Dear Editor,
The Hippo-YAP pathway is an evolutionally conserved signaling module that controls tissue growth during development and its dysregulation causes cancer1. Core components of the Hippo pathway include a kinase cascade comprising MST1/2 and LATS1/2 kinases, in which MST1/2 phosphorylates and activates LATS1/22. The major downstream effectors of the Hippo pathway are the transcriptional co-activators YAP and TAZ, which are phosphorylated and inhibited by LATS1/23,4. Unphosphorylated YAP/TAZ localizes in the nucleus and promotes target gene expression through binding to the TEAD family transcription factors5,6. Protein kinase C (PKC) controls a broad range of biological processes and can be classified into three sub-groups based on sequence homology and activation mechanisms: the Ca2+- and diacylglycerol (DAG)-dependent conventional PKC (cPKC α, βI, βII, and γ), the DAG-dependent novel PKC (nPKC δ, θ, ɛ, and η), and the Ca2+- and DAG-independent atypical PKC (aPKC ι and ζ)7. Recent studies have established that extracellular diffusible signals act through G-protein coupled receptors (GPCRs) to regulate the Hippo-YAP pathway8,9. PKC represents one of the major effectors downstream of GPCRs (especially Gq/11-coupled receptors). This led us to investigate whether PKC regulates the Hippo-YAP pathway.
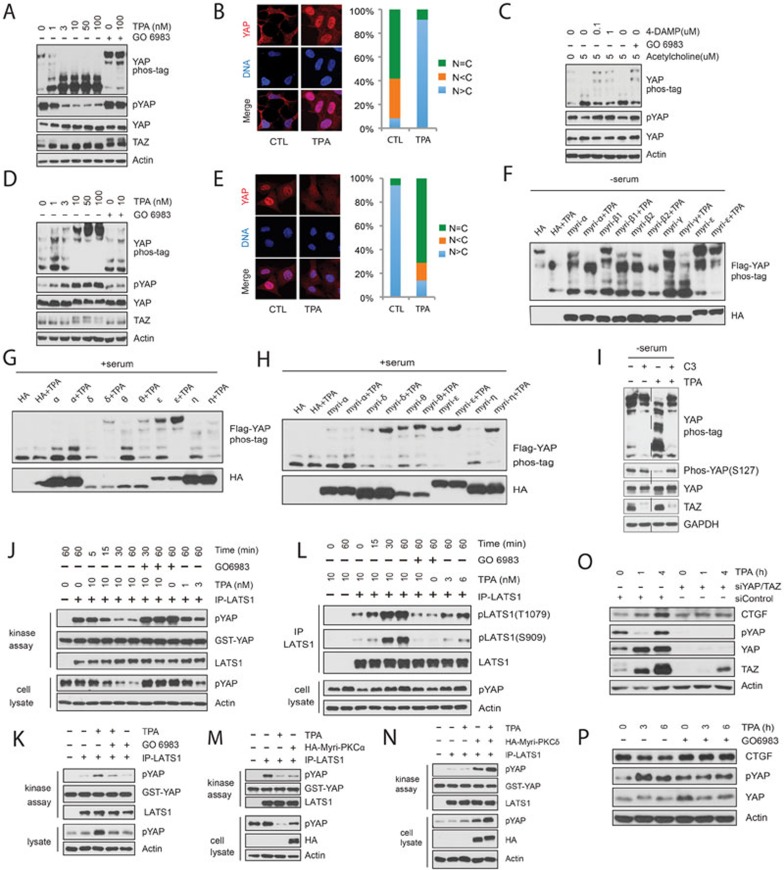
We used the DAG analog TPA to activate PKC in HEK293A cells and observed that TPA induced a rapid and robust YAP dephosphorylation as determined by western blotting using the phospho-specific (serine 127) YAP antibody and by differential electrophoretic mobility shift on phos-tag-containing gels. Similarly, TPA also induced TAZ dephosphorylation as indicated by a faster electrophoretic migration (Figure 1A). TPA-induced YAP dephosphorylation was also observed in HeLa and U251MG cells (Supplementary information, Figure S1A and S1B). Phosphorylated YAP localizes in the cytoplasm, whereas dephosphorylated YAP translocates to the nucleus to promote target gene expression. Consistent with this model, TPA induced YAP nuclear localization in HEK293A cells (Figure 1B). Moreover, GO6983, an inhibitor of both cPKC and nPKC, effectively blocked TPA-induced YAP/TAZ dephosphorylation (Figure 1A). These observations suggest a role of PKC in YAP/TAZ activation.
Figure 1.
Opposing roles of conventional and novel PKC isoforms in Hippo-YAP pathway regulation. (A) TPA induces YAP and TAZ dephosphorylation in HEK293A cells. Cells were starved with serum-free medium for 12 h and treated with various amounts of TPA for 1 h. Where indicated, cells were pretreated with 200 nM PKC inhibitor GO6983 for 30 min. Cell lysates were analyzed by western blotting with indicated antibodies. Yap phosphorylation status was also determined by differential electrophoretic mobility shift on phos-tag-containing gels. (B) TPA promotes YAP nuclear accumulation in HEK293A cells. (C) Acetylcholine acts through muscarinic acetylcholine receptor M3 and PKC to activate YAP. Experiments were performed similarly as described in A. (D) TPA promotes YAP/TAZ phosphorylation in Swiss3T3 cells. Cells were cultured in serum-containing medium and stimulated with various doses of TPA for 1 h as indicated. (E) TPA promotes YAP cytoplasmic localization in MEF cells. (F) The conventional PKC promotes YAP dephosphorylation. 500 ng of individual HA-tagged myri-PKC isoforms and 2 ng of Flag-YAP were co-transfected into HEK293A cells. 24 h after transfection, cells were serum starved for 12 h and stimulated with 10 nM TPA for 1 h. (G, H) Expression of novel PKC enhances YAP phosphorylation in HEK293A cells. Indicated plasmids were co-transfected into HEK293A cells. 36 h later, cells were stimulated with fresh 10% serum-containing medium for 40 min followed by 10 nM TPA for 1 h. (I) Exoenzyme C3 prevents TPA-induced YAP dephosphorylation in HEK293A cells. 24 h after serum starvation, cells were pretreated with 2 μg/ml C3 for 4 h and then stimulated with TPA for 1 h. (J) TPA inactivates LATS in HEK293A cells. Serum-starved cells were stimulated with TPA as indicated. Endogenous LATS1 was immunoprecipitated for the kinase assay using GST-YAP as the substrate in vitro. Phosphorylation states of endogenous YAP in cell lysates were shown for comparison. (K, L) TPA activates LATS in Swiss3T3 cells. Experiments were performed similarly as described in J except that Swiss3T3 cells were not serum starved and that endogenous LATS1 was immunoprecipitated for immunoblotting with phosphor-LATS antibodies as indicated in L. (M) Constitutively active PKCα decreases LATS kinase activity. HA-Myri-PKCα was transfected into HEK293A cells as indicated. Cells were serum starved for 12 h and treated with 10 nM TPA for 1 h. Endogenous LATS1 was immunoprecipitated for kinase activity assay. (N) Constitutively active PKCδ promotes LATS activation. Plasmid encoding HA-Myri-PKCδ was transfected into HEK293A cells. 36 h after transfection, cells were first stimulated with 10% serum for 40 min and then with 10 nM TPA for 1 h. Endogenous LATS1 was immunoprecipitated for kinase assay. (O) TPA induces a YAP/TAZ-dependent CTGF accumulation in HEK293A cells. Control or YAP/TAZ-knockdown HEK293A cells were serum-starved for 12 h and then stimulated with TPA for various time points as indicated. Cell lysates were analyzed by immunoblotting. Notably, YAP phosphorylation recovered 4 h after TPA stimulation. This could be due to a transient LATS inhibition by TPA or a negative feedback induced by YAP/TAZ activation. (P) TPA decreases CTGF expression level in Swiss3T3 cells. Swiss3T3 cells were cultured in the presence of 10% serum for 12 h and then stimulated with TPA for the indicated time. Cell lysates were analyzed by immunoblotting.
Next, we examined whether activation of PKC by physiological stimuli would activate YAP/TAZ. We found that addition of acetylcholine to U251MG cells, which express the Gq/11-coupled muscarinic acetylcholine receptor M3, resulted in significant YAP dephosphorylation (Figure 1C). Inhibition of either M3 receptor by 4-DAMP or PKC by GO6983 suppressed acetylcholine-induced YAP dephosphorylation (Figure 1C), suggesting that YAP activation by acetylcholine is mediated by the M3 receptor and PKC. Moreover, Gq/11 knockdown in U251MG cells suppressed YAP dephosphorylation by acetylcholine (Supplementary information, Figure S1C). Collectively, these data indicate that PKC acts downstream of Gq/11-coupled receptors to activate YAP.
In the efforts to examine the effect of TPA on YAP/TAZ phosphorylation in different cell types, we were surprised that TPA induced a dramatic YAP/TAZ phosphorylation in Swiss3T3 cells (Figure 1D), a response completely opposite to what was observed in HEK293A and several other cell lines (Figure 1A and Supplementary information, Figure S1A and S1B). Notably, TPA-induced YAP/TAZ phosphorylation could be blocked by GO6983 (Figure 1D), indicating that PKC activation was also responsible for the increase of YAP/TAZ phosphorylation in Swiss3T3 cells. Similar effects were observed in MEF cells and the lung cancer A549 cells (Supplementary information, Figure S1D and S1E). Consistent with the increased YAP phosphorylation, TPA treatment promoted a YAP cytoplasmic localization in MEF cells (Figure 1E). Although seemingly paradoxical, the above results are very interesting and show that PKC can either positively or negatively regulate YAP activity in a cell-type-dependent manner.
The opposing effects of TPA on YAP phosphorylation observed in different cell types prompted us to speculate that different PKC isoforms may exert opposite effects on YAP regulation. To test this, individual cPKC or nPKC isoform was co-transfected with YAP into HEK293A cells and TPA-induced YAP phosphorylation/dephosphorylation was measured. Overexpression of cPKC (α, β1, β2, or γ) had minor effects on YAP phosphorylation in HEK293A cells (Supplementary information, Figure S1F). We then fused the Src membrane-targeting sequence (myristoylation signal) to the N terminus of each individual PKC to render them constitutively active (also referred to as myri-PKC). Overexpression of myristoylated cPKC (α, β1, β2, or γ) was sufficient to induce YAP dephosphorylation even in the absence of TPA. Stimulation with TPA led to further dephosphorylation of YAP (Figure 1F). These results indicate that cPKCs promote YAP dephosphorylation and activation. In contrast to the cPKC, overexpression of nPKC (δ, θ, or ɛ) blocked TPA-induced YAP dephosphorylation in HEK293A cells (Supplementary information, Figure S1G). Although PKCη expression decreased YAP phosphorylation in the absence of TPA, it also suppressed TPA-induced YAP dephosphorylation. The above results indicate that nPKCs promote YAP phosphorylation. Consistent with our previous studies, serum induced YAP dephosphorylation (comparing the first lanes of Figure 1F and 1G). We examined the effect of nPKC overexpression on YAP phosphorylation in the presence of serum. Ectopic expression of PKCɛ strongly increased YAP phosphorylation in the presence of serum and expression of PKCδ, PKCθ, and PKCη had resulted in a mild-to-moderate increase of YAP phosphorylation. As expected, TPA had little effect on YAP phosphorylation in the control or PKCα-transfected cells because YAP was already largely dephosphorylated in the presence of serum (Figure 1G). Remarkably, TPA further increased YAP phosphorylation levels in nPKC-transfected HEK293A cells (Figure 1G). These data demonstrate that nPKC activation by TPA induces YAP phosphorylation. Consistently, expression of constitutively active nPKC, increased YAP phosphorylation in the presence of serum (Figure 1H). Collectively, our results show that cPKC and nPKC have opposite effects on YAP phosphorylation, leading to YAP activation and inhibition, respectively. Moreover, ectopic expression of nPKC in HEK293A cells can reverse the cellular response from YAP activation to YAP inhibition in response to TPA.
The above observation suggests that the relative expression levels of cPKC and nPKC may account for the cell type-specific TPA response. mRNA levels of PKC isoforms were quantified in representative cell lines (Supplementary information, Figure S1H). However, no simple correlation could be observed between the levels of cPKC vs nPKC mRNA and the TPA-induced YAP dephosphorylation vs phosphorylation. We speculate that YAP regulation by PKC may also be influenced by additional factors that are differentially expressed in a cell type-dependent manner.
Rho GTPases have been established as key mediators in transducing GPCR signals to YAP activation8,9. We used botulinum toxin C3 to inactivate RhoA and found that C3 blocked TPA-induced YAP dephosphorylation in HEK293A cells (Figure 1I). Consistently, expression of Rho GDI, which inhibits Rho GTPases, also blocked TPA-induced YAP dephosphorylation (Supplementary information, Figure S1I). Furthermore, overexpression of an active RhoA Q63L mutant suppressed the effect of PKC inhibition (Supplementary information, Figure S1J). Collectively, these data suggest a pathway that cPKC modulates YAP activity through Rho GTPases.
We next examined the role of MST and LATS in PKC-induced YAP activation in HEK293A cells. MST1/2 double knockout slightly decreased YAP phosphorylation. However, TPA-induced YAP dephosphorylation was not affected, indicating that MST1/2 are not required for YAP regulation by PKC (Supplementary information, Figure S1K). Moreover, TPA-induced YAP dephosphorylation was blunted by ectopic LATS2 expression (Supplementary information, Figure S1L), supporting a role of LATS in mediating the PKC signal to YAP regulation. The involvement of MST-LATS kinase cascade in PKC-mediated YAP regulation was also examined in MEF cells. TPA-induced YAP phosphorylation was completely abolished in LATS1/2-knockout, but not MST1/2-knockout, MEF cells (Supplementary information, Figure S1M).
To test whether PKC modulates LATS activity, LATS1 was immunoprecipitated from HEK293A cells stimulated with TPA for various periods and then subjected to the in vitro kinase assay using GST-YAP as the substrate. As shown in Figure 1J, TPA rapidly decreased LATS1 kinase activity in HEK293A cells. The time course of LATS inhibition by TPA paralleled that of endogenous YAP dephosphorylation, consistent with a role of LATS in TPA response. LATS1 and 2 are activated by phosphorylation of the activation loop (S909 for LATS1) and the hydrophobic motif (T1079 for LATS1). We found that TPA decreased the phosphorylation level of LATS1 on T1079 (Supplementary information, Figure S1N). Interestingly, TPA treatment significantly enhanced LATS1 kinase activity in Swiss3T3 cells, as evidenced by both increased kinase activity and elevated phosphorylation levels of LATS1 on T1079 and S909 (Figure 1K and 1L). To further test the role of cPKC and nPKC, constitutively active myri-PKCα and myri-PKCδ were transfected into HEK293A cells and LATS1 kinase activity was measured. Overexpression of myri-PKCα significantly suppressed LATS1 kinase activity along with the reduced YAP phosphorylation level (Figure 1M). On the contrary, overexpression of myri-PKCδ markedly increased LATS1 kinase activity. Full activation of myri-PKCδ by TPA treatment further enhanced LATS1 kinase activity and YAP phosphorylation (Figure 1N). Taken together, we conclude that cPKC inhibits LATS kinase activity whereas nPKC activates LATS.
We examined the expression of YAP target gene CTGF. TPA increased CTGF expression in HEK293A cells in a YAP/TAZ-dependent manner (Figure 1O). On the contrary, TPA decreased CTGF expression level in Swiss3T3 cells and inhibition of PKC with GO6983 blocked the effect of TPA on CTGF protein levels (Figure 1P). These results are consistent with the effects of TPA on YAP/TAZ phosphorylation in HEK293A and Swiss3T3 cells, and support a role of YAP/TAZ in PKC-induced gene regulation, especially cell type-dependent induction or repression.
The Hippo-YAP pathway plays a major role in development and organ size control, and its dysregulation is widely observed in human cancers1,10,11. Given the importance of this pathway, it must be tightly controlled. In this study, we have demonstrated that PKC mediates GPCR signaling, likely downstream of Gq/11 activation, to modulate YAP activity. Notably, Gq/11-activating mutation is found in ∼70% of uveal melanoma, the most common adult eye cancer. Consistent with our findings, YAP has been shown to be critical in mutant Gq/11-induced tumorigenesis and PKC inhibitors suppresses uveal melanoma in a mouse model12,13,14. An interesting and surprising finding of this study is that different PKC isoforms have different effects on LATS and YAP phosphorylation. The cPKC activates YAP by inducing its dephosphorylation while the nPKC inhibits YAP by stimulating its phosphorylation. These opposing effects are achieved by cPKC-induced LATS inhibition and nPKC-induced LATS activation. A fascinating and challenging issue in biological research is cell type-specific response. For example, one hormone may induce opposite effects in different tissues or cell types. We know very little about the molecular basis for cell type-specific responses. This study provides one possible underlying mechanism by which different cell types can produce different or even opposite responses in response to the same stimulus. Remarkably, a simple ectopic expression of nPKC can actually convert the TPA-induced YAP activation to inhibition in HEK293A cells, which normally show YAP activation upon TPA treatment. Our study provides an intriguing example of molecular engineering the specificity of cellular response to a given signal and reveals a biochemical basis for cell type-specific responses frequently observed in cell biology.
Acknowledgments
We thank Drs Frank B Furnari, Nikhil Rao, and Alexandra Newton (UCSD, USA) for reagents and insightful discussion. This work was supported by NIH grants (GM51586 and EY022611 to KLG, and GM7752 to AWH and SWP) and the National Natural Science Foundation of China (31030019, YX). FXY was supported in part by China “Thousand Youth Talents” and Shanghai “Oriental Scholar” grants.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Conventional and novel PKC oppositely regulate the Hippo-YAP pathway.
References
- Yu FX, Guan KL. Genes Dev. 2013. pp. 355–371. [DOI] [PMC free article] [PubMed]
- Wu S, Huang J, Dong J, Pan D. Cell. 2003. pp. 445–456. [DOI] [PubMed]
- Dong J, Feldmann G, Huang J, et al. Cell. 2007. pp. 1120–1133. [DOI] [PMC free article] [PubMed]
- Zhao B, Wei X, Li W, et al. Genes Dev. 2007. pp. 2747–2761. [DOI] [PMC free article] [PubMed]
- Zhao B, Ye X, Yu J, et al. Genes Dev. 2008. pp. 1962–1971. [DOI] [PMC free article] [PubMed]
- Lei QY, Zhang H, Zhao B, et al. Mol Cell Biol. 2008. pp. 2426–2436. [DOI] [PMC free article] [PubMed]
- Newton AC. Am J Physiol Endocrinol Metab. 2010. pp. E395–E402. [DOI] [PMC free article] [PubMed]
- Yu FX, Zhao B, Panupinthu N, et al. Cell. 2012. pp. 780–791. [DOI] [PMC free article] [PubMed]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Genes Dev. 2012. pp. 2138–2143. [DOI] [PMC free article] [PubMed]
- Harvey K, Tapon N. Nat Rev Cancer. 2007. pp. 182–191. [DOI] [PubMed]
- Pan D. Dev Cell. 2010. pp. 491–505. [DOI] [PMC free article] [PubMed]
- Yu FX, Luo J, Mo JS, et al. Cancer Cell. 2014. pp. 822–830. [DOI] [PMC free article] [PubMed]
- Feng X, Degese MS, Iglesias-Bartolome R, et al. Cancer Cell. 2014. pp. 831–845. [DOI] [PMC free article] [PubMed]
- Sagoo MS, Harbour JW, Stebbing J, et al. Oncogene. 2014. pp. 4722–4723. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conventional and novel PKC oppositely regulate the Hippo-YAP pathway.