Introduction
Complement is part of the innate immune system and plays a fundamental role in the clearance of immune complexes and cell debris. The main effector mechanisms of complement activation are induction of inflammatory response and phagocytosis and cell lysis. However, complement activation is a double-edged sword and has the potential to damage self-tissues. In order to avoid self-damage, there is an absolute need for strict control by fluid-phase and membrane-bound regulatory proteins. Thus, an underperforming regulatory system (due to either genetic or acquired abnormalities) can shift the balance between regulation and activation toward the latter and lead to tissue injury in response to otherwise innocuous stimuli. Deposition of both actived complement fragments from plasma in glomeruli and complement locally produced and activated in the kidney may contribute to many kidney disorders, including lupus nephritis, postinfectious glomerulonephritis, membranous nephropathy, antineutrophil cytoplasmic antibody vasculitis, and anti–glomerular basement membrane (anti-GBM) glomerulonephritis. Interest in the complement system has been boosted in the past 15 years by the discovery that rare devastating kidney diseases, including atypical hemolytic uremic syndrome (aHUS) and membranoproliferative glomerulonephritis (MPGN), are disorders of complement regulation.
Additional Readings
-
»
Nangaku M. Complement regulatory proteins in glomerular diseases. Kidney Int. 1998;54(5):1419-1428.
-
»
Ricklin D, Hajishengallis G, Yang K, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785-797.
-
»
Thurman JM. Complement in kidney disease: core curriculum 2015. Am J Kidney Dis. 2015;65(1):156-168.
-
»
Vieyra MB, Heeger PS. Novel aspects of complement in kidney injury. Kidney Int. 2010;77(6):495-499.
The complement system
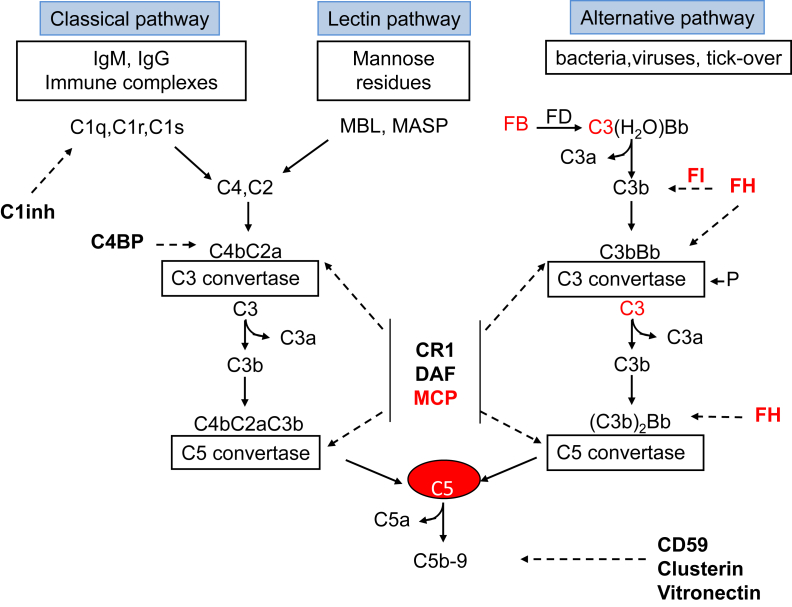
The complement system is organized in 3 activation pathways (the alternative, classical, and lectin pathways) and a common terminal pathway (Fig 1). Activation of the 3 pathways leads to the formation of protease complexes (C3 convertases) that cleave C3 into C3a and C3b.
Figure 1.
The 3 complement activation pathways. Bold text denotes complement-regulatory molecules; red text, proteins with genetic defects that have been associated with atypical hemolytic uremic syndrome (aHUS) and/or membranoproliferative glomerulopathy (MPGN)/C3 glomerulopathy (C3G). Abbreviations and definitions: C3(H2O)Bb, alternative pathway initiation convertase; C1inh, C1 inhibitor (inactivates C1r and C1s, MASP-1, and MASP-2); FB, complement factor B; FD, complement factor D; FH, complement factor H (binds C3b, exerts cofactor activity for FI-mediated C3b cleavage, prevents the formation of the alternative pathway C3 convertase, and destabilizes (decay accelerating activity) the alternative pathway C3 and C5 convertases); C4BP, C4b-binding protein (binds to C4b and has decay accelerating activity for the classical pathway C3 convertase and cofactor activity for FI-mediated C4b cleavage); CD59, protectin (with vitronectin and clusterin, prevents C5b-9 formation); CR1, complement receptor 1 (has decay accelerating activity as well as cofactor activity for FI-mediated C3b and C4b cleavage); DAF, decay accelerating factor (has decay accelerating activity on C3/C5 convertases of the classical and alternative pathways); FI, complement factor I (degrades C3b and C4b, aided by cofactors); Ig, immunoglobulin; MASP, MBL-associated serine proteases; MBL, mannose binding lectin; MCP, membrane cofactor protein (exerts cofactor activity for FI-mediated C3b cleavage); P, properdin.
The classical and lectin pathways are triggered by recognition of pathogens or damaged cell surfaces by antibodies and recognition molecules (Fig 1).
The alternative pathway undergoes constant low-grade activation in the fluid phase by spontaneous hydrolysis of C3 that, through the formation of the alternative pathway initiation C3 convertase (C3[H2O]Bb), is responsible for deposition of a low amount of C3b onto cell surfaces (Fig 1). C3b deposited on bacterial cells binds receptors on leukocytes and initiates phagocytosis. In addition, C3b binds factor B (CFB) and forms the surface-localized C3 convertase C3bBb, which cleaves additional C3 molecules into C3b and C3a, resulting in a positive-feedback loop. The binding of additional C3b to C3 convertases forms C5 convertases, which cleave C5, producing C5a and C5b. C5a and C3a are potent chemoattractants for phagocytes and induce endothelial activation. C5b together with C6, C7, C8, and C9 form the terminal pathway complex (C5b-9), which leads to cell lysis (Fig 1).
Self-surfaces are protected from complement damage by a set of regulators (Fig 1) that are either membrane anchored or in the fluid phase. Regulatory proteins prevent formation of the C3 convertases, foster inactivation of C3b (iC3b) by factor I (CFI; cofactor activity), dissociate C3 convertase and C5 convertase (decay acceleration activity), or prevent C5b-9 complex assembly (Figs 1 and 2). Perturbation of the balance between complement activators and regulators provides the molecular basis of aHUS and MPGN/C3 glomerulopathy (C3G).
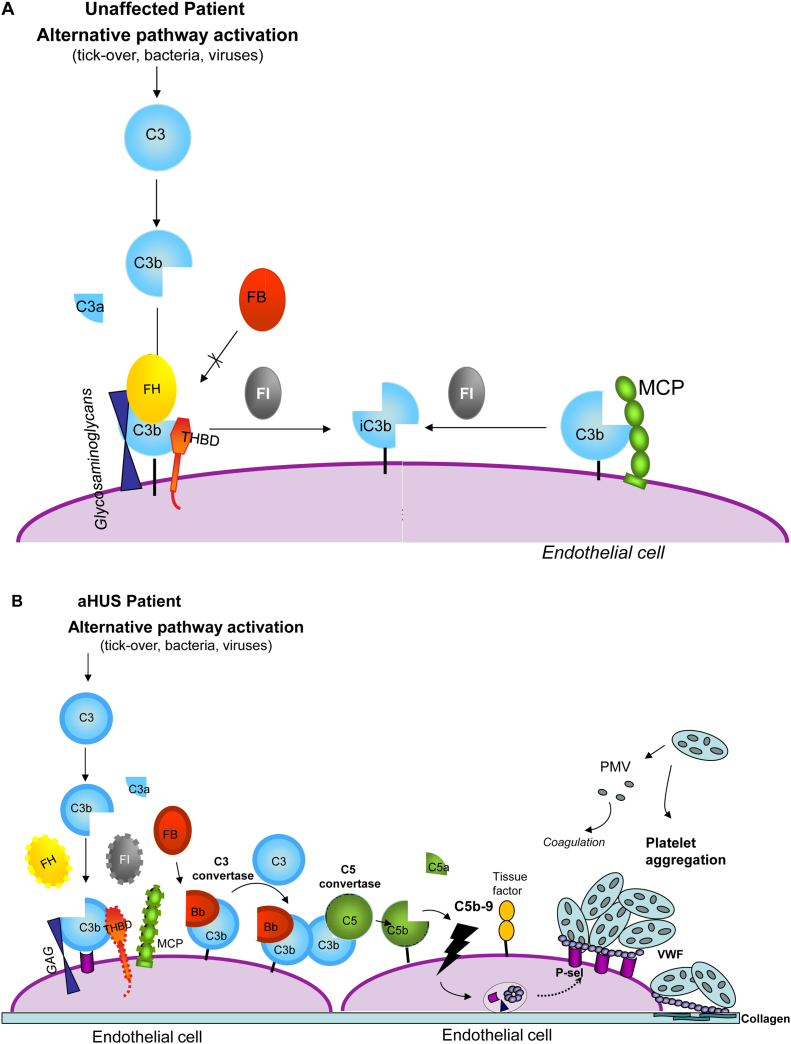
Figure 2.
Model for the mechanisms leading from impaired regulation of the alternative pathway to hemolytic uremic syndrome (HUS). (A) Pathway in unaffected individuals. Factor H (FH) binds to the glycosaminoglycans and to C3b on endothelial surface and prevents the interaction of C3b with factor B (FB) to form the alternative pathway C3 convertase. In addition, together with membrane cofactor protein (MCP), FH acts as cofactor for factor I (FI)-mediated cleavage of C3b. FH also dissociates the C3 convertase of the alternative pathway (not shown). Thrombomodulin (THBD) enhances FI-mediated inactivation of C3b in the presence of FH. (B) Pathway in patients with mutations in complement regulatory genes for FH, MCP, FI, and THBD (loss-of-function mutants are indicated with dashed lines). C3b is not degraded efficiently and forms the C3 and C5 convertases of the alternative pathway. C5b initiates assembly of the terminal complement complex (C5b-9), leading (together with C5a) to cell injury and activation, with exocytosis of adhesion molecules (P-selectin) and von Willebrand factor (VWF), which favor platelet adhesion and aggregation, and expression of tissue factor, which activates coagulation. Subsequent cell detachment exposes the subendothelial matrix, followed by VWF binding to collagen, which amplifies the prothrombotic state. Aggregated platelets release procoagulant microvesicles (PMV) containing tissue factor, which facilitate assembly of clotting enzymes. A similar picture applies to patients with gain-of-function mutations in FB and C3 (indicated with thick lines). Mutant C3b does not bind FH and MCP and is resistant to degradation by FI. Both mutant C3 and mutant FB form a superconvertase that is resistant to dissociation by surface complement regulators. Abbreviations: aHUS, atypical HUS; GAG, glycosaminoglycans.
Additional Readings
-
»
Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33(6):479-492.
-
»
Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol. 2008;151(2):210-230.
-
»
Thurman JM. Complement in kidney disease: core curriculum 2015. Am J Kidney Dis. 2015;65(1):156-168.
-
»
Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058-1066.
-
»
Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729-740.
Atypical hemolytic uremic syndrome
Hemolytic uremic syndrome (HUS) is a rare disease defined by the triad of nonimmune (Coombs negative) microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury. In children, the disease is most commonly associated with infection by Escherichia coli strains that produce Shiga toxins (STEC-HUS; Table 1). Approximately 5% of HUS cases in children result from infection by neuroaminidase-producing Streptococcus pneumoniae (pneumococcal-HUS or neuraminidase-associated HUS, usually Coombs positive; Table 1).
Table 1.
Classification of HUS
| Name | Causes |
|---|---|
| STEC-HUS | Infection with Shiga toxin–secreting strains of Escherichia coli or Shigella dysenteriae |
| Pneumococcal-HUS | Infection with neuroaminidase-producing Streptococcus pneumoniae |
| Atypical HUS | |
| Complement-mediated | Mutations (mostly heterozygous) in CFH, CFI, MCP, C3, CFB, and THBD genes; genomic rearrangements of CFH/CFHR loci; anti-CFH autoantibodies |
| DGKE-deficiency associated | Homozygous and compound heterozygous mutations in the DGKE gene |
| Cobalamin deficiency–associated HUS | Homozygous mutations in the MMACHC gene |
| Secondary HUS | Drugs (quinine, VEGF inhibition, antiviral, cancer chemotherapy), autoimmune diseases (SLE, scleroderma, antiphospholipid syndrome), malignancy, bone marrow transplantation |
Abbreviations: CFB, complement factor B; CFH, complement factor H; CFHR, complement factor H–related; CFI, complement factor I; DGKE, diacylglycerol kinase ε; HUS, hemolytic uremic syndrome; MCP, membrane cofactor protein; MMACHC, methylmalonic aciduria and homocystinuria type C; STEC-HUS, Shiga-like toxin–producing Escherichia coli HUS; SLE, systemic lupus erythematosus; THBD, thrombomodulin; VEGF, vascular endothelial growth factor.
aHUS has been used to describe those rare cases (<10%) in which infections by Shiga toxin–producing bacteria or S pneumoniae or other secondary causes (Table 1) can be excluded. aHUS is an extremely rare disease. The annual incidence is about 0.5 to 2 per million adults and 3.3 per million children or adolescents.
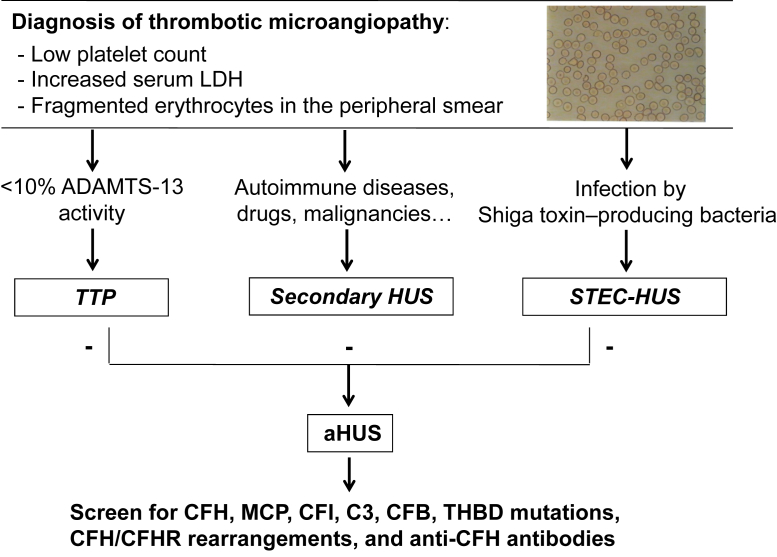
A differential diagnosis among the different forms of HUS is often not feasible based only on age of onset or clinical symptoms. In infants younger than 6 months, complement-mediated aHUS is most common, but pneumococcal-HUS may also occur. STEC-HUS largely predominates in infants and children from 6 months to 5 years, but may occur at any age. The presence of diarrhea, a characteristic symptom of STEC-HUS, cannot be used to differentiate between STEC-HUS and aHUS because ∼30% of patients with aHUS experience diarrhea, often bloody. However, microbiological, biochemical, and genetic studies may be of help for differential diagnosis (Fig 3).
Figure 3.
Diagnostic algorithm for screening and diagnosis of the different forms of thrombotic microangiopathy. In patients presenting with low platelet count, increased lactate dehydrogenase (LDH), and hemolytic anemia, plasma ADAMTS-13 activity should be measured: if <10%, this leads to diagnosis of thrombotic thrombocytopenic purpura (TTP). Positive tests for Shiga-toxin–producing bacteria in stools are consistent with a diagnosis of Shiga-like toxin–producing Escherichia coli hemolytic uremic syndrome (STEC-HUS). Diagnosis of atypical HUS (aHUS) is done by exclusion in patients in whom these tests are negative and with no evidence of secondary conditions. When aHUS is suspected, full screening for complement disease–associated abnormalities (complement factor H [CFH], membrane cofactor protein [MCP], complement factor I [CFI], C3, complement factor B [CFB], and thrombomodulin [THBD] mutations; CFH/CFHR rearrangements; and anti-CFH autoantibodies) should be performed. Genetic screening is being performed at the diagnostic level in several centers in Europe (http://www.orpha.net/consor/cgi-bin/ClinicalLabs_Search_Simple.php?lng=EN) and the United States, while detection of genomic rearrangements and anti-CFH antibodies is done only in few specialized centers for research purposes.
In contrast to STEC-HUS, which is characterized by full recovery in >80% of patients, aHUS presages a poorer prognosis. The majority of patients need dialysis at admission, and until very recently (following the introduction of the anti-C5 monoclonal antibody eculizumab), ∼50% of patients never recovered kidney function. Mortality after the first episode has been reported to be higher in children than in adults, but progression to end-stage renal disease is more frequent in adults. At 5 years after onset, 48% of children and 67% of adults have died or reached end-stage renal disease.
Additional Readings
-
»
Campistol JM, Arias M, Ariceta G, et al. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2013;33(1):24-45.
-
»
George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666.
-
»
Kavanagh D, Goodship TH, Richards A. Atypical hemolytic uremic syndrome. Semin Nephrol. 2013;33(6):508-530.
-
»
Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60.
-
»
Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(17):1676-1687.
Histologic Features in aHUS
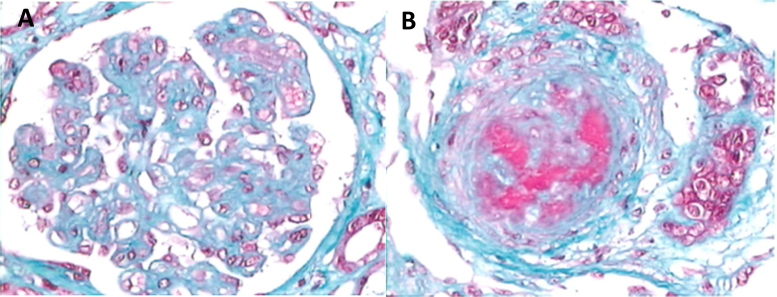
Broadly, 3 types of lesions are seen in patients with aHUS. These lesions are not mutually exclusive and all involve glomerular injury. In the most common type, glomerular thrombotic microangiopathy (TMA), glomerular capillary walls are thickened with widening of the subendothelial space. Endothelial cells may appear swollen, obstructing the capillary, or detached from the basement membrane. These lesions are mainly observed in glomerular capillaries and preglomerular arterioles, whereas larger arteries are only rarely involved. Glomeruli may be enlarged, with capillaries distended by red blood cells and platelet-fibrin thrombi that may also be observed in the afferent arterioles (Fig 4A).
Figure 4.
(A) Glomerulus from a patient with a glomerular form of atypical hemolytic uremic syndrome (aHUS). (B) Interlobular artery in a case of aHUS with severe vascular involvement. The vascular lumen is nearly fully occluded. Changes include myointimal proliferation. Thrombotic material and erythrocytes can be seen in the lumen and within the vascular wall.
The second type, arterial TMA, also involves arterioles and interlobular arteries. Intimal edema and proliferation, necrosis of the arterial wall, luminal narrowing, and thrombosis are observed (Fig 4B). Glomeruli appear ischemic and shrunken, with wrinkling of the basement membranes of the collapsed capillaries. Arterial TMA is seen more often in relapsing forms with severe hypertension and is associated with a poor prognosis.
The third lesion type, cortical necrosis, is patchy or rarely diffuse to the whole superficial cortex and is due to the acute cortical ischemia after obstruction of the local microcirculation. This lesion is more common in severe cases of STEC-HUS, but is also observed in aHUS and correlates with chronic kidney disease.
Glomerular deposits of fibrin or fibrinogen are seen by immunofluorescence microscopy. Deposits of complement activation products have also been found along glomerular capillaries and on the endothelium of arterioles and small arteries.
Although in aHUS, by definition, the TMA predominantly affects kidney vessels, the lesion can involve the microvasculature of other organs (heart, intestine, pancreas, lungs, and especially the brain) in ∼20% of patients.
Additional Readings
-
»
Habib R. Pathology of the hemolytic uremic syndrome. In: Kaplan B, Moake J, eds. Hemolytic Uremic Syndrome and Thrombotic Thrombocytopenic Purpura. New York, NY: Marcel Dekker; 1992:315-353.
-
»
Mele C, Remuzzi G, Noris M. Hemolytic uremic syndrome. Semin Immunopathol. 2014;36(4):399-420.
Pathogenesis
Genetic or acquired abnormalities in the complement system have been documented in ∼60% of patients with aHUS (Table 2).
Table 2.
Genetic Abnormalities Associated With aHUS
| Gene | Abnormality | Main Effect | Frequency in aHUS |
|---|---|---|---|
| CFH | Heterozygous and (rarely) homozygous mutations mainly in the last 2 exons | Impaired cell-surface complement regulation | 25%-30% |
| CFH/CFHRs | Nonallelic homologous recombinations | Impaired cell-surface complement regulation | 3%-5% |
| CFHR1 | Deletion and formation of anti-CFH antibodies | Impaired cell-surface complement regulation | 5%-10% |
| CD46a | Heterozygous and (rarely) homozygous mutations | Reduced surface expression | 8%-10% |
| CFI | Heterozygous mutations | Low cofactor activity | 4%-8% |
| C3 | Heterozygous mutations | Resistance to C3b inactivation, C3 convertase stabilization | 4%-8% |
| CFB | Heterozygous mutations | C3 convertase stabilization | 1%-4% |
| THBD | Heterozygous mutations | Reduced TAFI activation, reduced C3b inactivation | 3%-4% |
| DGKE | Homozygous or compound heterozygous mutations | Protein truncation, proinflammatory and prothrombotic endothelial phenotype, increased endothelial apoptosis | 2%-27% of infantile casesb |
Abbreviations: aHUS, atypical hemolytic uremic syndrome; CFB, complement factor B; CFH, complement factor H; CFHR, complement factor H–related; CFI, complement factor I; DGKE, diacylglycerol kinase ε; MCP, membrane cofactor protein; TAFI, thrombin-activatable fibrinolysis inhibitor; THBD, thrombomodulin.
CD46 encodes MCP.
Onset of the disease before 1 to 2 years of age.
CFH Mutations, CFH-CFHR Hybrid Genes, and Anti-CFH Autoantibodies
Complement factor H (CFH) is the most important plasma regulator of the alternative pathway. It is composed of 20 domains called short consensus repeats (SCRs) encoded by 23 exons. The 4 amino-terminal SCRs mediate the complement-regulatory functions of the protein (cofactor activity and decay accelerating activity). Besides regulating complement in the fluid phase, CFH protects host cells by binding through its carboxy-terminal SCRs to polyanions and C3 activation compounds on the cell surface (Fig 2A).
In 1998, Warwicker and colleagues described for the first time mutations in the CFH gene in aHUS. CFH mutations are the most common genetic abnormalities in aHUS, with a prevalence of 20% to 30% (http://www.FH-HUS.org; Table 2). The mutations are of all types: nonsense, missense, and small indels (insertions or deletions). Some patients have homozygous mutations but most are heterozygous. More than 50% of mutations cluster in SCR19-20. Some mutations are associated with a quantitative deficit of CFH, while the majority are associated with normal CFH levels and result in a mutant protein that is unable to bind to and regulate complement at the cell surface (Fig 2B). These mutants have also been shown to have impaired binding to platelets, resulting in increased complement-mediated platelet activation and release of tissue factor–expressing microvesicles. Plasma C3 is decreased in 30% to 50% of patients with heterozygous CFH mutations.
CFH is in close proximity to the genes CFHR1 to CFHR5, which encode 5 CFH-related proteins. CFH and the CFHR genes share a high degree of sequence identity. This homology predisposes to gene conversion and genomic rearrangements due to nonallelic homologous recombination and microhomology-mediated end joining. The most common rearrangement results in a hybrid gene comprising the first 21 CFH exons and the last 2 CFHR1 exons. Another hybrid gene consisting of the first 22 CFH exons and the last 5 CFHR3 exons has been reported in aHUS. More recently, a novel nonallelic homologous recombination event that forms a reverse hybrid gene consisting of the first 5 exons of CFHR1 and the last exon of CFH has been reported in 2 patients with familial aHUS. Overall, hybrid genes account for 3% to 5% of patients with aHUS (Table 2) and result in gene products with decreased complement-regulatory activity on endothelial surfaces.
An acquired CFH defect due to anti-CFH immunoglobulin G (IgG) autoantibodies accounts for 5% to 10% and 25% to 50% of adult and pediatric aHUS cases, respectively (Table 2). Ninety percent of patients with anti-CFH antibodies have a complete deficiency of CFHR1 and CFHR3 associated with a homozygous deletion of CFHR1 and CFHR3, a polymorphism also observed in 4% of healthy individuals of European ancestry who do not develop anti-CFH antibodies. In more recent studies, some patients with aHUS with CFHR1 deficiency resulting from CFHR1 mutations or from a deletion including CFHR1 and CFHR4 have been reported to develop anti-CFH antibodies. This may suggest that CFHR1 deficiency is the predominant predisposing factor for autoantibodies (Table 2).
Epitope mapping initially suggested that the anti-CFH antibodies bound predominantly to SCR19-20; however, it recently has been reported that the antibody response is polyclonal, involving multiple epitopes throughout CFH. Cross-reactivity with CFHR1 and CFHR2 has also been reported. Functional consequences of anti-CFH antibodies include reduced CFH binding to C3b and other C3 fragments and perturbed CFH-mediated cell-surface protection. C3 levels are decreased in 40% to 60% of patients with anti-CFH antibodies.
Membrane Cofactor Protein Mutations
Membrane cofactor protein (MCP) is a surface complement regulator that exerts cofactor activity for the cleavage of C3b and C4b by CFI (Fig 2A). The association between mutations in the MCP gene (CD46) and aHUS were first described by Noris et al and Richards et al in 2003. MCP gene mutations account for 8% to 10% of cases, and most are heterozygous (http://www.FH-HUS.org; Table 2). The majority cluster in SCR1 to 4, which are extracellular domains critical for regulation. Most patients (∼75%) have decreased MCP expression on peripheral leukocytes. Less frequently, MCP expression is normal, but the mutant proteins bind C3b weakly and have low cofactor activity. C3 levels in patients with mutant forms of MCP are most often normal, an expected finding because MCP alterations result in local surface complement activation, with no substantial effect in the fluid phase (Fig 2B).
CFI Mutations
CFI is a serum serine protease that cleaves C3b and C4b in the presence of its cofactors. CFI mutations were first described in 2004 and account for 4% to 8% of patients with aHUS (Table 2). Eighty percent of mutations cluster in the serine protease domain. Approximately 50% block protein secretion; some mutants are secreted but have dysfunctional proteolytic activity with altered degradation of C3b/C4b in the fluid phase and on cell surfaces (Fig 2B). C3 levels can be decreased (in 20%-30% of patients).
Mutations in the Alternative Pathway C3 Convertase Components
Heterozygous gain-of-function mutations affecting genes encoding the alternative pathway C3 convertase components CFB and C3 were first described in 2007 and 2008, respectively. C3 mutations have been found in 4% to 8% of patients with aHUS (Table 2). Most C3 mutations induce a defect in the ability of complement regulators (in particular MCP) to bind to C3b, preventing its cleavage to iC3b (Table 2; Fig 2B). More recent studies described 2 C3 mutants that bind CFB with higher affinity, resulting in a more stable C3 convertase complex. C3 mutations result in increased complement activation on glomerular endothelium and platelets. Serum C3 levels are low in ∼70% of patients.
Mutations in CFB are rare (1%-4% of patients with aHUS; Table 2). These mutations result in a “super-B,” which forms a hyperactive C3 convertase that is resistant to decay by complement regulators (Table 2; Fig 2B), with enhanced complement activation at endothelial cell surfaces. However, a more recent functional study revealed that 9 of 15 CFB genetic changes identified in patients with aHUS lead to a normal C3 convertase in vitro. Patients with CFB mutations experience ongoing activation of the alternative pathway and usually have very low C3 levels.
Thrombomodulin Mutations
Thrombomodulin (THBD) is an endothelial glycoprotein that favors the thrombin-mediated activation of both protein C and TAFI, the thrombin-activatable fibrinolysis inhibitor responsible for inactivating C3a and C5a. THBD has also been shown to downregulate the alternative pathway by amplifying CFH cofactor activity (Fig 2A).
Heterozygous mutations in THBD were first reported in 2009 and have been found in 3% to 4% of patients with aHUS (Table 2). Cells expressing these mutants inactivate C3b and activate TAFI less efficiently than cells expressing wild-type THBD. These data document a functional link between complement and coagulation. C3 levels are decreased in half the patients with THBD mutations.
Combined Complement Abnormalities and Susceptibility Factors
Mutations in more than 1 complement gene have been reported in a few patients. In a report from 4 European cohorts, 27 of 795 (3.4%) patients with aHUS had combined mutations. Of note, only 8% to 10% of patients with CFH, C3, or CFB mutations also had a mutation in other genes. These data indicate that CFH, C3, or CFB mutations alone may be enough to cause aHUS. By contrast, about a quarter of patients with a CD46 (MCP) or CFI mutation had a second or third mutation in other complement genes. Thus, screening of all known disease-associated genes is recommended in patients with aHUS.
A number of single-nucleotide polymorphisms (SNPs; polymorphisms are defined as genetic changes with >1% frequency in ethnically matched control groups) have been associated with aHUS. The CFH haplotype H3 (Table 3) increases the risk of aHUS 2- to 4-fold. Functional studies have documented that one of the polymorphisms within this haplotype, a variant in which there is a valine at amino acid 62, leads to slightly decreased cofactor activity compared with proteins in which isoleucine is at this position. The MCP2 haplotype of MCP (Table 3) has been associated with a 2- to 3-fold increased risk of aHUS. In reporter gene studies, this haplotype reduced transcriptional activity by 25%. A CFHR1 polymorphism (CFHR1*B) is strongly associated with aHUS in the homozygous state (Table 3). Because CFHR1*B renders SCR3 of CFHR1 identical to SCR18 of CFH, it has been suggested that CFHR1*B variant may compete with CFH at the glomerular endothelium, thus impairing complement regulation.
Table 3.
Risk and Protective SNPs and Haplotypes for aHUS and DDD
| Gene | Variant (SNP) | Haplotype | Effect | Disease |
|---|---|---|---|---|
| CFH | −332T (c.−332C>T) | Risk | aHUS | |
| 184G (c.184G>A, p.V62I) | Risk | DDD | ||
| 1204C (c.1204T>C, p.Y402H) | Risk | DDD | ||
| 2016G (c.2016A>G, p.Q672Q) | Risk | aHUS | ||
| 2808T (c.2808G>T, p.E936D) | Risk | aHUS | ||
| H1 (CGCAG) | Risk | DDD | ||
| H2 (CATAG) | Protective | aHUS and DDD | ||
| H3 (TGTGT) | Risk | aHUS | ||
| CFHR1 | 469T (c.469C>T, p.H157Y) 475G (c.475C>G, p.L159V) 523C (c.532G>C, p.E175Q) |
CFHR1*B (TGC) | Risk | aHUS |
| MCP | −652G (c.−652A>G) | Protective | C3GN and IC-MPGN | |
| *897C (c.*897T>C) | Risk | aHUS | ||
| MCP1 (AT) | Risk | C3GN and IC-MPGN | ||
| MCP2 (GC) | Risk | aHUS | ||
| MCP3 (GT) | Protective | C3GN and DDD | ||
| C3 | 304G (c.304C>G, p.R102G) | Risk | DDD | |
| 941T (c.941C>T, p.P314L) | Risk | DDD |
Note: Descriptions of SNPs first include a shorthand for referring to the variant, and in parentheses, its full description at the coding DNA (c) level and, if applicable, at the protein (p) level; example: 184G (c.184G>A, p.V62I) means a guanine to adenine substitution at the 184th nucleotide of the coding sequence, leading to a substitution of an isoleucine for a valine at amino acid 62. A minus sign indicates a position upstream of the translation start site, while an asterisk indicates a position downstream of the translation stop codon (ie, these upstream and downstream variants would both be in noncoding regions of the gene). The letters in parentheses after each haplotype (ie, a set of variants inherited together) give the identity of the nucleotide at each SNP listed for the gene in this table. For example, haplotype H3 (TGTGT) comprises a thymine at c.−332C>T, a guanine at c.184G>A, a thymine at c.1204T>C, a guanine at c.2016A>G, and a thymine at c.2808G>T. Data are from Abrera-Abeleda et al (J Am Soc Nephrol. 2011;22:1551-1559), Pickering et al (J Exp Med. 2007;204:1249-1256), and Servais et al (Kidney Int. 2012;82:454-464).
Abbreviations: aHUS, atypical hemolytic uremic syndrome; C3GN, C3 glomerulonephritis; CFH, complement factor H; CFHR, complement factor H–related; DDD, dense deposit disease; IC-MPGN, immune-complex–mediated membranoproliferative glomerulonephritis; MCP, membrane cofactor protein; SNP, single-nucleotide polymorphism.
Diacylglycerol Kinase ε Mutations
In 2013, homozygous or compound heterozygous mutations in the gene encoding diacylglycerol kinase ε (DGKE) were reported to cosegregate with aHUS in 9 unrelated kindreds (Table 2). Mutation carriers who presented with aHUS before 1 year of age had persistent hypertension, hematuria, and proteinuria and developed chronic kidney disease with age. DGKE is expressed in endothelium, platelets, and podocytes. It is apparently unrelated to the complement cascade, and a very recent study has documented that DGKE silencing in human cultured endothelial cells induces a proinflammatory and prothrombotic phenotype, increases endothelial apoptosis, and impairs migration and angiogenesis. DGKE knockdown is reported to decrease the expression of the complement regulator MCP on endothelial cells, but does not induce complement deposition.
Nevertheless, there is some evidence for a connection between DGKE and complement systems. In a family with 2 affected children, one child had a lower-than-normal C3 level at onset. In addition, DGKE mutations combined with mutations in THBD or C3 have been reported in children with severe aHUS who responded to anticomplement therapy. These observations suggest that in patients with DGKE mutations, complement gene mutations play a role in determining aHUS severity and therapeutic response.
Additional Readings
-
»
Bresin E, Rurali E, Caprioli J, et al. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24(3):475-486.
-
»
Bruneau S, Neel M, Roumenina LT, et al. Loss of DGKε induces endothelial cell activation and death independently of complement activation. Blood. 2015;125(6):1038-1046.
-
»
Caprioli J, Castelletti F, Bucchioni S, et al. Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet. 2003;12(24):3385-3395.
-
»
Delvaeye M, Noris M, De Vriese A, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(4):345-357.
-
»
Dragon-Durey MA, Loirat C, Cloarec S, et al. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16(2):555-563.
-
»
Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, et al. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet. 2004;41(6):e84.
-
»
Fremeaux-Bacchi V, Miller EC, Liszewski MK, et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112(13):4948-4952.
-
»
Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2007;104(1):240-245.
-
»
Lemaire M, Fremeaux-Bacchi V, Schaefer F, et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet. 2013;45(5):531-536.
-
»
Marinozzi MC, Vergoz L, Rybkine T, et al. Complement factor B mutations in atypical hemolytic uremic syndrome, disease-relevant or benign? Blood. 2014;25(9):2053-5065.
-
»
Noris M, Brioschi S, Caprioli J, et al. Familial haemolytic uraemic syndrome and an MCP mutation. Lancet. 2003;362(9395):1542-1547.
-
»
Richards A, Kemp EJ, Liszewski MK, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2003;100:12966-12971.
-
»
Schramm EC, Roumenina LT, Rybkine T, et al. Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood. 2015;125:2359-2369.
-
»
Valoti E, Alberti M, Tortajada A, et al. A novel atypical hemolytic uremic syndrome-associated hybrid CFHR1/CFH gene encoding a fusion protein that antagonizes factor H-dependent complement regulation. J Am Soc Nephrol. 2015;26(1):209-219.
-
»
Venables JP, Strain L, Routledge D, et al. Atypical haemolytic uraemic syndrome associated with a hybrid complement gene. PLoS Med. 2006;3(10):e431.
-
»
Warwicker P, Goodship TH, Donne RL, et al. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998;53(4):836-844.
Restricted Cell-Surface Complement Activation in aHUS Causes Microvascular Thrombosis
aHUS-associated mutant forms of CFH, MCP, CFI, and THBD cannot fully regulate the alternative pathway on host cells, resulting in C3 and C5b-9 deposition on cell surfaces (Fig 2B). By contrast, aHUS-associated mutant proteins effectively regulate complement in the fluid phase, which would explain the normal or near-normal circulating C3 levels in many mutation carriers. Gain-of-function mutations of CFB and C3 form a C3 convertase resistant to decay by endothelial cell regulators. In an ex vivo test, we documented that serum from patients with aHUS induced intense C5b-9 deposition on cultured endothelial cells; this occurred in 100% of patient samples tested, independent of the type of mutation and the gene involved. Such data confirm that local complement activation on endothelial cells rather than in fluid phase plays a pathogenetic role in aHUS. Of note, sera from patients without identified mutations also induce C5b-9 endothelial deposits, indicating that there are still unrecognized genetic or acquired complement abnormalities leading to aHUS.
Besides its well-known lytic effect, C5b-9 can mediate endothelial cell injury and dysfunction by many mechanisms. Sublytic C5b-9 induces secretion of multimers of von Willebrand factor, stimulates prothrombinase and tissue factor activity, and upregulates adhesion molecules, which generate a prothrombotic endothelial cell surface that activates platelets and fibrin deposition (Fig 2B). Furthermore, C5b-9 induces cell retraction, exposing the underlying prothrombotic matrix, and can directly activate platelets. The pathogenetic role of C5b-9 and the other C5 activation products in aHUS was eventually confirmed by clinical trials showing that the monoclonal antibody eculizumab, through blockade of C5 cleavage, protects against microvascular thrombosis and radically improves the outcome of patients with aHUS.
Additional Readings
-
»
Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264(15):9053-9060.
-
»
Kerr H, Richards A. Complement-mediated injury and protection of endothelium: lessons from atypical haemolytic uraemic syndrome. Immunobiology. 2012;217(2):195-203.
-
»
Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181.
-
»
Manuelian T, Hellwage J, Meri S, et al. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111(8):1181-1190.
-
»
Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124(11):1715-1726.
-
»
Roumenina LT, Jablonski M, Hue C, et al. Hyperfunctional C3 convertase leads to complement deposition on endothelial cells and contributes to atypical hemolytic uremic syndrome. Blood. 2009;114(13):2837-2845.
-
»
Saadi S, Holzknecht RA, Patte CP, Stern DM, Platt JL. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182(6):1807-1814.
-
»
Ståhl AL, Vaziri-Sani F, Heinen S, et al. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood. 2008;111(11):5307-5315.
Incomplete Penetrance and Triggering Factors
Penetrance of aHUS has been found to be ∼50% for individuals who carry complement gene mutations. Thus, it can be inferred that although the genetic alterations are important, they are not sufficient to cause aHUS. As discussed previously, patients may carry mutations in multiple genes or have both genetic abnormalities and autoantibodies; in addition, they may carry a mutation in combination with common at-risk genetic variants in CFH or MCP. In family studies, it has been observed that the penetrance of aHUS increases as the number of alterations increases in a given individual. Even in individuals with multiple genetic/acquired risk factors, aHUS may not occur until adulthood, indicating that a triggering event that activates the alternative pathway is required for disease manifestation in genetically susceptible individuals (the multiple hits theory).
Additional Readings
-
»
Esparza-Gordillo J, Goicoechea de Jorge E, Buil A, et al. Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum Mol Genet. 2005;14(5):703-712.
-
»
Fakhouri F, Roumenina L, Provot F, et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. 2010;21(5):859-867.
-
»
Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124(11):1715-1726.
HUS associated with cobalamin deficiency
A rare autosomal recessive form of HUS results from mutations in the MMACHC gene, which is involved in cobalamin C metabolism (Table 1). The biochemical characteristics are hyperhomocysteinemia and methylmalonic aciduria. Patients generally present within days or months of birth with symptoms of failure to thrive, poor feeding, and vomiting. This is followed by rapid deterioration, caused by metabolic acidosis, gastrointestinal bleeding, hemolytic anemia, thrombocytopenia, severe respiratory and liver failure, and decreased kidney function. It has been suggested that the marked elevation in homocysteine level may contribute to the pathogenesis of the vascular lesions.
Additional Reading
-
»
Geraghty MT, Perlman EJ, Martin LS, et al. Cobalamin C defect associated with hemolytic uremic syndrome. J Pediatr. 1992;120(4):934-937.
Secondary forms of HUS
A variety of conditions have been reported in association with HUS, including viral (HIV [human immunodeficiency virus] and H1N1 influenza A), parasitic, and other bacterial infections; oral contraceptives; calcineurin inhibitors; illicit drugs; cancer chemotherapy and ionizing radiation; malignant hypertension; bone marrow or solid-organ transplantation; autoimmune disorders; malignancy; pregnancy; and HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. These forms are called secondary HUS (Table 1), although this classification does not account for the evidence that some of these conditions often act as a trigger of aHUS in individuals who have a genetic background predisposing to complement activation. For instance, a substantial proportion of HUS cases occurring during pregnancy, postpartum, or de novo in the kidney transplant have been found to be associated with complement gene mutations. Along with this, triggering or underlying clinical conditions have been reported in up to 70% of patients with complement gene mutations, providing evidence that disease development requires both a genetic predisposition and a triggering event.
Additional Reading
-
»
Besbas N, Karpman D, Landau D, et al. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int. 2006;70(2):423-431.
Membranoproliferative Glomerulonephritis
MPGN is an uncommon cause of chronic proteinuric nephropathy (the incidence is ∼5 per million persons per year) that may be primary or secondary to hepatitis C virus and other infections, autoimmune diseases, and malignancies. The onset of MPGN can occur from childhood to late adulthood. The clinical presentation is variable and ranges from asymptomatic hematuria and proteinuria to acute nephritic syndrome, nephrotic syndrome, chronic kidney disease, or even rapidly progressing glomerulonephritis resulting in end-stage renal disease.
The high variability of clinical presentation and course is likely caused by differences in the pathogenesis of the disease, the histologic lesion in the kidney, and the timing of the diagnosis (that is based on kidney biopsy) relative to the clinical course.
Additional Readings
-
»
Doutrelepont JM, Adler M, Willems M, et al. Hepatitis C infection and membranoproliferative glomerulonephritis. Lancet. 1993;341(8840):317.
-
»
Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348.
Histologic Features and Classification
MPGN is characterized by mesangial hypercellularity and matrix expansion, with thickening of glomerular capillaries (with the formation of double contours), interposition of leukocytes and mesangial cells, and synthesis of new GBM resulting in lobular accentuation of glomerular tufts. This pattern of injury results from deposition of immune complexes and/or complement factors in the glomerular mesangium and along the glomerular capillary walls and is easily recognized by light and immunofluorescence microscopy.
Traditionally, on the basis of electron microscopy findings, MPGN was classified as type I, with subendothelial electron-dense deposits; type II (also called dense deposit disease [DDD]), with intramembranous highly electron-dense deposits; and type III, with both subendothelial and subepithelial deposits. These categories had limited prognostic value because of their complexity and the occurrence of features suggestive of more than 1 type in the same biopsy samples.
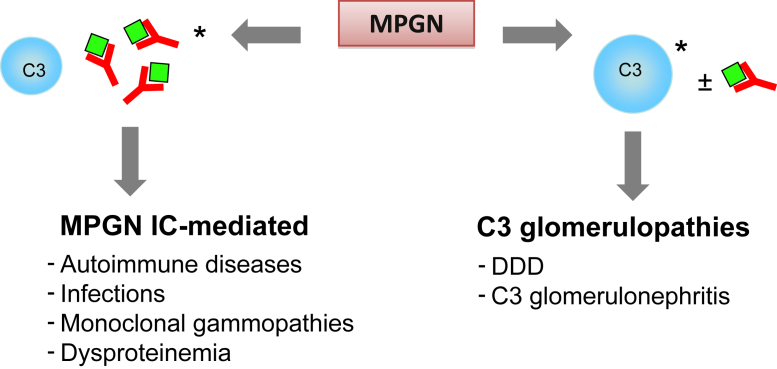
A new classification based on the pathogenesis and composition of glomerular deposits as analyzed by immunofluorescence microscopy has been proposed. MPGN associated with substantial immunoglobulin deposits has been termed immune-complex–mediated MPGN and is commonly associated with chronic infections or autoimmune diseases (Fig 5). In these secondary forms, careful characterization of deposits can often help identify the underlying cause. Deposits of IgM, IgG, C3, and κ and λ light chains are typically found in MPGN associated with hepatitis C virus infection. Multiple immunoglobulins and complement proteins (IgG, IgM, IgA, C1q, C3, and κ and λ light chains) are also observed in MPGN associated with autoimmune diseases. However, monotypic immunoglobulin with κ and λ light chain restriction is observed in MPGN associated with monoclonal gammopathy. Immune-complex–mediated MPGN has been considered to include most cases of MPGN types I and III according to the older classification, with electron microscopy typically revealing mesangial and subendothelial deposits and, in some cases, intramembranous and subepithelial deposits.
Figure 5.
Schematic representation of membranoproliferative glomerulonephritis (MPGN) reclassification based on immunofluorescence findings. ∗Prevalent. Abbreviations: DDD, dense deposit disease; IC-mediated, immune complex–mediated.
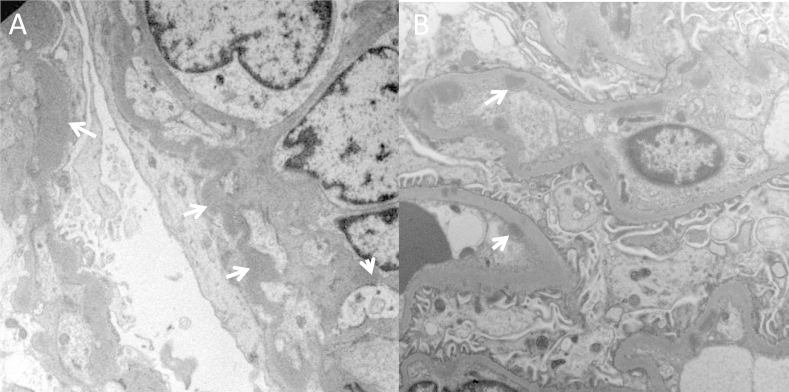
MPGN cases that present clear glomerular C3 staining with absent or scanty immunoglobulins are considered complement-mediated MPGN and are referred to as C3Gs (Fig 5). C3G is less common than immune-complex–mediated MPGN, has a prevalence of about 1-2 cases per million inhabitants, and is further divided on the basis of the quality of the deposits seen in glomeruli on electron microscopy (Fig 5). DDD is diagnosed in cases with distinctive highly electron-dense osmiophilic deposits that are typically found within the GBM. These deposits extend along the central part of the GBM, but may also involve the subendothelial and subepithelial region. Globular deposits appear in the mesangium and in about half the patients with DDD, are also seen in Bowman capsule and the tubular basement membrane (Fig 6A).
Figure 6.
Electron microscopy images of glomeruli from patients with dense deposit disease (DDD) or C3 glomerulonephritis. (A) DDD. Osmiophilic wavy dense deposits occurring in the glomerular basement membrane and also the mesangium (white arrows). (B) C3 glomerulonephritis. Intramembranous and subendothelial deposits (white arrows).
Cases of C3G that lack the electron-dense deposits of DDD are called C3 glomerulonephritis. In C3 glomerulonephritis, deposits have subendothelial and sometimes subepithelial and intramembranous localization (Fig 6B), morphologic characteristics likely resembling MPGN types I and III. Thus, with the exception of DDD, electron microscopy cannot distinguish between immune-complex–mediated MPGN and C3G.
However, immunofluorescence microscopy cannot always confirm a diagnosis because immunoglobulins may be present in glomeruli of patients with C3G. Small amounts of immunoglobulin may become trapped in areas of sclerosis or in podocytes of patients with proteinuric glomerular diseases, and about one-third of patients with DDD have glomerular IgG staining. Thus, a subsequent broader classification of C3G proposed by a recent consensus report suggested including all cases that have dominant immunofluorescence microscopy staining of C3 of 2 or more orders of magnitude greater than any other immune reactants (using a scale of 0-3). Even with this broader classification, a substantial proportion (20%) of patients with DDD would not be classified as having C3G. Conversely, isolated staining for C3 on immunofluorescence microscopy may be observed in cases of postinfectious glomerulonephritis. Therefore, the issue of MPGN classification has not yet been fully resolved.
Additional Readings
-
»
Anders D, Thoenes W. Basement membrane-changes in membranoproliferative glomerulonephritis: a light and electron microscopic study. Virchows Arch A Pathol Anat Histol. 1975;369(2):87-109.
-
»
Barbour TD, Pickering MC, Terence Cook H. Dense deposit disease and C3 glomerulopathy. Semin Nephrol. 2013;33(6):493-507.
-
»
Cook HT, Pickering MC. Histopathology of MPGN and C3 glomerulopathies. Nat Rev Nephrol. 2015;11(1):14-22.
-
»
Hou J, Markowitz GS, Bomback AS, et al. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int. 2014;85(2):450-456.
-
»
Pickering MC, D'Agati VD, Nester CM, et al. C3 glomerulopathy: consensus report. Kidney Int. 2013;84(6):1079-1089.
-
»
Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med. 2012;366(12):1119-1131.
Pathophysiology of Immune-Complex–Mediated MPGN
Infectious Causes of Complement Activation
Most cases of immune-complex–mediated MPGN in the adult population are attributable to chronic viral infections such as those due to hepatitis B or C virus. Chronic bacterial infections, like those caused by staphylococcus, Mycobacterium tuberculosis, and streptococci, and protozoal diseases, such as malaria and schistosomiasis, are also associated with MPGN, particularly in developing countries.
These conditions are characterized by persistent antigenemia and the formation of antigen-antibody immune complexes. It is generally accepted that immune complexes initiate the classical pathway of complement by activating C1, which triggers formation of the C3 convertase of the classical pathway, resulting in deposition of immune complexes and C3 activation fragments in the glomerulus, and finally to the generation of the terminal C5b-9 complex. This phase is followed by an influx of leukocytes, favored by formation of the C3a and C5a anaphylatoxins, that damages capillary walls by the release of cytokines and proteases, leading to proteinuria and hematuria. As a reparative reaction, new basement membrane is then formed, which is associated with entrapment of immune complexes, complement products, and cell debris.
Autoimmune Causes of Complement Activation
MPGN can also be triggered by circulating immune complexes associated with autoimmune diseases such as systemic lupus erythematosus, mixed cryoglobulinemia, Sjögren syndrome, mixed cryoglobulinemia, and scleroderma. Immune complexes activate the classical complement pathway and initiate the same cascade of events observed in MPGN forms secondary to chronic infections.
Complement Activation by Monoclonal Immunoglobulins
MPGN can be associated with deposition of monoclonal immunoglobulins, which may occur in the setting of monoclonal gammopathy of undetermined significance, chronic lymphocytic leukemia, low-grade B-cell lymphoma, and multiple myeloma. Monoclonal gammopathies are associated with complement activation. The mechanism underlying this effect is poorly understood, but likely involves activation of the alternative pathway by the aberrant immunoglobulin. Of note, monoclonal λ light chains from a patient with DDD have been reported to activate the alternative pathway directly through an interaction with CFH.
Additional Readings
-
»
Jokiranta TS, Solomon A, Pangburn MK, et al. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol. 1999;163(8):4590-4596.
-
»
Sethi S, Nester CM, Smith R. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 2012;81(2):434-441.
-
»
Sethi S, Zand L, Leung N, et al. Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol. 2010;5(3):770-782.
Pathophysiology of C3G
Hyperactivation of the alternative pathway of complement has been proposed as a primary cause of C3G and may be associated with autoantibodies like the C3 nephritic factors (C3Nefs) or with defects in genes encoding complement proteins and regulators.
However, the distinction between immune-complex–mediated MPGN and C3G as diseases of classical and alternative pathway activation has been recently questioned by the observation that alternative pathway abnormalities with low C3, C3Nef, and/or mutations in complement genes are present as frequently in patients with immune-complex–mediated MPGN as in those with C3G. These findings indicate that the 2 diseases may show more commonality than previously recognized, with dysregulation of the alternative pathway playing a primary pathogenetic role. This possibility is further supported by reports of immune-complex–mediated MPGN associated with complete CFH deficiency. It has been hypothesized that in predisposed individuals with genetic or acquired complement alternative pathway dysregulation, immune complex deposition initially triggers the classical pathway and then chronic complement activation and kidney injury is sustained through the alternative pathway.
Autoimmune Alternative Pathway Abnormalities
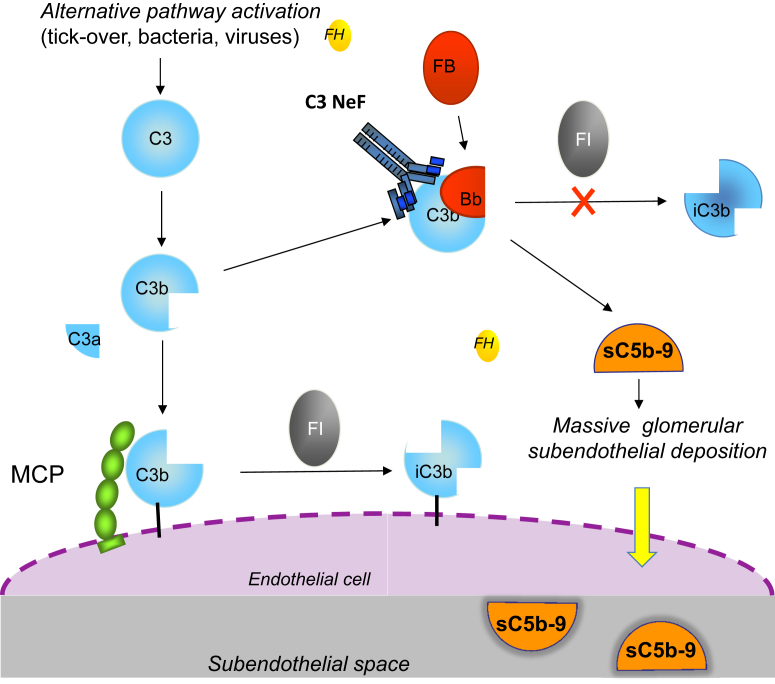
Upregulated activity of the complement alternative pathway can be caused by autoantibodies targeted at either C3 convertase or its components or at regulatory proteins. The first described autoantibody was C3Nef, which binds to a neoepitope on the C3 convertase of the alternative pathway component C3bBb, stabilizing it against CFH-mediated decay and prolonging its C3 cleaving action (Fig 7). A second type of C3Nef was found to display slower C3 activation and dependence on properdin for convertase stabilization. Properdin-independent C3Nefs have been found to have no effect on C5 cleavage and terminal pathway activity, whereas properdin-dependent C3Nef enhances C5 convertase activity. C3Nefs are found in >80% of patients with DDD and 40% to 50% of patients with C3 glomerulonephritis or immune-complex–mediated MPGN. However, in most studies, the presence of C3Nefs has not been found to correlate with disease outcome. In a family with MPGN and partial lipodystrophy, C3Nef was detected in all members with lipodystrophy but did not segregate with the renal phenotype. In addition, C3NeFs have been also found in patients with meningococcal meningitis, lupus nephritis, and even healthy individuals and asymptomatic family members of patients with DDD, indicating that the presence of C3NeFs alone is not sufficient for the development of C3G. Whether C3Nefs are the primary causes of the disease or are secondarily formed as consequences of the disease process is still unclear. Additional studies are needed to define the significance of C3NeFs in the pathophysiology of immune-complex–mediated MPGN and C3G and their relationship to disease course and treatment.
Figure 7.
Model for mechanisms leading from fluid-phase impaired regulation of the alternative pathway to C3 glomerulopathies. Mutations in the factor H (FH) gene causing very low FH levels or impaired FH cofactor activity, or C3 nephritic factor (C3NeF) and C3 gain-of-function mutations that render the alternative pathway C3 convertase resistant to FH-mediated dissociation; all result in uncontrolled fluid-phase alternative pathway activation and finally in the formation of the fluid-phase terminal complement pathway complex (with vitronectin or clusterin bound; sC5b-9). The injury likely develops upon deposition in the subendothelial space of C3 activating products and the terminal complement complex after transfer through the fenestrated endothelium (dashed line). Abbreviations: FB, factor B; FI, factor I; MCP, membrane cofactor protein.
Autoantibodies that bind to native CFB and stabilize the alternative pathway C3 convertase, or target both CFB and C3b, have been described in patients with DDD. Anti-CFH autoantibodies have been identified in a few patients with immune-complex–mediated MPGN, DDD, and C3 glomerulonephritis. Of note, the autoantibodies bind predominantly to the amino-terminal complement regulatory domain of CFH, in contrast to aHUS-associated autoantibodies, which mostly bind the CFH carboxy-terminal surface recognition domain. Altogether, both C3Nef and autoantibodies against either C3 convertase components or CFH stabilize the convertase and prolong its half-life, preventing its dissociation and degradation and thereby resulting in uncontrolled alternative pathway activation in the fluid phase (Fig 7).
Genetic Alternative Pathway Abnormalities
The reports of familial cases of C3G and immune-complex–mediated MPGN (MPGN types I and III) indicate a genetic basis of the diseases, although the genetic defect has been characterized in only a minority of patients.
Homozygous nonsense and missense CFH mutations, causing either premature truncation of the protein or impaired protein secretion, respectively, have been reported in a few familial cases of autosomal recessive DDD or C3 glomerulonephritis and are associated with very low or undetectable CFH plasma levels. Homozygous carriers present with kidney disease during infancy and have very low serum C3, CFB, and C5 levels, consistent with massive fluid-phase alternative pathway activation. In 2 young daughters with C3 glomerulonephritis from consanguineous Turkish parents, a homozygous CFH mutation caused a deletion of the lysine at amino acid 224 (in SCR4). These patients had very low C3 levels but only slightly reduced CFH levels. Functional studies revealed that the mutation produces a circulating CFH protein with impaired binding to C3b and defective decay accelerating and cofactor activities, while its cell-binding activity is unaffected. Thus, this mutation recapitulates the condition of dysregulated alternative pathway fluid-phase activation observed in patients with complete CFH deficiency.
A C3 gain-of-function heterozygous mutation leading to a 2–amino-acid deletion has been reported in a mother and her 2 identical twin boys, all with DDD. This C3 mutant is resistant to cleavage by C3 convertase and therefore cannot form activated C3b. However, it forms the alternative pathway initiation C3 convertase (C3[H2O]Bb; Fig 1) through the normal “tick-over” process. This convertase is capable of cleaving circulating wild-type C3 produced from the normal allele, but is resistant to CFH-mediated regulation, resulting in a dominant gain-of-function effect of the mutation. Importantly, decay of the mutant C3 convertase by the surface regulator decay accelerating factor and MCP cofactor activity for CFH-mediated C3b cleavage are not affected by the C3 mutation, providing evidence that DDD in this family results from alternative pathway dysregulation restricted to the fluid phase.
As a consequence of all the underlying abnormalities reported, dysregulation of the alternative pathway results in massive formation of complement products in the circulation (Fig 7), such as C3b and C5b-9, which are delivered indiscriminately to endothelial surfaces, including glomeruli. The transfer of these complement products and debris through the fenestrated endothelium to subendothelial region and the mesangium triggers glomerular inflammation and leads to MPGN (Fig 7). This scenario appears different from that associated with complement gene mutations or anti-CFH autoantibodies in aHUS, characterized by surface-restricted complement dysregulation.
Additional Readings
-
»
Bogdanović RM, Dimitrijević JZ, Nikolić VN, Ognjanović MV, Rodić BD, Slavković BV. Membranoproliferative glomerulonephritis in two siblings: report and literature review. Pediatr Nephrol. 2000;14(5):400-405.
-
»
Chen Q, Muller D, Rudolph B, et al. Combined C3b and factor B autoantibodies and MPGN type II. N Engl J Med. 2011;365(24):2340-2342.
-
»
Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, et al. Heterozygous and homozygous factor H deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol. 2004;15(3):787-795.
-
»
Habbig S, Mihatsch MJ, Heinen S, et al. C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int. 2009;75(11):1230-1234.
-
»
Martinez-Barricarte R, Heurich M, Valdes-Canedo F, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120(10):3702-3712.
-
»
Neary J, Dorman A, Campbell E, Keogan M, Conlon P. Familial membranoproliferative glomerulonephritis type III. Am J Kidney Dis. 2002;40(1):E1.
-
»
Paixao-Cavalcante D, Lopez-Trascasa M, Skattum L, et al. Sensitive and specific assays for C3 nephritic factors clarify mechanisms underlying complement dysregulation. Kidney Int. 2012;82(10):1084-1092.
-
»
Zhang Y, Meyer NC, Wang K, et al. Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol. 2012;7(2):265-274.
aHUS, MPGN, and C3G: Different Diseases or a Spectrum of Complement-Mediated Glomerular Diseases?
The paradigm of the distinct pathogenetic mechanisms of complement activation (cell surface vs fluid phase) leading to aHUS versus MPGN has been recently thrown into question by a number of findings. A heterozygous nonhomologous allelic recombination in the CFHR5 gene leading to internal duplication of the initial 2 amino-terminal repeats of CFHR5 (CFHR51212) has been detected in 2 families with autosomal dominant C3G from the Troodos Mountains of Cyprus; this disease was called CFHR5 nephropathy. A total of 91 carriers of the same founder mutation from 16 apparently unrelated Greek-Cypriot families was later reported, with disease penetrance of 90%. Of note, some carriers had a previous diagnosis of MPGN type I. The same abnormally large CFHR51212 protein was found in association with C3 glomerulonephritis in a family without Cypriot ancestry. However, the mutant protein derived from a genomic rearrangement distinct from that seen in Cypriot CFHR5 nephropathy. Another genomic duplication, in this case affecting the CFHR1 gene, results in internal duplication of the first 4 amino-terminal domains of CFHR1 (CFHR11414) and segregates with C3G in a pedigree with autosomal dominant transmission. CFHR1 to CFHR5 circulate in plasma as homo- and hetero-oligomeric complexes formed by interactions of their conserved amino-terminal domains. CFHR1 to CFHR5 homo- and hetero-oligomers can compete with CFH for binding to C3b and other C3 activation fragments. In mutant CFHR51212 and CFHR11414, the dimerization motifs are duplicated, which results in the formation of large multimeric complexes that exhibit increased affinity for surface-bound C3b and exert enhanced competition with CFH.
However, why did these patients develop C3G and not aHUS? It has been hypothesized that while CFH mutations and CFH/CFHR genomic rearrangements associated with aHUS impair binding of CFH to most self-surfaces, C3G-associated mutant CFHRs compete with CFH for binding to only a subset of self-surfaces, but not to endothelial cell surfaces. Of relevance, the mutant duplicated CFHR51212 interacts more with GBM-bound C3 than does wild-type CFHR5. It has been suggested that there is continuous activation of C3 along the GBM (spontaneous, following a trigger, or both) that in normal conditions is controlled by CFH. In the presence of abnormal CFHR proteins, competition with CFH enables C3 to accumulate along the GBM, causing accumulation of C3 activation products and C3G.
In addition, hybrid genes (including CFHR3-1, which encodes a fusion protein with the first 2 SCRs from CFHR3 and all 5 SCRs of CFHR1 [CFHR312-CFHR1]), and a CFHR2/CFHR5 hybrid gene (encoding the first 2 SCRs of CFHR2 and all 9 SCRs of CFHR5 [CFHR212-CFHR5]) have been reported in affected individuals of a family with autosomal dominant C3 glomerulonephritis (previously classified as MPGN type III) and a family with autosomal dominant DDD, respectively. The hybrid CFHR312-CFHR1 and CFHR212-CFHR5 proteins can compete with CFH for C3b binding and are able to deregulate CFH function and activate the alternative pathway C3 convertase both at the cell-surface level and in plasma.
Furthermore, in a series of patients with immune-complex–mediated MPGN and C3G, heterozygous mutations in the CFH, CFI, and MCP genes have been described in gene areas previously identified in patients with aHUS, and conversely, mutations affecting the CFH amino terminus and leading to defective regulation both in the fluid phase and at the cell surface have been reported in patients with aHUS.
Finally, a homozygous mutation in CFH associated with undetectable circulating CFH levels has been documented in a patient who first developed C3G and later aHUS. Conversely, 2 children with CFH deficiency and aHUS have been reported to develop C3G after kidney transplantation.
Altogether, these observations are suggestive of a pathogenetic link between C3G, MPGN, and aHUS.
Additional Readings
-
»
Chen Q, Wiesener M, Eberhardt HU, et al. Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest. 2014;124(1):145-155.
-
»
Gale DP, de Jorge EG, Cook HT, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376(9743):794-801.
-
»
Goicoechea de Jorge E, Caesar JJ, Malik TH, et al. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A. 2013;110(12):4685-4690.
-
»
Malik TH, Lavin PJ, Goicoechea de Jorge E, et al. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23(7):1155-1160.
-
»
Servais A, Noel LH, Roumenina LT, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82(4):454-464.
-
»
Tortajada A, Yebenes H, Abarrategui-Garrido C, et al. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest. 2013;123(6):2434-2446.
Incomplete Penetrance and Predisposing Factors
Complement gene mutations or rearrangements have been identified in only ∼20% of patients with immune-complex–mediated MPGN or C3G. In addition, the diseases do not develop in all mutation carriers in high-risk families, indicating that gene mutations are predisposing factors and additional genetic factors and environmental triggers are required to induce the disease and determine the phenotype.
Beside mutations, certain polymorphic variants have also been linked to immune-complex–mediated MPGN or C3G. Among these, the Y402H variant of CFH (Table 3), which displays reduced binding and complement regulation at cell surfaces, is over-represented in patients with DDD, but no significant association has been detected with immune-complex–mediated MPGN and C3 glomerulonephritis.
However, a significant association has been found between immune-complex–mediated MPGN and C3 glomerulonephritis and 1 SNP (c.-652A>G; Table 3) located in the MCP promoter, with the G variant under-represented in patients with immune-complex–mediated MPGN and C3 glomerulonephritis. Finally, 2 C3 SNPs (R102G and P314L; Table 3) have been associated with risk of DDD. Of relevance, in functional studies, the R102G variant activates the alternative pathway more efficiently than the wild-type protein. The likelihood of developing DDD increases with the presence of 2 or more risk alleles in CFH and C3. There have been previous reports not only of at-risk haplotypes combining multiple SNPs in the CFH, MCP, and C3 genes, but also of protective haplotypes related to the CFH and MCP genes (Table 3).
Notably, all the mentioned gene variants and haplotypes are different from those associated with aHUS. It has been proposed that specific combinations of risk and protective variants in alternative pathway genes, called complotypes, contribute to set levels of alternative pathway activity in plasma or at the cell surface, thereby influencing disease risk and phenotype. However, appropriately designed and powered association studies are required to define specific disease-associated complotypes.
Such studies will be relevant to refine the classification of immune-complex–mediated MPGN and C3G and also to predict response to therapies.
Additional Readings
-
»
Abrera-Abeleda MA, Nishimura C, Frees K, et al. Allelic variants of complement genes associated with dense deposit disease. J Am Soc Nephrol. 2011;22(8):1551-1559.
-
»
Heurich M, Martinez-Barricarte R, Francis NJ, et al. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc Natl Acad Sci U S A. 2011;108(21):8761-8766.
Therapeutic Perspectives
Limitation of complement activation is a major goal of current research into the treatment of C3G and immune-complex–mediated MPGN; however, the relative roles of upstream C3 activation products and of the terminal complement complex are still not well understood. At variance with the almost universal response to eculizumab that is observed in aHUS, case reports and a small trial have shown a clinical response (reduction in serum creatinine and/or proteinuria and histopathologic improvement) in some but not all patients with C3G or immune-complex–mediated MPGN either in native kidneys or recurring in the kidney transplant. Elevated plasma sC5b-9 levels are reported as a potential marker of responsiveness. A trial comprising a greater number of well-characterized patients is warranted to establish the potential role of eculizumab in the treatment of C3G and immune-complex–mediated MPGN.
Additional Readings
-
»
Daina E, Noris M, Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med. 2012;366(12):1161-1163.
-
»
Herlitz LC, Bomback AS, Markowitz GS, et al. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23(7):1229-1237.
-
»
Nester CM, Smith RJ. Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens. 2013;22(2):231-237.
-
»
Radhakrishnan S, Lunn A, Kirschfink M, et al. Eculizumab and refractory membranoproliferative glomerulonephritis. N Engl J Med. 2012;366(12):1165-1166.
-
»
Vivarelli M, Emma F. Treatment of C3 glomerulopathy with complement blockers. Semin Thromb Hemost. 2014;40(4):472-477.
-
»
Vivarelli M, Pasini A, Emma F. Eculizumab for the treatment of dense-deposit disease. N Engl J Med. 2012;366(12):1163-1165.
Conclusions
The last decade has seen great advances in the knowledge of the pathophysiologic role of complement activation in aHUS, immune-complex–mediated MPGN, and C3G and has led to the discovery of an effective therapy, at least in aHUS. However, there are still a number of issues to be addressed.
In aHUS, we need to clarify whether and which secondary forms are related to complement dysregulation. Whether eculizumab should be a life-long treatment or could be safely discontinued in a subgroup of patients with aHUS is another crucial issue in view of the impressively high cost of the drug and the potential side effects. In this regard, the recent identification of a specific test of aHUS serum-induced endothelial complement activation could be of help to monitor disease activity and personalize treatment.
In immune-complex–mediated MPGN and C3G, biomarkers of alternative pathway activation more specific than C3 deposits on immunofluorescence microscopy are needed to better discriminate immune forms that are related to antigen–immune complex reactions in glomeruli from complement-mediated forms associated with alternative pathway activation. The role of C3Nef as a cause or a consequence of the disease process should be clarified. Additional genetic studies are required to clarify the role of complement gene variants in determining disease predisposition. It is likely that a subgroup of patients will benefit from C5 blockade, and biomarkers are required for early identification of potentially eculizumab-responsive patients. However, emerging therapies that target C3 convertase activity will be of major interest in these diseases. Better understanding of the different pathogenetic mechanisms underlying these conditions will help optimize trial design with these agents.
Additional Reading
-
»
Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124(11):1715-1726.
Acknowledgements
Support: This work was supported by Fondazione ART per la Ricerca sui Trapianti ART ONLUS (Milano, Italy), the European Union Seventh Framework Programme FP7-EURenOmics project number 305608, and by Telethon (grant GGP09075).
Financial Disclosure: Dr Noris has received honoraria from Alexion Pharmaceuticals (manufacturer of eculizumab) for giving lectures and participating in advisory boards. Dr Remuzzi has consultancy agreements with AbbVie, Alexion Pharmaceuticals, Bayer Healthcare, Reata Pharmaceuticals, Novartis Pharma, AstraZeneca, Otsuka Pharmaceutical Europe, and Concert Pharmaceuticals (no personal remuneration is accepted for these agreements, compensation is paid to his institution for research and educational activities).