, Branch WJ, Jenkins DJ. The effect of meat protein and dietary fiber on colonic function and metabolism. I. Changes in bowel habit, bile acid excretion, and calcium absorption. Am J Clin Nutr. 1979;32:2086–2093. doi: 10.1093/ajcn/32.10.2086. [DOI] [PubMed] [Google Scholar]
- 42.Kretsch MJ, Crawford L, Calloway DH. Some aspects of bile acid and urobilinogen excretion and fecal elimination in men given a rural Guatemalan diet and egg formulas with and without added oat bran. Am J Clin Nutr. 1979;32:1492–1496. doi: 10.1093/ajcn/32.7.1492. [DOI] [PubMed] [Google Scholar]
- 43.Heller SN, Hackler LR, Rivers JM, Van Soest PJ, Roe DA, Lewis BA, Robertson J. Dietary fiber: the effect of particle size of wheat bran on colonic function in young adult men. Am J Clin Nutr. 1980;33:1734–1744. doi: 10.1093/ajcn/33.8.1734. [DOI] [PubMed] [Google Scholar]
- 44.Stasse-Wolthuis M, Albers HF, van Jeveren JG, Wil de Jong J, Hautvast JG, Hermus RJ, Katan MB, Brydon WG, Eastwood MA. Influence of dietary fiber from vegetables and fruits, bran or citrus pectin on serum lipids, fecal lipids, and colonic function. Am J Clin Nutr. 1980;33:1745–1756. doi: 10.1093/ajcn/33.8.1745. [DOI] [PubMed] [Google Scholar]
- 45.Stephen AM, Cummings JH. Mechanism of action of dietary fibre in the human colon. Nature. 1980;284:283–284. doi: 10.1038/284283a0. [DOI] [PubMed] [Google Scholar]
- 46.Kurzer MS, Calloway DH. Nitrate and nitrogen balances in men. Am J Clin Nutr. 1981;34:1305–1313. doi: 10.1093/ajcn/34.7.1305. [DOI] [PubMed] [Google Scholar]
- 47.Kies C, Balters S, Kan S, Lo B, Westring M, Fox H. Impact of corn bran on nutritional status and on gastrointestinal tract function of humans. Maize: Recent Progress in Chemistry and Technology Nebraska Agriculture Station Journal Series; 1982. pp. 33–43. [Google Scholar]
- 48.Eastwood MA, Robertson JA, Brydon WG, MacDonald D. Measurement of water-holding properties of fibre and their faecal bulking ability in man. Br J Nutr. 1983;50:539–547. doi: 10.1079/bjn19830125. [DOI] [PubMed] [Google Scholar]
- 49.Fleming SE, Marthinsen D, Kuhnlein H. Colonic function and fermentation in men consuming high fiber diets. J Nutr. 1983;113:2535–2544. doi: 10.1093/jn/113.12.2535. [DOI] [PubMed] [Google Scholar]
- 50.Van Dokkum W, Pikaar NA, Thissen JT. Physiological effects of fibre-rich types of bread. 2. Dietary fibre from bread: digestibility by the intestinal microflora and water-holding capacity in the colon of human subjects. Br J Nutr. 1983;50:61–74. doi: 10.1079/bjn19830072. [DOI] [PubMed] [Google Scholar]
- 51.Wrick KL, Robertson JB, Van Soest PJ, Lewis BA, Rivers JM, Roe DA, Hackler LR. The influence of dietary fiber source on human intestinal transit and stool output. J Nutr. 1983;113:1464–1479. doi: 10.1093/jn/113.8.1464. [DOI] [PubMed] [Google Scholar]
- 52.Fedail SS, Badi SE, Musa AR. The effects of sorghum and wheat bran on the colonic functions of healthy Sudanese subjects. Am J Clin Nutr. 1984;40:776–779. doi: 10.1093/ajcn/40.4.776. [DOI] [PubMed] [Google Scholar]
- 53.Spiller GA, Wong LG, Nunes JD, Story JA, Petro MS, Furumoto EJ, Alton-Spiller M, Whittam JH, Scala J. Effect of four levels of hard wheat bran on fecal composition and transit time in healthy young women. Fed Proc. 1984;43:392. doi: 10.1093/jn/116.5.778. [DOI] [PubMed] [Google Scholar]
- 54.Eastwood MA, Elton RA, Smith JH. Long-term effect of wholemeal bread on stool weight, transit time, fecal bile acids, fats, and neutral sterols. Am J Clin Nutr. 1986;43:343–349. doi: 10.1093/ajcn/43.3.343. [DOI] [PubMed] [Google Scholar]
- 55.Miyoshi H, Okuda T, Oi Y, Koishi H. Effects of rice fiber on fecal weight, apparent digestibility of energy, nitrogen and fat, and degradation of neutral detergent fiber in young men. J Nutr Sci Vitaminol (Tokyo) 1986;32:581–589. doi: 10.3177/jnsv.32.581. [DOI] [PubMed] [Google Scholar]
- 56.Spiller GA, Story JA, Wong LG, Nunes JD, Alton M, Petro MS, Furumoto EJ, Whittam JH, Scala J. Effect of increasing levels of hard wheat fiber on fecal weight, minerals and steroids and gastrointestinal transit time in healthy young women. J Nutr. 1986;116:778–785. doi: 10.1093/jn/116.5.778. [DOI] [PubMed] [Google Scholar]
- 57.Balasubramanian R, Johnson EJ, Marlett JA. Effect of wheat bran on bowel function and fecal calcium in older adults. J Am Coll Nutr. 1987;6:199–208. doi: 10.1080/07315724.1987.10720182. [DOI] [PubMed] [Google Scholar]
- 58.Jenkins DJ, Peterson RD, Thorne MJ, Ferguson PW. Wheat fiber and laxation: dose response and equilibration time. Am J Gastroenterol. 1987;82:1259–1263. [PubMed] [Google Scholar]
- 59.Reddy BS, Sharma C, Simi B, Engle A, Laakso K, Puska P, Korpela R. Metabolic epidemiology of colon cancer: effect of dietary fiber on fecal mutagens and bile acids in healthy subjects. Cancer Res. 1987;47:644–648. [PubMed] [Google Scholar]
- 60.Stevens J, VanSoest PJ, Robertson JB, Levitsky DA. Comparison of the effects of psyllium and wheat bran on gastrointestinal transit time and stool characteristics. J Am Diet Assoc. 1988;88:323–326. [PubMed] [Google Scholar]
- 61.Tomlin J, Read NW. Comparison of the effects on colonic function caused by feeding rice bran and wheat bran. Eur J Clin Nutr. 1988;42:857–861. [PubMed] [Google Scholar]
- 62.Melcher EA, Levitt MD, Slavin JL. Methane production and bowel function parameters in healthy subjects on low- and high-fiber diets. Nutr Cancer. 1991;16:85–92. doi: 10.1080/01635589109514147. [DOI] [PubMed] [Google Scholar]
- 63.Ziegenhagen DJ, Tewinkel G, Kruis W, Herrmann F. Adding more fluid to wheat bran has no significant effects on intestinal functions of healthy subjects. J Clin Gastroenterol. 1991;13:525–530. doi: 10.1097/00004836-199110000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Lampe JW, Slavin JL, Melcher EA, Potter JD. Effects of cereal and vegetable fiber feeding on potential risk factors for colon cancer. Cancer Epidemiol Biomarkers Prev. 1992;1:207–211. [PubMed] [Google Scholar]
- 65.Lampe JW, Wetsch RF, Thompson WO, Slavin JL. Gastrointestinal effects of sugarbeet fiber and wheat bran in healthy men. Eur J Clin Nutr. 1993;47:543–548. [PubMed] [Google Scholar]
- 66.Lupton JR, Morin JL, Robinson MC. Barley bran flour accelerates gastrointestinal transit time. J Am Diet Assoc. 1993;93:881–885. doi: 10.1016/0002-8223(93)91526-v. [DOI] [PubMed] [Google Scholar]
- 67.Bingham SA, Pignatelli B, Pollock JR, Ellul A, Malaveille C, Gross G, Runswick S, Cummings JH, O’Neill IK. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17:515–523. doi: 10.1093/carcin/17.3.515. [DOI] [PubMed] [Google Scholar]
- 68.Cherbut C, Aube AC, Mekki N, Dubois C, Lairon D, Barry JL. Digestive and metabolic effects of potato and maize fibres in human subjects. Br J Nutr. 1997;77:33–46. doi: 10.1017/s0007114500002865. [DOI] [PubMed] [Google Scholar]
- 69.Chen HL, Haack VS, Janecky CW, Vollendorf NW, Marlett JA. Mechanisms by which wheat bran and oat bran increase stool weight in humans. Am J Clin Nutr. 1998;68:711–719. doi: 10.1093/ajcn/68.3.711. [DOI] [PubMed] [Google Scholar]
- 70.Kanauchi O, Mitsuyama K, Saiki T, Fushikia T, Iwanaga T. Germinated barley foodstuff increases fecal volume and butyrate production in humans. Int J Mol Med. 1998;1:937–941. doi: 10.3892/ijmm.1.6.937. [DOI] [PubMed] [Google Scholar]
- 71.Jenkins DJ, Kendall CW, Vuksan V, Augustin LS, Li YM, Lee B, Mehling CC, Parker T, Faulkner D, Seyler H, et al. The effect of wheat bran particle size on laxation and colonic fermentation. J Am Coll Nutr. 1999;18:339–345. doi: 10.1080/07315724.1999.10718873. [DOI] [PubMed] [Google Scholar]
- 72.Vuksan V, Jenkins DJ, Vidgen E, Ransom TP, Ng MK, Culhane CT, O’Connor D. A novel source of wheat fiber and protein: effects on fecal bulk and serum lipids. Am J Clin Nutr. 1999;69:226–230. doi: 10.1093/ajcn/69.2.226. [DOI] [PubMed] [Google Scholar]
- 73.Gråsten SM, Juntunen KS, Poutanen KS, Gylling HK, Miettinen TA, Mykkänen HM. Rye bread improves bowel function and decreases the concentrations of some compounds that are putative colon cancer risk markers in middle-aged women and men. J Nutr. 2000;130:2215–2221. doi: 10.1093/jn/130.9.2215. [DOI] [PubMed] [Google Scholar]
- 74.McRorie J, Kesler J, Bishop L, Filloon T, Allgood G, Sutton M, Hunt T, Laurent A, Rudolph C. Effects of wheat bran and Olestra on objective measures of stool and subjective reports of GI symptoms. Am J Gastroenterol. 2000;95:1244–1252. doi: 10.1111/j.1572-0241.2000.02017.x. [DOI] [PubMed] [Google Scholar]
- 75.Hovey AL, Jones GP, Devereux HM, Walker KZ. Whole cereal and legume seeds increase faecal short chain fatty acids compared to ground seeds. Asia Pac J Clin Nutr. 2003;12:477–482. [PubMed] [Google Scholar]
- 76.Li J, Kaneko T, Qin LQ, Wang J, Wang Y. Effects of barley intake on glucose tolerance, lipid metabolism, and bowel function in women. Nutrition. 2003;19:926–929. doi: 10.1016/s0899-9007(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 77.McIntosh GH, Noakes M, Royle PJ, Foster PR. Whole-grain rye and wheat foods and markers of bowel health in overweight middle-aged men. Am J Clin Nutr. 2003;77:967–974. doi: 10.1093/ajcn/77.4.967. [DOI] [PubMed] [Google Scholar]
- 78.Muir JG, Yeow EG, Keogh J, Pizzey C, Bird AR, Sharpe K, O’Dea K, Macrae FA. Combining wheat bran with resistant starch has more beneficial effects on fecal indexes than does wheat bran alone. Am J Clin Nutr. 2004;79:1020–1028. doi: 10.1093/ajcn/79.6.1020. [DOI] [PubMed] [Google Scholar]
- 79.Gråsten SM, Juntunen KS, Mättö J, Mykkänen OT, El-Nezami H, Adlercreutz H, Poutanen KS, Mykkänen HM. High-fiber rye bread improves bowel function in postmenopausal women but does not cause other putatively positive changes in the metabolic activity of intestinal microbiota. Nutr Res. 2007;27:454–461. [Google Scholar]
- 80.Bird AR, Vuaran MS, King RA, Noakes M, Keogh J, Morell MK, Topping DL. Wholegrain foods made from a novel high-amylose barley variety (Himalaya 292) improve indices of bowel health in human subjects. Br J Nutr. 2008;99:1032–1040. doi: 10.1017/S000711450783902X. [DOI] [PubMed] [Google Scholar]
- 81.Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C, Gibson GR, Tuohy KM. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr. 2008;99:110–120. doi: 10.1017/S0007114507793923. [DOI] [PubMed] [Google Scholar]
- 82.Holmes AD. Experiments on the digestibility of wheat bran in a diet without wheat flour. Bulletin US Dept Agic. 1919;751:1–20. [Google Scholar]
- 83.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Panel on Macronutrients Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Available from: http://www.nal.usda.gov/fnic/DRI/DRI_Energy/energy_full_report.pdf (accessed 2013)
- 84.Canadian Food Inspection Agency. Guide to food labelling and advertising, Chapter 8: Section 8.10.2, Laxative and laxation claim. Available from: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/health-claims/eng/1392834838383/1392834887794?chap=7 (accessed December 12, 2013)
- 85.EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the substantiation of health claims related to wheat bran fibre and increase in faecal bulk (ID 3066), reduction in intestinal transit time (ID 828, 839, 3067, 4699) and contribution to the maintenance or achievement of a normal body weight (ID 829) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA J 2010; 8: 1817. Available from: http://www.efsa.europa.eu/en/scdocs/doc/1817.pdf (accessed 26 March 2015)
- 86.World Health Organization and Food and Agriculture Organization of the United Nations. Physiological effects of dietary fibre. In: Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Corporate Document Repository; Rome, Italy, 1998. Available from: http://www.fao.org/docrep/W8079E/W8079E00.htm (accessed March 26, 2015)
- 87.Lawton CL, Walton J, Hoyland A, Howarth E, Allan P, Chesters D, Dye L. Short term (14 days) consumption of insoluble wheat bran fibre-containing breakfast cereals improves subjective digestive feelings, general wellbeing and bowel function in a dose dependent manner. Nutrients. 2013;5:1436–1455. doi: 10.3390/nu5041436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, Scott MS, Simren M, Soffer E, Szarka L. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8–23. doi: 10.1111/j.1365-2982.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organization and Food and Agricultural Organization of the United Nations. Diet, nutrition and the prevention of chronic diseases. Report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series No. 916. Geneva: World Health Organization; 2003. [PubMed] [Google Scholar]
- 90.Piantadosi S. Clinical Trials: A Methodological Perspective, 2nd Edition. Volume 593 of Wiley Series in Probability and Statistics. New York: Wiley; 2005. [Google Scholar]


