Abstract
Background
Echinacea has antiviral activity against influenza viruses in vitro and has traditionally been used for treatment of colds and flu.
Objectives
This randomized, double-blind, double-dummy, multicenter, controlled clinical trial compared a new echinacea formulation with the neuraminidase inhibitor oseltamivir, the gold standard treatment for influenza.
Methods
Following informed consent, 473 patients with early influenza symptoms (≤48 hours) were recruited in primary care in the Czech Republic and randomized to either 5 days of oseltamivir followed by 5 days of placebo, or 10 days of an Echinacea purpurea-based formulation called Echinaforce Hotdrink (A. Vogel Bioforce AG, Roggwil, Switzerland). The proportion of recovered patients (influenza symptoms rated as absent or mild in the evening) was analyzed for noninferiority between treatment groups using a generalized Wilcoxon test with significance level α = 0.05 (2-sided) and using a CI approach in the per-protocol sample.
Results
Recovery from illness was comparable in the 2 treatment groups at 1.5% versus 4.1% after 1 day, 50.2% versus 48.8% after 5 days, and 90.1% versus 84.8% after 10 days of treatment with Echinaforce Hotdrink and oseltamivir, respectively. Noninferiority was demonstrated for each day and overall (95% CI, 0.487–0.5265 by generalized Wilcoxon test). Very similar results were obtained in the group with virologically confirmed influenza virus infections and in a retrospective analysis during the peak influenza period. The incidence of complications was lower with Echinaforce Hotdrink than with oseltamivir (2.46% vs 6.45%; P = 0.076) and fewer adverse events (particularly nausea and vomiting) were observed with Echinaforce Hotdrink.
Conclusions
Echinaforce Hotdrink is as effective as oseltamivir in the early treatment of clinically diagnosed and virologically confirmed influenza virus infections with a reduced risk of complications and adverse events. It appears to be an attractive treatment option, particularly suitable for self-care. Clinical trial identifier: Eudra-CT: 2010-021571-88. (Curr Ther Res Clin Exp. 2015; 77:66–72)
Key words: Complications, echinacea, influenza, noninferiority, oseltamivir, recovery
Introduction
Influenza presents a potential threat to human beings with seasonal epidemics and occasional severe pandemics, following reassortment of viral antigens. The World Health Organization estimates a total of 25 to 50 million cases each year resulting in 150,000 hospitalizations and 30,000 to 40,000 deaths in the United States alone, due to epidemic influenza. During pandemics the mortality and morbidity may be much higher, putting enormous pressure on basic health service provisions.1,2
Influenza illness is characterized by involvement of the lower respiratory tract (cough) accompanied with systemic complaints, including headache, myalgia, and fever.3,4 Acute onset of cough and fever are good clinical predictors, associated with positive tests for influenza virus in 79% to 88% of patients during acute epidemics. Results depend to a large extent on detection and sampling methods as well as the severity of epidemic.5,6 Influenza viruses replicate predominantly in the airway epithelia, but also in various other host organs and tissues leading to airway congestion, inflammation, and necrosis.7 Complications are frequent and severe complications include encephalitis, myelitis, myocarditis, intravascular coagulation, and septic shock.8,9 Patients with underlying respiratory or cardiovascular disorders, children, older adults, and immunocompromised individuals display higher rates of severe illness and mortality.10 Overall, acute respiratory tract infections severely influence morbidity and mortality during the winter and influenza plays a central role in this context.11,12
Neuraminidase inhibitors are recommended for the early treatment of influenza by the Centers for Disease Control and Prevention, the German Gesellschaft für Virologie, and the UK National Institute for Health and Care Excellence (NICE). The neuraminidase inhibitors oseltamivir and zanamivir have been demonstrated to reduce the duration and intensity of illness, but early intervention appears critical.13,14 Success of therapy depends on the sensitivity of causative viruses to these drugs. During the 2008–2009 winter season most seasonal H1N1 influenza subtypes had developed reduced sensitivity to oseltamivir. In subsequent years this resistant virus type was again replaced by more sensitive strains like H1N1pdm2009 or H3N2.15 Neuraminidase inhibitors are associated with adverse effects, including nausea, vomiting, psychiatric effects, and renal events. Safety issues, the importance of early administration, availability, and the potential emergence of resistance compromise the broad applicability of neuraminidase inhibitors as recently highlighted by Jefferson et al.16
Antiviral activities are found in plants, including echinacea. A hydroethanolic extract prepared from freshly harvested Echinacea purpurea has demonstrated strong activity against a series of influenza viruses (eg, H1N1, H3N2, H5N1, H7N7, and H1N1pdm2009).17,18 The extract exhibited no potential to induce resistance and inactivated oseltamivir-resistant H5N1 influenza viruses. In addition to the direct inhibition of influenza viruses, anti-inflammatory activities and modulation of the immune system may contribute to echinacea’s pharmacologic spectrum.19,20
We tested efficacy and safety of a newly developed preparation of Echinacea purpurea called Echinaforce Hotdrink (A. Vogel Bioforce AG, Roggwil Switzerland) for the treatment of acute influenza symptoms compared with the neuraminidase inhibitor oseltamivir.13,14 Patients with clinically diagnosed influenza were recruited as early as possible after symptom onset. The inclusion criteria were matched with those for which the comparator had demonstrated superior over placebo treatment in previous trials. The inclusion period corresponded with the circulation period of influenza viruses in the community. Nasal swab virus testing further enhanced diagnostic specificity.
Material and Methods
Study design
This was a randomized, double-blind, parallel, double-dummy clinical trial conducted at 29 general practices in the Prague area of the Czech Republic. It was conducted in accordance with the ethical principles of the Declaration of Helsinki/Good Clinical Practice Guideline and the applicable local regulatory requirements. The clinical trial (Eudra-CT: 2010-021571-88) was authorized by the competent national authorities (Štátny ústav pre kontrolu lieciv 16.11.2011) and a favorable opinion was granted by University Hospital Hradec Kralove, Ethics Committee (1.9.2011). Directive 2001/20/EC and the related detailed guidance ENTR CT1 and CT2 were applied, as described in national legal provisions.
From November 2011 to April 2013 eligible patients were approached and after informed, written consent was obtained, patients were randomly allocated to receive either Echinaforce Hotdrink for 10 days treatment or oseltamivir treatment for 5 days, followed by another 5 days of treatment with oseltamivir placebo. Rescue medication (paracetamol and dextromethorphan) was issued for treatment of very severe symptoms. Nasal samples were taken from participants at inclusion using midturbinate nasal swabs by the study personnel and were placed into a tube containing transport medium (CyMol; Copan, Brescia, Italy). The samples were kept at 4°C before shipment to the Provincial Health Services Authority British Columbia Center for Disease Control in Vancouver, Canada, for influenza virus detection via reverse transcription polymerase chain reaction. Participants received a diary to record influenza symptoms daily over the treatment period. After the treatment period patients were requested to return to the study center for a final visit. Returned medication was counted and the use of rescue medication was assessed. Blood samples were taken for analysis of hematologic and metabolic parameters before and after treatment.
Treatment
Echinaforce Hotdrink verum contains a hydroethanolic extract (65% v/v) of freshly harvested Echinacea purpurea. The tinctures from the herb (drug extraction ratio 1:12) and from the roots (drug extraction ratio 1:11) are combined at a ratio of 95% to 5%. Two hundred forty milligrams of the above active ingredient was concentrated to extractum spissum, which was supplemented with 276.5 mg Sambucus fructus succus recentis (elderberry), and excipients were added sufficient to give 1 mL Echinaforce Hotdrink. On analysis, the batch (No. 033492) was found to contain 883 μg/100 mL dodecatetraenoic acid isobutylamide and 101 mg/100 mL rutoside. The Echinaforce Hotdrink placebo contained the same excipients as verum plus colorants and flavors (Günter Aroma GmbH, Beinwil, Switzerland) for masking (batch No. 033493). Echinafore Hotdrink verum and corresponding placebo were filled into 200-mL dark-brown glass bottles by A. Vogel Bioforce AG under good manufacturing practice conditions.
The comparator was manufactured by overencapsulation of original oseltamivir capsules (Tamiflu 75 mg, batch No. 01130082; Hoffmann-La Roche AG, Basel, Switzerland) using optically dense, dark green, hard gelatine capsules, size 0 (Capsugel, Bornem, Belgium). Corden Pharma GmbH (Plankstadt, Germany) manufactured comparator capsules packed in high-density polyethylene bottles each containing 10 capsules. The corresponding placebo consisted of hard gelatine capsules filled with microcrystalline cellulose and were indistinguishable from verum capsules. The investigational products were manufactured under good manufacturing practice conditions and were batch released by Corden Pharma GmbH. Paracetamol and dextromethorphan were provided as rescue medication in form of Paralen 500 mg tablets and Stopex 30 mg tablets, respectively, sourced from Czech Republic, and were used according to the respective manufacturers’ instructions.
Dosing
Participants in the Echinaforce Hotdrink group were instructed to take, on the first 3 days, 5 × 5 mL Echinaforce Hotdrink verum syrup dissolved in approximately 150 mL hot water, and 3 × 5 mL on the following 7 days. Accurate dosing was ensured by using a measuring spoon, calibrated at 5 mL. In parallel, they were instructed to take oseltamivir placebo capsules twice a day over 10 days. The oseltamivir verum group were instructed to take the same regimen of Echinaforce Hotdrink placebo and 5 days of oseltamivir verum capsules, followed by 5 days of oseltamivir placebo capsules, twice daily.
Compliance was measured by weighing returned Echinaforce Hotdrink syrup and by counting capsules and rescue medication tablets.
Study participants and randomization
After identification of influenza activity by Euroflu.org and the Global Influenza Surveillance and Response System in the Czech Republic during November 2011, children and adults aged 12 to 70 years with acute influenza symptoms were screened for eligibility at 29 general practices around Prague until the end of the influenza season in April 2013. After obtaining informed consent, inclusion and exclusion criteria were checked. Clinical diagnosis of influenza was based on the presence of at least 1 respiratory symptom (eg, cough, sore throat, or nasal symptom), 1 constitutional symptom (eg, headache, malaise, myalgia, sweats and/or chills, and fatigue), and fever (≥37.8°C), with symptoms not present for more than 48 hours. A negative pregnancy test, body weight >40 kg, good general health, and a signed informed consent were prerequisites for inclusion.
The following exclusion criteria were applied: intake of antimicrobial agents during the past month; influenza vaccination during the past 12 months; suspected bacterial infection; bronchitis; intake of steroid or immune-suppressive medication; pregnancy or breastfeeding; chronic cardiac diseases; known endocrine disorders like diabetes mellitus; liver, kidney, and respiratory disorders; asthma; and serious chronic diseases influencing absorption, metabolism, and the elimination of the investigational product. Patients with known AIDS, autoimmune disease, as well as clinically significant chronic disease; illness requiring hospitalization; known allergy to plants of the Compositae family, paracetamol, or dextromethorphan; psychiatric disorders; neurologic and neurodevelopment conditions; planned surgical intervention during the trial period; alcohol or drug abuse; nicotine addiction; and participation in another trial were excluded. Women without effective contraception were also excluded.
In eligible patients, a physical examination was carried out, blood pressure and heart rate were assessed, and finally blood as well as a sample of nasal secretions were taken.
A randomization list was prepared by D.S.H. Statistical Services (Rohrbach, Germany) using Rancode Professional 3.6 (IDV, Gauting, Germany) with a block size of 4. The medication was distributed to the study sites in multiples of the randomization block. Every participant received medication on the basis of its identification number and in order of presentation.
Randomization codes were retained by the statistician in a sealed envelope and a copy was kept by the investigator. Only in case of emergency was the envelope to be opened to identify the treatment. At the final visit, the study patients were asked if they thought they knew which treatment they had received. Of the patients, 16.2% in the Echinaforce Hotdrink and 18.2% in oseltamivir group guessed which therapy they had received, whereas 83.8% and 81.4% admitted that they did not know. Successful blinding therefore was achieved.
Sample size calculation
With 200 evaluable patients in each group, the lower limit of the observed 1-sided 97.5% CI was expected to exceed the noninferiority limit of 0.42 (corresponding to a Cohen’s standardized difference of 15%21) with 89% power if the percentage of alleviated patients in the oseltamivir group was 80% and the Echinaforce Hotdrink was 78%. This is based on 10,000 simulations using the Newcombe-Wilson score method to construct the confidence interval.22 Because a generalized Wilcoxon test was used to evaluate all primary parameters combined (Day 1, 5, and 10), a power of at least 80% was expected for the combined hypothesis as well. Sample size was calculated with nQuery Advisor 7.0 (lower confidence limit for difference in proportions) (Statistical Solutions Ltd, Cork, Ireland).
Clinical outcomes and statistical analysis
Participants received a symptom diary to record their influenza symptoms in the morning and evening and throughout the study period or until recovery. Cough, nasal obstruction, sore throat, fatigue, headache, myalgia, feverishness, malaise, sweats, and/or chills were rated as 0 = not present, 1 = mild, 2 = moderate, or 3 = severe. Axillary body temperature was measured by electronic thermometer in degrees Celsius, sleeping disorders for the preceding night and ability to return to regular daily activities were scored yes/no, and intake of study medication as well as rescue and comedication and cotherapies was recorded. Occurrence of complications (eg, pneumonia, sinusitis, bronchitis, or other) was recorded at the close-out visit as was the need for intermed visits/contacts, hospitalization, and for antibiotic treatment. Patients and physicians rated tolerability as well as efficacy on a subjective basis using a Likert scale where 1 = very good, 2 = good, 3 = moderate, and 4 = poor. Finally, patients gave their opinions whether they would take the same medication again.
The primary end point of the study was the cumulative proportion of patients with influenza symptoms alleviated (recovered) after 1 day, 5 days, and 10 days of treatment. Recovery was defined as the first day when cough, nasal obstruction, sore throat, fatigue, headache, myalgia, and feverishness were rated as absent or mild in the evening. The analysis of noninferiority between treatments based on the per-protocol cohort, defined as those who fulfilled inclusion and exclusion criteria, took at least 80% of study medication, and did not take unauthorized concomitant medication.
Secondary variables included evaluation of further influenza symptoms, number of days with sleep disturbance, time point of return to normal activity, evolution of body temperature, use of rescue medication, and additional health care contacts. The proportion of patients experiencing respiratory respective gastrointestinal complications that required premature treatment stop was analyzed.
Statistical analysis used standard noninferiority methods. The combined hypothesis (inferiority of Echinaforce Hotdrink to oseltamivir on Days 1, 5, and 10 in terms of the primary end point) at the level of α = 0.05 was first tested. If this hypothesis was rejected single hypotheses were to be tested. These additional hypotheses were inferiority on single Days 1, 5, and 10 for Hypothesis 1, Hypothesis 2, and Hypothesis 3, and at a level of α/2 and α/3 with Bonferroni correction. Noninferiority for the individual measurements was considered to have been demonstrated when the lower confidence limit was above 0.42 in the Mann-Whitney statistics.
To detect adverse events (AEs) patients were asked, “Did you experience any unusual or unexpected symptom since your last visit, apart from the symptoms recorded in the diary?" Changes in concomitant diseases and medications as well as laboratory parameters were also taken into account before judgment of AE causality. Lowest level terms were chosen, which were translated into preferred terms before classification into system organ class using MedDRA Dictionary (version 13.1) (McLean, Virginia, USA). Blood samples were analyzed for erythrocytes, hematocrit, hemoglobin, leucocytes, Mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), thrombocytes, aspartate aminotransferase, cholesterol, creatinine, total bilirubin, and gamma-glutamyl transferase.
Results
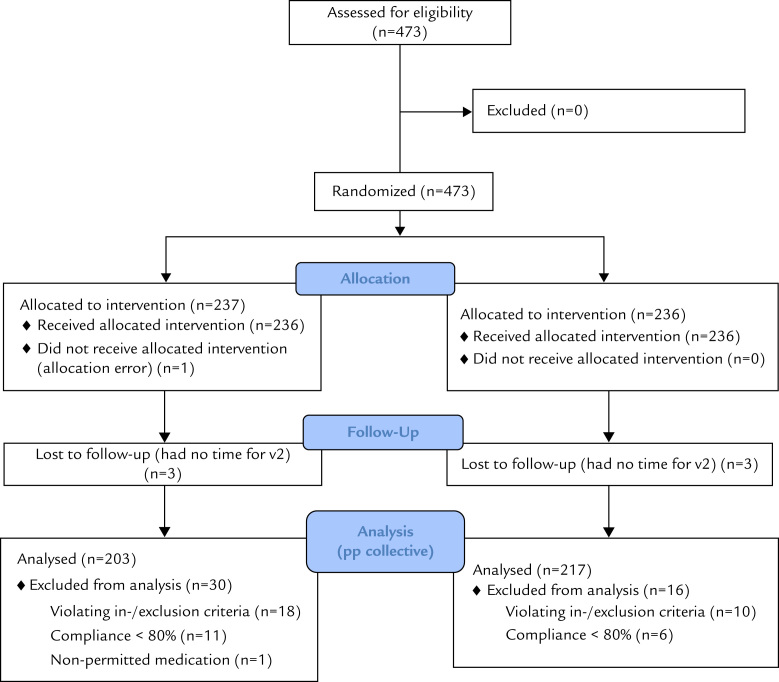
In total, 473 patients, all Caucasian with early acute influenza symptoms, were screened and enrolled in the study from November 22, 2011, until April 29, 2013. Of the 237 and 236 patients allocated to the 2 treatment groups (Echinaforce Hotdrink and oselatmivir, respectively), 6 were lost to follow-up because they did not attend the close-out appointment, and 1 patient did not receive the allocated intervention. Another 18 and 10 patients had major protocol violations, and compliance in 11 and 6 cases was <80% in the Echinaforce Hotdrink and comparator groups, respectively. These patients were excluded from the efficacy analysis. One patient took nonpermitted medication. Finally, a total of 203 and 217 patients were included in the per-protocol group for analysis of efficacy (Figure 1).
Figure 1.
Patient flow diagram.
Demographic data and other baseline characteristics
Patients in the 2 treatment groups were comparable with regard to age, body weight, body height, body mass index, and sex distribution (Table 1). Most participants (n = 464) were adults more than 18 years in age; mean age was ~37 years. Nine children and adolescents aged between 12 and 17 years were included in the study. Physical baseline values, including heart rate and blood pressure, were similar in the treatment groups. Obtained pregnancy tests were all negative and nasal secretions were collected from all but 1 patient (in the Echinaforce Hotdrink group).
Table 1.
Patient characteristics in the analysis collective (per protocol).
| Characteristic* | Echinaforce Hotdrink† (n = 203) | Oseltamivir (n = 217) | P value (Wilcoxon) |
|---|---|---|---|
| Age (y) | 37.7 (13.9) | 36.7 (13.1) | > 0.05 |
| Body weight (kg) | 77.7 (16.9) | 74.9 (15.5) | > 0.05 |
| Body height (cm) | 173.3 (9.9) | 173.2 (9.3) | > 0.05 |
| Body mass index | 25.8 (4.8) | 24.9 (4.1) | > 0.05 |
| Sex | |||
| Female | 94 (46.3) | 116 (53.5) | > 0.05 |
| Male | 109 (53.7) | 101 (46.5) |
Values for age, body weight, and body mass index are presented as mean (SD). Values for sex are presented as n (%).
A. Vogel Bioforce AG, Roggwil, Switzerland.
The most prominent respiratory symptom was cough, present in 87.2% and 85.3% of patients at inclusion. Congruent with the case definition and study indication, influenza lead symptoms, cough, myalgia, headache, and feverishness, were prominent, and the typical common cold symptom of sore throat less so (Table 2). There was a small but almost significant difference in the mean axillary temperature of patients at baseline: in the Echinaforce Hotdrink group it was 38.3°C (± 0.8°C) and 38.2°C (± 0.8°C) in the comparator group (P = 0.065). At inclusion, headache and cough showed a trend to greater severity in the Echinaforce Hotdrink group (P < 0.1). The mean (SD) duration of exposure to medication for Echinaforce Hotdrink was 10.1 (1.2) days and 9.9 (1.8) days in the comparator group.
Table 2.
Baseline influenza symptoms. Percentage of patients rating individual symptoms as “moderate” or “severe.”
| Symptom | Echinaforce Hotdrink* (n = 203) | Oseltamivir (n = 217) | P value (Mantel-Haenszel χ2) |
|---|---|---|---|
| Cough | 82.82 | 76.15 | 0.017 |
| Fatigue | 80.21 | 75.64 | 0.682 |
| Myalgia | 70.31 | 58.88 | 0.181 |
| Feverishness | 69.27 | 69.03 | 0.651 |
| Headache | 67.19 | 54.31 | 0.061 |
| Nasal obstruction | 66.67 | 59.39 | 0.131 |
| Sweats and chills | 59.16 | 61.42 | 0.576 |
| Sore throat | 44.79 | 48.22 | 0.414 |
| Malaise | 20.84 | 20.82 | 0.742 |
A. Vogel Bioforce AG, Roggwil, Switzerland.
Compliance and blinding
Among patients, 91.7% from the Echinaforce Hotdrink group and 90.5% of those in the comparator group took at least 80% of the planned study medication. Less than 80% of the study medication was taken by 8.3% and 9.5% of Echinaforce Hotdrink and comparator patients, respectively. For 3 patients in each group this was due to alleviation of symptoms before Day 5, and for 5 patients (2.2%) with Echinaforce Hotdrink and 13 patients (5.6%) with oseltamivir failure to take all of the medication was due to occurrence of an AE. Blinding was effective, with 83.8% and 91.4% not knowing whether they received Echinaforce Hotdrink or oseltamivir verum in the respective groups.
Efficacy analysis
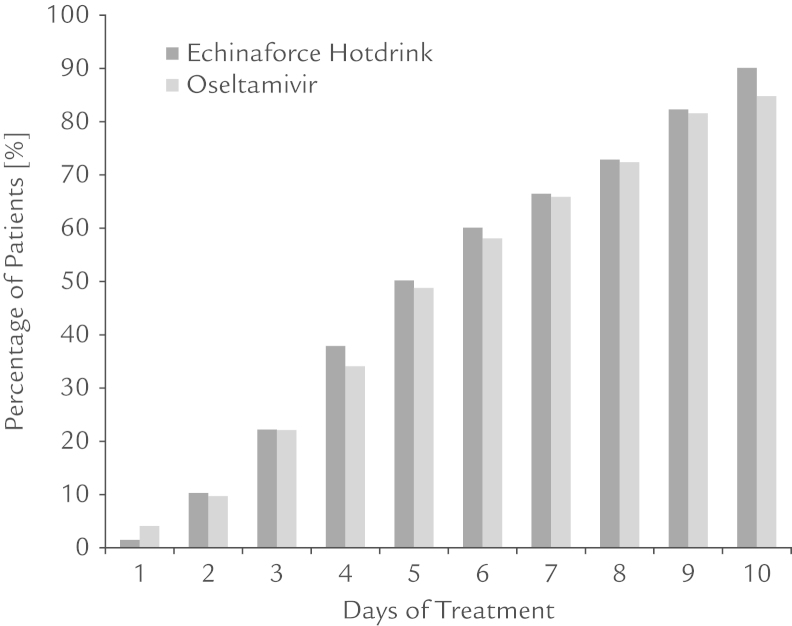
Primary and secondary variables of efficacy are displayed for the per-protocol group. Unless otherwise stated, the data correspond to the intention to treat group. Figure 2 shows the proportion of recovered patients through the treatment period; recovery rates were very similar for the 2 treatment groups. Alleviation of symptoms after 1, 5, and 10 days of treatment was observed in 1.5% and 4.1%; 50.2% and 48.8%; and 90.1% and 84.8% at 10 days in patients treated with Echinaforce Hotdrink and oseltamivir, respectively (as demonstrated in Table 3). Noninferiority of Echinaforce Hotdrink to oseltamivir was demonstrated for each day (Mann-Whitney statistics) as well as in the generalized analysis over all days of measurement (generalized Wilcoxon test, P[x<Y] = 0.5068; 95% CI, 0.4871–0.5265). The percentage of treatment failures after 10 days showed a nonstatistically significant trend favoring Echinaforce Hotdrink: 15.2% in the oseltamivir group versus 9.9% with Echinaforce Hotdrink (P > 0.05).
Figure 2.
Proportion of patients with alleviated influenza symptoms in the per-protocol group (n = 420). A total of 9.9% did not respond to Echinaforce Hotdrink (A. Vogel Bioforce AG, Roggwil, Switzerland) treatment in comparison to 15.2% who did not respond to oseltamivir treatment until Day 10.
Table 3.
Mann-Whitney statistics (MWS) and confidence limits of the noninferiority analysis are given for individual treatment days and the global test, applying alpha levels of 0.017 and 0.05, respectively (per protocol analysis, N = 420).
| Criterion | Alleviation after 1 day | Alleviation after 5 days | Alleviation after 10 days | Global test (Days 1, 5, and 10) |
|---|---|---|---|---|
| MWS | 0.4867 | 0.5070 | 0.5268 | 0.5068 |
| Confidence limits | 98.3% CI, (0.4676–0.5057) | 98.3% CI, (0.4487–0.5652) | 98.3% CI, (0.4884–0.5651) | 95% CI, (0.4871–0.5265) |
The above analysis concerned the total of patients with clinically diagnosed influenza illness. Next we tested for the primary variable in the subgroup with confirmed influenza virus in nasal samples at inclusion. Positive samples were obtained from a total of 41 patients (24.4% with influenza type A [H3 strain], 41.5% nontypeable influenza A, 31.7% influenza B viruses, and 2.4% of samples with coinfection of influenza A and B). The rates of recovery of patients with confirmed influenza virus infection were very similar to those in clinically diagnosed influenza patients (primary parameter). Alleviation of symptoms after 1, 5, and 10 days of treatment was observed in 0% and 0%; 45.0% and 42.9%; and 95.0% and 76.2% of patients treated with Echinaforce Hotdrink and oseltamivir, respectively. Again, inferiority of Echinaforce Hotdrink compared with oseltamivir was not detected in the generalized analysis or for the individual days of measurement. In contrast, at Day 10 Echinaforce Hotdrink was significantly superior in the 1-sided Wilcoxon test (P = 0.0365).
In another sensitivity analysis we compared outcomes during the period of peak influenza activity according to Euroflu and Global Influenza Surveillance and Response System data (calendar week 5–16 [2012] and calendar week 50 [2012]–calendar week 17 [2013]). Subgroups comprised a sample size of 347 and 367 patients, which represented a good part of the overall population, indicating selective recruiting during influenza season. In this group the recovery curves matched well with those of the whole group and again noninferiority could be demonstrated (data not shown).
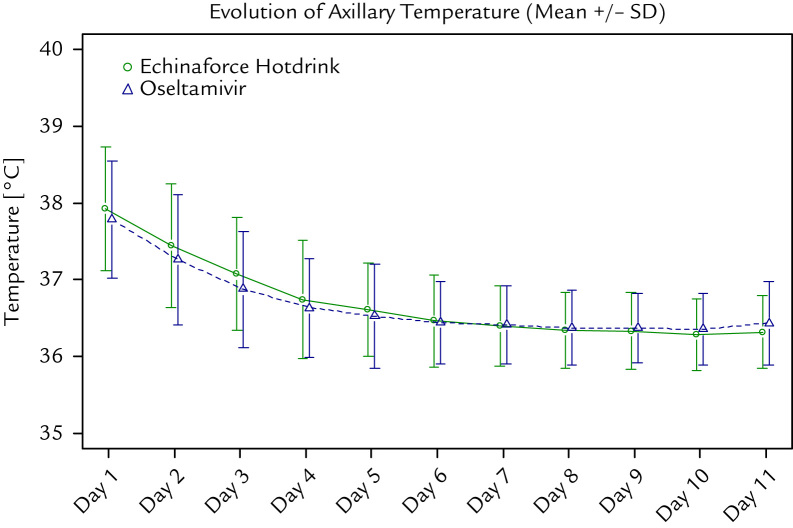
In the overall study group the individual symptoms resolved quickly and there was no noticeable difference between the treatment groups. The influenza lead symptoms cough, headache, and myalgia showed a marked reduction, which was slightly more marked in the Echinaforce Hotdrink group without reaching statistical significance (P > 0.05). Body temperature normalized to afebrile within 2 days of treatment in both groups (median, 2 days; P = 0.193). Figure 3 shows the evolution of axillary temperature in the 2 treatment groups. No difference between groups was observed in terms of return to normal daily activities and nights with sleeping disturbance (data not shown).
Figure 3.
Evolution of mean (SD) body temperature (˚C) during the study period.
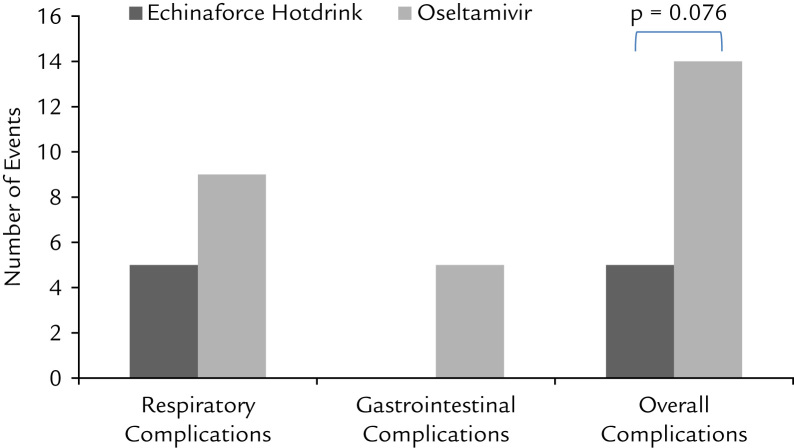
Complications are frequently seen in patients with influenza, and are a major reason for hospitalization and mortality. In previous studies, oseltamivir was shown to reduce incidence of lower respiratory tract complications from 10.3% to 4.6% (P < 0.001).23 A similar, relatively low incidence of complications was recorded in this study. In the oseltamivir group a total of 14 (6.5%) complications occurred. Nine (4.6%) concerned respiratory complications, including pneumonia (n = 2), sinusitis (n = 4), bronchitis (n = 2), and rhinopharyngitis (n = 1). In the Echinaforce Hotdrink group the incidence of complications was lower at 2.5% and this difference approaches statistical significance (χ2 test P = 0.079) (Figure 4). Antibiotic use was very low in both treatment groups at 1.8% and 2.0% in the oseltamivir and Echinaforce Hotdrink groups, respectively (P = 1.00).
Figure 4.
Occurrence of complications accompanying use of Echinaforce Hotdrink (A. Vogel Bioforce AG, Roggwil, Switzerland) and oseltamivir treatment.
No hospitalizations were reported during the investigational period in either treatment group. Patients had the option to contact the physician during the treatment period for any reason. About 7% of patients from the Echinaforce Hotdrink group and about 10% from the oseltamivir group requested an additional health care contact between the inclusion and close-out visits. The use of rescue medication was counted at the close-out visit, and no statistical difference was detected. The Echinaforce Hotdrink group took, over a mean of 3.1 and 3.5 days, a mean total of 7.1 and 7.6 g paracetamol and dextromethorphan, respectively. The comparator group took, over a mean of 3.1 and 3.8 days, a mean total of 6.3 and 8.1 g of the same medications (P = 0.17 to 0.86). At the end of the study, 85.2% and 86.6% of patients, respectively, were able to return to normal daily activities. There was no statistically significant difference between groups.
Finally, physicians and participants made a personal judgment of the efficacy of the treatment at the end of the study. One hundred eighty-six investigators (91.6%) judged Echinaforce Hotdrink as “good” or “very good” compared with 198 (91.2%) for the comparator. There was no statistically significant difference between groups (per-protocol population). Patient assessment was similarly positive with 87.2% and 86.2%, respectively, rating treatment efficacy as “good” or “very good.”
Safety analysis
Overall, 31 AEs were reported by 26 patients (11.4%) in the Echinaforce Hotdrink group (n = 229), and 44 AEs were reported by 32 patients (13.9%) in the comparator group (n = 231). Ten events were assessed to be related to the study medication—4 events from 4 patients (1.7%) treated with Echinaforce Hotdrink and 6 events from 5 patients (2.2%) treated with oseltamivir. Five (2.2%) and 12 (5.2%) patients stopped Echinaforce Hotdrink and comparator treatment because of AEs. The higher incidence of AEs in the oseltamivir group was largely due to gastrointestinal disorders like nausea and vomiting. Nausea and vomiting were 5 times more frequent with oseltamivir (15 reports) than with Echinaforce Hotdrink (3 reports) and in 9 cases led to treatment cessation. No serious AEs such as encephalitis, myelitis, myocarditis, or septic shocks were reported. No clinically relevant changes in metabolic or hematologic laboratory parameters (erythrocytes, leucocytes, thrombocytes, hemoglobin, hematocrit, MCV, MCH, MCHC, creatinine, cholesterol, total bilirubin, aspartate aminotransferase, and gamma-glutamyl transferase) were observed between the inclusion and close-out visits.
Two hundred nineteen patients (95.6%) from the Echinaforce Hotdrink group and 214 patients (93%) from the oseltamivir group judged the tolerability of the treatment as “good” or “very good.” There was no statistically significant difference between groups.
Discussion
The clinical efficacy and safety of a novel formulation of Echinacea purpurea, Echinaforce Hotdrink, was compared with oseltamivir in patients with early symptoms of influenza. The study design and conditions matched with large clinical trials showing superiority of oseltamivir over placebo treatment. An early inclusion of patients (mean 25.1 hours after symptom onset) was achieved by recruiting patients in primary care. Under these conditions, noninferiority of Echinaforce Hotdrink to oseltamivir was demonstrated in terms of recovery from illness.
The specificity and robustness of effects was further examined, first in virologically confirmed influenza virus infections and second by focusing on infections during peak influenza activity. The detection efficiency of influenza viruses was relatively low but comparable with reports from the Canadian Respiratory Virus Detection Surveillance System. They found a consistent synchronization of negative with positive tests for influenza (but not with other respiratory viruses), which was associated with a high number of false negative results using a Poisson regression model.24 The predominance of influenza viruses finally can be concluded from the predominance of influenza cardinal symptoms over the negative predictor sore throat in the included sample of our study. The therapeutic effects of Echinaforce Hotdrink appear robust and consistent in varying scenarios; for example, clinically diagnosed influenza, during influenza peak activity, as well as in virologically confirmed influenza virus infections.
The results of this double-blind randomized controlled trial are consistent with in vitro results obtained from the studied echinacea extract (Echinaforce). A variety of influenza A and B virus types, including the strains observed in this study (H3N2, H5N1, H1N1pdm09, and influenza B), are sensitive to the extract at low concentrations of 0.1 to 50 μg/mL.17,18 Likewise, echinaforce significantly prevented membranous virus infections, including influenza, during 4 months of application of echinacea extract in comparison to placebo treatment.25 Emergence of drug resistance was not observed even after serial passaging of H1N1 viruses in vitro.17
Despite slightly higher baseline values of pyrexia, cough, headache, and myalgia in the Echinaforce Hotdrink group, equalization of symptoms between groups was achieved after 2 to 3 days. Fever resolved within 2 days of treatment and cardinal influenza symptoms were effectively treated with both therapies. At the end of the treatment period, more patients had recovered in the Echinaforce Hotdrink group, although the difference was borderline significant.
Previous studies of oseltamivir of comparable design have yielded results comparable to ours. Complications are frequently observed in patients with influenza and are a major reason for hospitalization and mortality. In comparable clinical trials, complications were observed in 5.3% to 18.5% in placebo groups, depending on the general health.23 In these studies oseltamivir reduced incidence of lower respiratory tract complications from 10.3% to 4.6% on average (P < 0.001).
Similar incidences were detected in our study: with oseltamivir, a total of 6.5% patients developed any complication (4.6% affecting the respiratory tract). With Echinaforce Hotdrink treatment complications, were very low at 2.5% and the difference to the comparator was almost statistically significant. There were no hospitalizations in either treatment group.
In comparison with the existing literature, both therapies performed well and were similarly effective in the treatment of influenza illness and its complications. Likewise, a reduced need for antibiotics was observed; this has been up to 19% in placebo-treated patients in similar trials.23 In our study, in both treatment groups the percentage of patients requiring antibiotics was low (2% and 1.8%) and not significantly different between groups.
The presence of oseltamivir-resistant influenza virus strains (ie, seasonal influenza A H1N1) might undermine the assay sensitivity of our study because of reduced efficacy of the comparator with these strains. The World Health Organization-collaborating Centre for Reference and Research on Influenza, which reviewed the susceptibility of recent human and avian influenza virus types, found the H1N1pdm09, H3N2, and influenza B strains to be fully sensitive to neuraminidase inhibitors in general, and oseltamivir in particular, during this time frame.26
Gastrointestinal disorders have been reported for oseltamivir treatment with an incidence of 10.7%. These include nausea, vomiting, and diarrhea (summary of product characteristics, Tamiflu, Hoffmann-La Roche AG, Basel, Switzerland). In our study, gastrointestinal complaints in 9 patients lead to cessation of therapy in the oseltamivir group, which in 5 cases prevented recovery from influenza illness. No such event occurred with Echinaforce Hotdrink, and safety was clearly in favor of the latter therapy.
In view of the comparable benefits (recovery from illness), possible advantages (lack of induction of drug resistance and complications), and the lower incidence of AEs, treatment of influenza with Echinaforce Hotdrink overall outperformed treatment with oseltamivir in the studied patient cohort. Further studies are warranted to show the extent to which our results are applicable to patients with concomitant diseases and at-risk populations, which were not studied here.
Conclusions
Neuraminidase inhibitors are the main current therapeutic option for the treatment of influenza and are recommended by authoritative bodies in several countries. Risk of drug resistance, safety issues, and limited availability challenge their usefulness for the early influenza therapy in reality. Echinaforce Hotdrink has been demonstrated to be an attractive therapy for acute influenza treatment with a better safety and a comparable efficacy profile to the neuraminidase inhibitor oseltamivir. Its availability as an over-the-counter medicine allows for a very early treatment start, which is central for treatment success with any intervention. Further studies are warranted.
Conflicts of Interest
This study was sponsored by A. Vogel Bioforce AG, Roggwil, Switzerland, manufacturer of Echinaforce Hotdrink. R. Schoop is an employee of Bioforce AG, and K. Rauš and P. Klein have received honorarium funds from the study sponsor. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
P. Fischer and S. Pleschka were involved in data interpretation and writing of the manuscript, K. Rauš was involved in planning and conducting the clinical study, P. Klein provided power calculation and developed the study protocol and the statistical analysis; and R. Schoop was involved in writing the study protocol and manuscript.
Footnotes
Supplementary data associated with this article can be at 10.1016/j.curtheres.2015.04.001.
Supplementary Materials
Supplementary Material
References
- 1.Fleming D.M., Elliot A.J., Nguyen-van Tam J.S. A winter’s tale: Coming to terms with winter respiratory illnesses. Health Protection Agency; London: 2005. [Google Scholar]
- 2.Weber O. The role of viruses in the etiology and pathogenesis of common cold. In: Eccles R.W.O., editor. Common Cold. Birkhäuser Verlag; Basel, Switzerland: 2009. pp. 132–133. [Google Scholar]
- 3.Call S.A., Vollenweider M.A., Hornung C.A. Does this patient has influenza? JAMA. 2005;293:987–997. doi: 10.1001/jama.293.8.987. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson K.G. Clinical features of influenza. Semin Respir Infect. 1992;7:26–37. [PubMed] [Google Scholar]
- 5.Monto A.S., Gravenstein S., Elliott M. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 6.Boivin G., Hardy I., Tellier G. Predicting influenza infections during epidemics with use of a clinical case definition. Clin Infect Dis. 2000;31:1166–1169. doi: 10.1086/317425. [DOI] [PubMed] [Google Scholar]
- 7.Guarner J., Paddock C.D., Shieh W.H. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003-2004 season. Clin Infect Dis. 2006;43:132–140. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- 8.Studahl M. Influenza virus and CNS manifestations. J Clin Virol. 2003;28:225–232. doi: 10.1016/s1386-6532(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 9.Jaimovich D.G., Kumar A., Shabino C.L., Formoli R. Influenza B virus infections associated with non-bacterial septic shock-like illness. J Infect. 1992;25:311–315. doi: 10.1016/0163-4453(92)91659-y. [DOI] [PubMed] [Google Scholar]
- 10.Cate T.R. Clinical manifestations and consequences of influenza. Am J Medicine. 1987;82:15–19. doi: 10.1016/0002-9343(87)90555-9. [DOI] [PubMed] [Google Scholar]
- 11.Reichert T.A., Simonsen L., Sharma A. Influenza and the winter increase in mortality in the United States, 1959-1999. Am J Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson K.G., Kent J., Hammersley V., Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ. 1996;313:1119–1123. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treanor J.J., Hayden F.G., Vrooman P.S. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 14.Aoki F.Y., Macleod M.D., Paggiaro P., IMPACT Study Group Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123–129. doi: 10.1093/jac/dkg007. [DOI] [PubMed] [Google Scholar]
- 15.Whitley R.J., Boucher C.A., Lina B. Global assessment of resistance to neuraminidase inhibitors, 2008-2011: the Influenza Resistance Information Study (IRIS) Clin Infect Dis. 2013;56:1197–1205. doi: 10.1093/cid/cis1220. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson T., Jones M.A., Doshi P. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014:4. doi: 10.1002/14651858.CD001265.pub3. CD008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pleschka S., Stein M., Schoop R., Hundson J.B. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV) Virol J. 2009;6:197. doi: 10.1186/1743-422X-6-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma M., Anderson S.A., Schoop R., Hudson J.B. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antiviral Res. 2009;83:165–170. doi: 10.1016/j.antiviral.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Sharma M., Schoop R., Hudson J.B. Echinacea as an anti-inflammatory agent: the influence of physiologically relevant parameters. Phytother Res. 2009;23:863–867. doi: 10.1002/ptr.2714. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie M.R., Gertsch J., Klein P., Schoop R. Effects of Echinaforce® treatment on ex vivo-stimulated blood cells. Phytomedicine. 2011;18:826–831. doi: 10.1016/j.phymed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Colditz G.A., Miller J.N., Mosteller F. Measuring gain in the evaluation of medical technology: the probability of a better outcome. Int J Technol Assess Health Care. 1988;4:637–642. doi: 10.1017/s0266462300007728. [DOI] [PubMed] [Google Scholar]
- 22.Newcombe R.G. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1988;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser L., Wat C., Mills T. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Internal Med. 2003;163:1667–1672. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- 24.Schanzer D.L., Garner M.J., Hatchette T.F. Estimating Sensitivity of Laboratory Testing for Influenza in Canada through Modelling. PLoS ONE. 2009;4:1–7. doi: 10.1371/journal.pone.0006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jawad M., Schoop R., Suter A. Safety and efficacy profile of echinacea purpurea to prevent common cold episodes: a randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2012:841315. doi: 10.1155/2012/841315. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh D.Y., Hurt A.C. A review of the antiviral susceptibility of human and avian influenza viruses over the last decade. Scientifica (Cairo) 2014:430629. doi: 10.1155/2014/430629. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material