Highlights
-
•
80% of bulk milk samples from 600 UK dairy farms tested positive for F. hepatica.
-
•
Farm level risk factors explained 39% of variation in F. hepatica exposure.
-
•
Raised F. hepatica antibodies were associated with a 15% reduction in milk yield.
Keywords: Fasciola hepatica, Dairy cattle, Milk production, Risk factors
Abstract
The liver fluke Fasciola hepatica is a trematode parasite with a worldwide distribution and is the cause of important production losses in the dairy industry. The aim of this observational study was to assess the prevalence of exposure to F. hepatica in a group of high yielding dairy herds, to determine the risk factors and investigate their associations with production and fertility parameters. Bulk milk tank samples from 606 herds that supply a single retailer with liquid milk were tested with an antibody ELISA for F. hepatica. Multivariable linear regression was used to investigate the effect of farm management and environmental risk factors on F. hepatica exposure. Higher rainfall, grazing boggy pasture, presence of beef cattle on farm, access to a stream or pond and smaller herd size were associated with an increased risk of exposure. Univariable regression was used to look for associations between fluke exposure and production-related variables including milk yield, composition, somatic cell count and calving index. Although causation cannot be assumed, a significant (p < 0.001) negative association was seen between F. hepatica exposure and estimated milk yield at the herd level, representing a 15% decrease in yield for an increase in F. hepatica exposure from the 25th to the 75th percentile. This remained significant when fertility, farm management and environmental factors were controlled for. No associations were found between F. hepatica exposure and any of the other production, disease or fertility variables.
1. Introduction
Fasciola hepatica, also known as the common liver fluke, is a trematode parasite of sheep and cattle with a widespread distribution worldwide and in the UK, where fluke prevalence in adult dairy cattle has been reported to be 48% to 76% (Salimi-Bejestani et al., 2005a; McCann et al., 2010b). Elsewhere in Western Europe, prevalence estimates of 37%, 50% and 61% are reported in Belgium, Germany, and Spain, respectively (Mezo et al., 2008; Bennema et al., 2009; Kuerpick et al., 2012b). The prevalence of F. hepatica in the UK has increased over recent years, and fasciolosis has been reported in new areas, thought to be as a result of wetter summers and warmer winters (Mitchell, 2002; Pritchard et al., 2005; Kenyon et al., 2009; Fox et al., 2011). Fluke prevalence remains high also in countries where flukicide is used routinely (Charlier et al., 2014). Moreover, there are reports of resistance to commonly used anthelmintics making fluke a threat to animal health, welfare and agricultural productivity (Sargison and Scott, 2011; Daniel et al., 2012; Gordon et al., 2012; Sargison, 2012; Hodgkinson et al., 2013).
Climatic and geographical variables are known to be important in determining the risk of fluke infection, because of their effect on the survival and rate of development of the parasite on pasture and in the intermediate host, Galba truncatula, and on the intermediate host itself. A predictive model based on climate data first developed by Ollerenshaw and Rowlands (1959) is still used on the National Animal Disease Information Service (NADIS) website to predict years when fluke infection related losses will be heaviest, enabling farmers to plan ahead (NADIS, 2014). More recent work on climate showed that geographical and climate variables can explain 70–76% of variation in fluke infection at the level of postcode area (McCann et al., 2010a).
Farm management factors may affect the chances of cattle coming into contact with infective metacercariae (Morgan and Wall, 2009; Bennema et al., 2011). Examples found to be important in previous studies include the presence of snail habitats on pasture, length of grazing season, proportion of grazed grass in the diet, stocking rate, type of drinking water supply and grazing on mowed pastures, whilst other factors such as herd size affect risk of fluke infection risk via an unknown or combination of mechanisms (Bennema et al., 2011; Charlier et al., 2011). These factors have been found to vary between studies, depending on the local environment and farming systems (Charlier et al., 2014).
Fluke control should be aimed at reducing infection levels in snails as well as in cattle (Parr and Gray, 2000; Knubben-Schweizer et al., 2010). So far, few studies have looked at how grazing management can be used to control fluke, either alone or in conjunction with flukicides. Control of snail populations by use of molluscicides is not permitted in the UK due to their adverse effects on the environment. Pasture drainage is another option, however in most cases this is impractical, prohibitively expensive (Roberts and Suhardono, 1996) and is discouraged in the UK for environmental reasons (Natural England, 2011a,b).
Most fluke infections in adult cattle are sub-clinical, yet are of economic importance (Dargie, 1987; Schweizer et al., 2005). There is considerable evidence from around the world that fluke infection has an adverse effect on production in dairy cattle. Decreased milk yields of between 8 and 15% are reported, equivalent to between 0.7 and 4.2 kg per cow per day (Donker, 1970; Horchner et al., 1970; Ross, 1970; Randell and Bradley, 1980; Schweizer et al., 2005; Charlier et al., 2007; Khan et al., 2011; Mezo et al., 2011; Charlier et al., 2012; Kuerpick et al., 2012a). Some studies found a reduction in butterfat content (Horchner et al., 1970; Black and Froyd, 1972; Charlier et al., 2007; Khan et al., 2009, 2011), although others did not (Mezo et al., 2011; Charlier et al., 2012). Reported effects on fertility vary, with some studies reporting an increased calving interval, or delayed puberty in young animals (Lopez-Diaz et al., 1998; Charlier et al., 2007), whereas others found no difference (Simsek et al., 2007; Mezo et al., 2011). The magnitude of the effect depends on the breed of cow and the husbandry system used, which varies greatly between countries studied, even within western Europe (Bennema et al., 2010; Mezo et al., 2011). To date, no studies have investigated the effect of fluke infection on the UK dairy industry, which is predominantly made up of high yielding Holstein Friesian cattle.
This study aimed to determine the prevalence, and economic significance, of fluke infection at the herd level, and to ascertain which climate, environmental and farm management risk factors are important in this group of high yielding dairy herds.
2. Methods
2.1. Study population
The study population was a group of 606 high yielding herds contracted to supply milk to a major supermarket chain. These herds were located in England, Wales and Scotland and the mean herd size was 153 adult cows. Farmers consented to their milk test results and herd information being used for research as a condition of their milk supply contract.
2.2. Determination of F. hepatica exposure levels
Bulk milk tank (BMT) samples were obtained from all herds via National Milk Laboratories (NML) during October to December 2012. A BMT sample from each herd was submitted daily by the milk processing company (Arla or Müller Wiseman) to NML, where they were stored for 5 days at 4 °C in case required for milk quality random testing. Following addition of bronopolnatamyin (MSI, Nottingham) as preservative, samples from participating herds were sent by courier to the University of Liverpool (UoL). On arrival they were centrifuged at 1000 g for 20 min and aliquots of skimmed milk were taken for testing.
The BMT samples were tested using a F. hepatica excretory–secretory (ES) antibody-detection ELISA developed at UoL, according to Salimi-Bejestani et al. (2005b). Results were expressed as percent positivity (PP) of a positive control. Individual results were considered valid if the duplicate PP values were within 10% of each other. A PP of 27 or above defines a positive result, and corresponds with more than 25% of the herd being infected (Salimi-Bejestani et al., 2007). The sensitivity and specificity of this ELISA to detect herds in which more than 25% of the cows are infected are 96% (95% CI 89–100%) and 80% (95% CI 66–94%), respectively (Salimi-Bejestani et al., 2005a). Fifteen percent of samples were retested on separate plates to ensure repeatability. As a further test for the validity of the results, a commercial ELISA kit, the Fasciola Verification test (IDEXX, Montpellier, France) was used according to the manufacturer's instructions to test 40 (6.6%) randomly selected samples.
2.3. Data sources
Variables relating to milk yield and quality, fertility, disease, pasture quality, soil type, climate and farm management were obtained either by farmer questionnaire, directly from the dairies or from various databases (Table 1). The questionnaire contained mainly closed questions, with open questions used to obtain further detail for some questions (Supplementary information 1). It was validated by asking two farmers to complete it, before it was posted to the study farms, and they could complete it either on paper or online (SurveyMonkey Inc., USA). Milk yield (kg produced per cow per year) was available for 32 herds. An estimated yield was calculated for 475 other herds by dividing the total herd milk production for November 2012 by the number of cows in milk in the same month, and multiplying this number by 10 for a typical lactation length of ten months per year. Estimated yield was analysed as a separate outcome from yield as the two are not equivalent. Most herds calved all year round, as the milk supply contract requires a constant monthly volume throughout the year. The small number of seasonal calving herds (as determined by farmer questionnaire, n = 24) were excluded from the production analysis to avoid error due to seasonal production fluctuations in these herds. No information on milk yield was available for the remaining 99 herds.
Table 1.
Sources and details of farm management and environmental data about UK dairy herds (n=606)
| Variable | Available for | Resolution | Source | Date |
|---|---|---|---|---|
| Climate | ||||
| Raindays >1mm 5 year mean for MJJ and ASOa | All farms | 5 km grid | Met Officeb | 2007–2011 |
| Rainfall 5 year mean for MJJ, ASO, NDJ | All farms | 5 km grid | Met Officeb | 2007–2011 |
| Min Temp 5 year mean FMA, MJJ, NDJ | All farms | 5 km grid | Met Officeb | 2007–2011 |
| Max Temp 5 year mean FMA, MJJ, NDJ | All farms | 5 km grid | Met Officeb | 2007–2011 |
| Land quality | ||||
| Average slope of land in degrees | All farms | 1 km grid | CEH CISc | 1995 |
| Altitude | All farms | 1 km grid | CEH CISc | 1995 |
| Improved grassland | All farms | 1 km grid | CEH CISc | 2000 |
| ALC Grade | England only (485 farms) | <50 m | Natural Englandd | 1988 |
| Soil | ||||
| pH | England and Wales (544 farms) | 5 km grid | NSRI LandISe | 1983 |
| Iron (Fe) | England and Wales (544 farms) | 5 km grid | NSRI LandISe | 1983 |
| Phosphorus (P) | England and Wales (544 farms) | 5 km grid | NSRI LandISe | 1983 |
| Very fine sand (soil texture) | England and Wales (544 farms) | 5 km grid | NSRI LandISe | 1983 |
FMA, MJJ, ASO and NDJ refer to 3 month periods of February, March, April; May, June, July; etc.
UK Meteorological Office (www.metoffice.gov.uk/climatechange/science/monitoring/ukcp09).
Centre for Ecology and Hydrology Countryside Information System (CEH CIS) (www.ceh.ac.uk/products/software/CEHSoftware-CIS.htm).
Natural England (www.gis.naturalengland.org.uk).
National Soil Research Institute Land Information System (NSRI LandIS) (http://www.cranfield.ac.uk/sas/nsri).
Climate data include monthly rainfall, days of rainfall >1 mm and maximum and minimum temperatures. These were converted to means of 3 month periods and then into 5-year (2007–2011) means, because a previous study found that aggregated data was more able to explain variation in fluke infection risk than data from individual years or months (McCann et al., 2010a). More detailed 2012 weather data were not available.
Agricultural land classification (ALC) grade is a scale from 1 to 5 where 1 is best, and is determined by combining several factors including climate, aspect (eg. north or south facing), elevation, exposure to wind and rain, frost risk, flood risk and stoniness. Some variables were not available for all farms depending on country.
2.4. Statistical analysis
True prevalence, overall and at Level 1 Nomenclature of Territorial Units for Statistics (NUTS) region, taking into account the sensitivity and specificity of the ELISA, were calculated using the Rogan–Gladen estimator (Rogan and Gladen, 1978). The ELISA has a sensitivity of 92% and specificity of 88% (Salimi-Bejestani et al., 2005a). Adjusted 95% Blaker’s confidence intervals for the prevalence were calculated according to Lang and Reiczigel (2014), in R (R Development Core Team, 2011), using the Hmisc package (Farrell, 2012) The Chi-square test was used to test for significant differences between regions. All statistical analyses were performed using SPSS Statistics 20 (2011, SPSS Inc, Chicago) and R (R Development Core Team, 2011). A p value of <.05 was considered statistically significant.
2.5. Risk factor modelling
Univariable linear regression was performed for every variable against ELISA PP. Variables with p < 0.2 were selected for use in the multivariable models. A causal web was used to select meaningful combinations of variables (Dohoo et al., 2009). Firstly two models were built using farm management and environmental data separately, to help to identify which variables to include in the final model. Natural logs of number of cows, young stock and stocking rate were used to avoid disproportionate influence from the few farms with high values.
From the 606 cases (herd records), 80% were randomly selected for model building with the remaining 20% used for model validation. Data was analysed to quantify the missing data by variable, case and value, using the multiple imputation function in SPSS. To deal with missing data, multiple imputation (MI) was used to replace missing values. This method is used to avoid losing information through the exclusion of cases where one or more observation is missing, by imputing a value which preserves the variability of the original dataset. The following variables were used to create the MI model: ELISA PP, estimated milk yield, x and y co-ordinates, water source, beef herd, grazing period, boggy grazing, rainfall, temperature, cows, heifers, calves, sheep, altitude, grazing acreage, stocking rate and slope. The following variables were imputed: water source, beef herd, grazing period, boggy grazing, cows, youngstock, sheep, grazing acreage and stocking rate. Ten MI datasets were created using the fully conditional specification method with 10 iterations. All further risk factor analyses were then performed using the 10 MI datasets. Backward stepwise entry was used for exploratory model building for the multivariable linear regression model, followed by fine tuning using forced entry of variables. Variables with correlations >|0.7| were not entered simultaneously into the model. All combinations of variables were tested as interaction terms. The best model was considered to be the one with the highest adjusted R2. Variables in the final model appeared in best models for 8 or more of the MI datasets. The final model co-efficients are the mean of the coefficients resulting from the analysis of the 10 MI datasets. The model assumptions of linearity, normality, homoscedasticity and independence of the residuals were checked.
The final model was tested on the holdout sample by using it to predict results and comparing with the observed results. The same variables as in the final model were used to make two further models: for complete cases only, and for the holdout sample. A sensitivity analysis was done to compare the effect of each variable between models.
2.6. Spatial analysis
Spatial analysis was performed in ArcGIS v 10 (ESRI, Redlands, CA, USA). All explanatory variables in the final model were mapped using the co-ordinates of the farm postcode, as were the ELISA results, predicted values and model residuals. To find out whether values were spatially clustered, Getis-Ord Gi cluster analysis was performed (Getis and Ord, 1992). A large neighbourhood search threshold (37.245 m) was used in order to compare the amount of clustering relative to the whole of the study area. A variogram with 95% confidence envelopes was drawn to establish whether residual clustering was statistically significant at different inter-farm distances. The distance between each pair of farms was plotted against the semivariance of their ELISA results. The 95% confidence envelopes were created in the geoR package by randomly permuting the data across the locations 999 times and plotting the envelopes within which 95% of the semivariances lay (Ribeiro and Diggle, 2001).
2.7. Production effects
Univariable linear or logistic regression with ELISA PP as the explanatory variable was performed separately for each production and fertility outcome variable. Cases with missing data were excluded. Yield was added into each model as an additional explanatory variable, to control for confounding, as there is known to be a relationship between yield and most production and fertility variables (Nebel and McGilliard, 1993). Risk factors for fluke infection were also added to the models because some of these, for example herd size and grazing management, could also have an effect on yield.
3. Results
3.1. Prevalence of F. hepatica
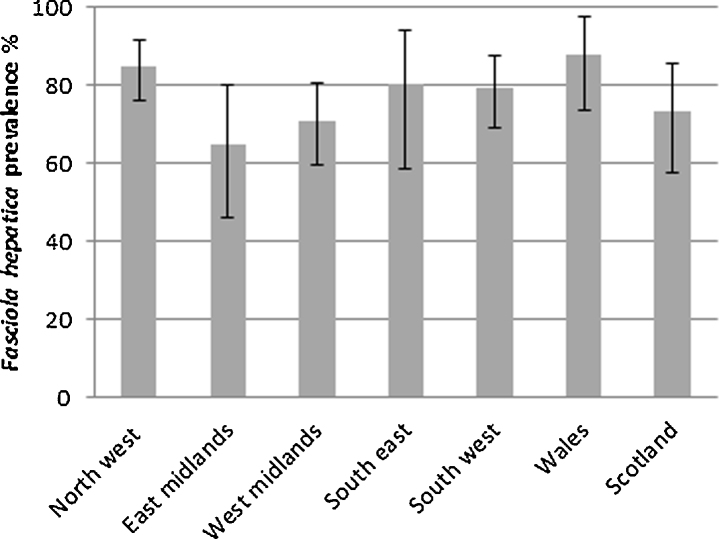
Across all herds, the apparent prevalence of fluke infection was 79.7% (estimated true prevalence 78.1%; 95% CI 73.6–82.2%). England and Scotland had a similar apparent prevalence at 78.8% (True Prev. 77.5%; 95% CI 72.4–82.1%) and 75.8% (True Prev. 73.4%; 95% CI 57.7–85.7%), respectively. Wales had a higher apparent prevalence at 86.9% (True Prev. 88%; 95% CI 73.4-97.6%). When categorized by NUTS region, North West England had the highest apparent prevalence of 85.1% (True Prev. 84.8%; 95% CI 76.1–91.8%) whilst the lowest was the East Midlands with 69.2% (True Prev. 64.8%; 95% CI 45.9–80.1%) (Fig. 1). There were no significant differences between NUTS regions.
Fig. 1.
True F. hepatica seroprevalence in UK dairy herds by NUTS region, taking into account the sensitivity and specificity of the ELISA. 95% confidence intervals are shown.
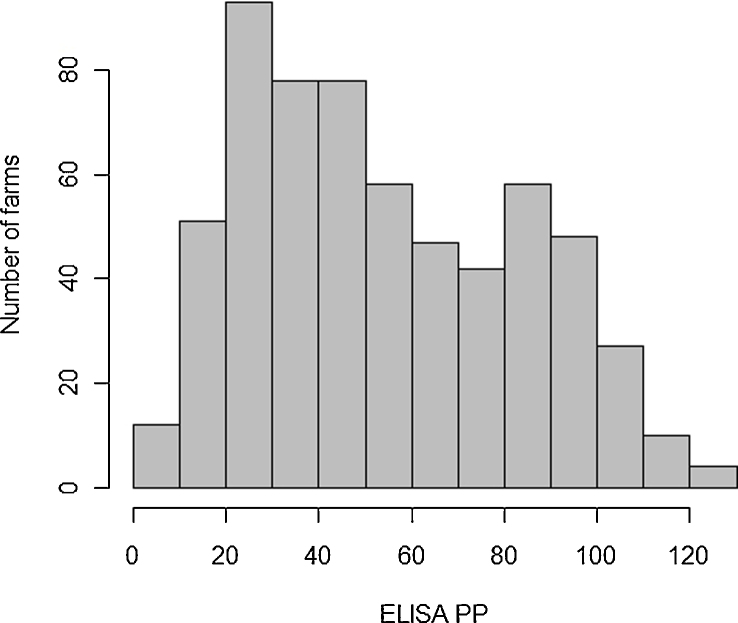
The PP values of the BMT samples varied between 9 PP and 126 PP, and were approximately normally distributed with a slight skew to the right (Fig. 2). Hartigan’s dip test statistic for unimodality showed that unimodality could not be rejected (p = 0.65) (Hartigan, 1985).
Fig. 2.
Distribution of the F. hepatica ELISA PP values from 606 UK dairy herds.
115 samples were retested and 95% gave the same result (positive/negative). Of the 40 samples tested with the Idexx kit, 37 (92.5%) gave the same result (positive/negative) as the UoL ELISA.
3.2. Fluke risk factor analysis
Three hundred and fifty-four (58%) of the questionnaires were returned. The mean ELISA PP value was not significantly different between those farms that did and did not return a questionnaire (Students t-test, p = 0.36). 46% of farmers treated with flukicide. Summary statistics for the variables are shown in Table 2.
Table 2.
Summary of variables included in the regression models for F. hepatica in UK dairy herds (n = 606) (Names in brackets are abbreviations)
| Categorical variables | % of farms positive for variable | Missing | ||
|---|---|---|---|---|
| Beef cattle on farm (Beef) | 47.4 | 266 | ||
| Boggy grazing land used (Boggy) | 63.2 | 288 | ||
| Water source used by cattle (Water) | 283 | |||
| Piped water supply only (coded 0 in analysis) | 73.7 | |||
| Access to river/stream/canal/ditch/pond (coded 1) | 26.3 | |||
| Agricultural Land Classification (ALC) Grade 1 | 0.8 | 121 | ||
| 2 | 11.2 | |||
| 3 | 73.0 | |||
| 4 | 14.5 | |||
| 5 | 0.4 | |||
| Continuous variables | Mean | SD | Range | Missing |
|---|---|---|---|---|
| Milking herd size. Log used for analysis (Cows) | 153.3 | 21.01 | 23-516 | 83 |
| Total youngstock. Log used for analysis (Youngstock) | 135.92 | 110.41 | 0-710 | 282 |
| Stocking rate. Log used for analysis (Stockrate) | 1.82 | 1.47 | 0-10 | 340 |
| Average daily rainfall (mm) over August, September and October, averaged over 2007-2011 (Rainfall) | 79.01 | 21.02 | 53.23-158.74 | 0 |
| Slope of land in degrees (Slope) | 2.29 | 2.02 | 0-15.1 | 0 |
| Texture of soil is very fine sand (Vfsand) | 7.42 | 6.04 | 0-43.3 | 0 |
| Average minimum temperature (°C) over May, June and July, averaged over 2007-2011 (T) | 9.86 | 0.67 | 7.40-11.65 | 0 |
| Improved grass | 47.93 | 20.84 | 0-97.6 | 0 |
The strongest single predictor of fluke exposure was Rainfall (F1,605 = 190.1, R2 = 24%, p < .001). The best model using only environmental/climate factors contained five predictors (vfsand, T, Improved grass, ALC Grade and Rainfall), (F5,481 = 35.9, R2 = 27%, p < .001) (Supplementary information 2). The best model using only farm management factors contained six predictors (Youngstock, Cows, Stockrate, Beef, Boggy, Water), (F6,209 = 9.33, R2 = 21%, p < .001) (Supplementary information 3).
Missing values analysis showed that between 0 and 57% of values were missing for each predictor variable. These were missing due to non-returned questionnaires or data being unavailable for some farms. Little’s MCAR (Missing Completely At Random) test was non-significant, indicating that the null hypothesis that data were missing completely at random cannot be rejected (p = .149).
The final model (F7,478 = 41.78, R2 = 37%, p < .001), containing a combination of all the risk factors included seven predictors (Rainfall, Beef, Boggy, Water, Slope, Youngstock, Cows). Of the farm management variables from the previous model, all but Stockrate were retained in this model, but of the environmental factors model, only Rainfall was retained, and a new variable, Slope, was added (Table 3). There were no significant interaction terms.
Table 3.
Regression model for F. hepatica ELISA PP in UK dairy herds (n=485) including climate and environmental and farm management variables (F7,478 = 41.78, R2 = 37%, p < .001).
| Unstandardised coefficients | t | Sig. | 95% Confidence interval for B |
|||
|---|---|---|---|---|---|---|
| B | Std. error | Lower bound | Upper bound | |||
| (Constant) | 56.80 | 12.71 | 4.47 | 0.00 | 31.64 | 81.96 |
| 5-yr mean Rainfall ASO | 0.66 | 0.06 | 10.70 | 0.00 | 0.54 | 0.78 |
| Slope of land | −2.35 | 0.59 | −3.98 | 0.00 | −3.51 | −1.19 |
| No. of cows | −8.13 | 2.43 | −3.34 | 0.00 | −12.93 | −3.32 |
| Water (access to river/stream/pond) (1 = yes) | 7.98 | 2.82 | 2.83 | 0.01 | 2.40 | 13.55 |
| Beef cattle on farm (1 = yes) | 8.40 | 3.42 | 2.46 | 0.02 | 1.39 | 15.41 |
| No. of youngstock | −4.30 | 1.77 | −2.43 | 0.03 | −8.01 | −0.59 |
| Boggy grazing land used (1 = yes) | 6.09 | 3.33 | 1.83 | 0.08 | −0.72 | 12.90 |
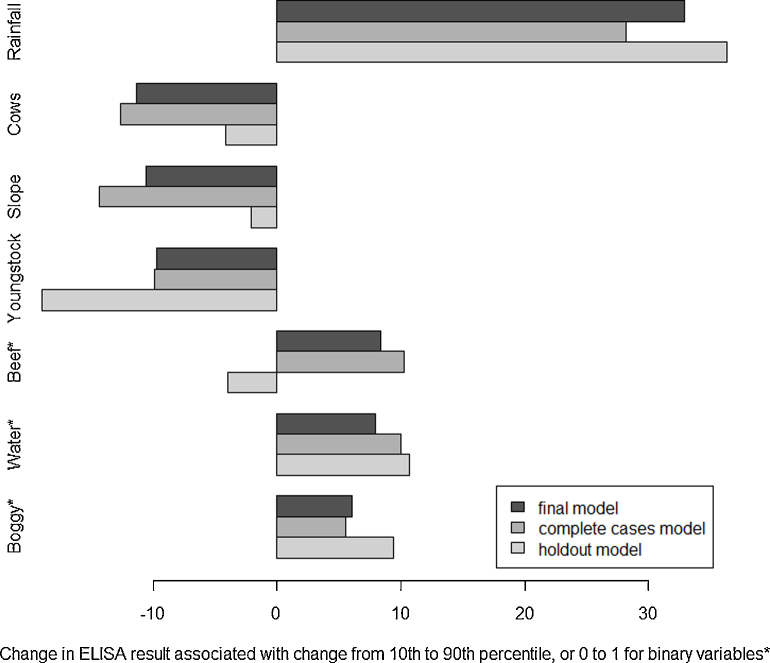
The hold out sample contained complete data for 55 farms. The final model was able to explain 49% of the variation in ELISA PP in this group of farms (Supplementary information 4). When the same variables were forced into a model for the holdout farms only, all co-efficients were of the same sign except Beef, although the effect size varied for some, particularly Youngstock, Slope and Cows (Fig. 3). Only Rainfall and Youngstock were significant F7,47 = 8.00, R2 = 54%, p < .001.
Fig. 3.
Sensitivity analysis for the final model (from 10 MI datasets), complete cases model and holdout model, showing effect size of each variable on F. hepatica ELISA in UK dairy herds (n = 485, 187 and 55, respectively). Binary variables are shown by *
The sensitivity analysis (Fig. 3) shows the effect of each model variable on ELISA result, and compares the final model (made from the MI datasets), the complete cases model, and the holdout model. The effect size for the final model and the complete cases model are similar for all variables. For five out of seven variables, the effect size for the final model falls between that of the holdout model and the complete cases model, indicating that imputing the data gave a more generalisable model than using complete cases alone.
3.3. Spatial analysis
Of the risk factor variables, rainfall and slope were highly spatially clustered and boggy ground and beef were moderately clustered, with slight clustering for the remaining three variables (Supplementary information 5).
The ELISA PP values were highly clustered with high values occurring in central Scotland and Lancashire, and low values in the East Midlands and Cheshire/Shropshire/Staffordshire. The residuals from the final model (containing the risk factors Beef, Boggy, Slope, Water, Rainfall, Cows and Youngstock) were not highly clustered but some small clusters occurred in Derbyshire (the actual values were higher than the predicted values) and in the East Midlands, South Wales and Devon, where the actual values were lower than the predicted values (Supplementary information 6). The variograms showed that there was no significant spatial dependence between the model residuals at a global or local distance (Supplementary information 7).
3.4. Associations between fluke exposure and production, fertility and disease
A summary of the data collected is shown in Table 4. Mean PP values in the herds for which estimated yield and yield were available were 54.3 PP (95% CI 51.7–56.9, range 9–127) and 56.3 PP (95% CI 47.4–65.2, range 8–99) respectively.
Table 4.
Descriptive statistics for the production, fertility and disease data for 606 UK dairy herds in 2012.
| Continuous variables | Range | Mean | SD | Missing | Source |
|---|---|---|---|---|---|
| Production | |||||
| Yield (mean kg /cow/year) | 4000–10800 | 7438 | 1276 | 574 | Dairy |
| Estimated yield (mean kg /cow/year) | 1182–11686 | 6994 | 1682 | 131 | Dairy |
| Protein% | 2.86–3.80 | 3.24 | 0.13 | 12 | Dairy |
| Butterfat% | 3.43–5.08 | 4.13 | 0.23 | 12 | Dairy |
| Fertility | |||||
| Calving interval (days) | 330–570 | 411.13 | 23.15 | 264 | Questionnaire |
| Calving to conception interval (days) | 40–249 | 124.42 | 30.76 | 308 | Questionnaire |
| Services per conception | 1.00–5.00 | 2.38 | 0.63 | 317 | Questionnaire |
| Abortion rate per cow in 2012 | 0–0.18 | 0.024 | 0.02 | 342 | Questionnaire |
| Disease | |||||
| Somatic cell count (x103 cells/ml) | 55.0–297.0 | 165.50 | 41.52 | 12 | Dairy |
| Bactoscan (×103 bacteria/ml) | 8–132 | 26.97 | 11.30 | 12 | Dairy |
| Categorical variables | n | % | Missing | Questionnaire |
|---|---|---|---|---|
| Previous herd diagnosis of infectious bovine rhinotracheitis (IBR) | 59 | 17.3 | 265 | Questionnaire |
| Previous herd diagnosis of leptospirosis | 17 | 5 | 264 | Questionnaire |
| Previous herd diagnosis of bovine viral diarrhoea (BVD) | 38 | 11.1 | 265 | Questionnaire |
| Previous herd diagnosis of Salmonella dublin | 2 | 0.6 | 265 | Questionnaire |
| Previous herd diagnosis of Johne’s disease | 167 | 49 | 265 | Questionnaire |
| TB status: Herd currently under restriction due to a bovine TB case | 37 | 10.8 | 261 | Questionnaire |
Using univariable analysis, ELISA PP was able to explain more than 10% of variation only for yield and estimated yield, although statistically significant relationships (p < 0.05) were found for five additional variables (Table 5). A change in ELISA PP from the 25th to the 75th percentile corresponds to a decrease in annual yield of 1042–1192 litres.
Table 5.
Results of univariable linear regression using F. hepatica ELISA PP as an explanatory factor for variations in milk composition and fertility in UK dairy herds, showing statistically significant relationships at p < 0.05 only.
| Outcome variable (Number of herds) | Univariable models |
||||||
|---|---|---|---|---|---|---|---|
| R2 | B | SE | Sig. | 95% confidence interval for B |
Effect of change in ELISA result from 25th to 75th quantile | ||
| Lower bound | Upper bound | ||||||
| Yield (kg) (32) | 0.245 | −24.61 | 7.88 | 0.004 | −40.69 | −8.52 | −1192.95 |
| Estimated yield (kg) (475) | 0.135 | −21.49 | 2.50 | <0.001 | −26.41 | −16.58 | −1042.12 |
| Butterfat% (594) | 0.019 | 0.00 | 0.00 | 0.001 | 0.00 | 0.00 | 0.05 |
| Somatic cell count (x103 cells/ml) (594) | 0.013 | 0.16 | 0.06 | 0.006 | 0.05 | 0.28 | 7.85 |
| Bactoscan (x103 cells/ml) (594) | 0.033 | 0.07 | 0.02 | <0.001 | 0.04 | 0.10 | 3.44 |
| Calving to conception interval (298) | 0.013 | −0.12 | 0.06 | 0.047 | −0.24 | −0.00 | −5.92 |
| Services per conception (289) | 0.040 | −0.01 | 0.00 | 0.001 | −0.01 | −0.00 | −0.19 |
Using multivariable analysis, when estimated yield was included as an additional explanatory variable in the models for calving to conception interval and services per conception, the latter two variables were no longer significant. When the variables from the risk factor model for fluke exposure (Table 3), somatic cell count and services per conception were added into the Yield model, ELISA PP remained significant at p < 0.001 (Supplementary information 8).
4. Discussion
This survey of 606 dairy farms revealed that the true prevalence of exposure to F. hepatica across the three regions of GB was: 77.5% (95% CI 72.4–82.1%) in England, 88% (73.4–97.6%) in Wales and 73.4% (57.7–85.7%) in Scotland. The prevalence for England is higher than two recent studies which found 72% and 48% (McCann et al., 2010b; Salimi-Bejestani et al., 2005a). Prevalence for Wales is similar to previous findings, although these studies did not adjust for the imperfect sensitivity and specificity of the ELISA (Salimi-Bejestani et al., 2005a; McCann et al., 2010b). No previous studies have been undertaken in Scotland. The fact that 46% of farmers treat adult cattle against fluke indicates that there is a good level of awareness of the parasite amongst farmers, and it is evident that F. hepatica is a common parasite which warrants close monitoring in this group of herds.
The risk factor modelling built on previous work by McCann et al. (2010a), and found that 39% of variation in exposure to F. hepatica between the herds can be explained by a combination of farm management factors, 5-year average rainfall during May, June and July, and slope. Of these, the most influential factor is rainfall, which on its own is responsible for 23% of variation between herds, whilst farm management factors on their own explain about 21% of variation. Four of the variables in the model relate to snail survival or contact between snails and cattle: the grazing of boggy pasture, use of a stream or pond as water source, rainfall and slope. The other three (presence of beef cattle, number of cows and number of young stock) are not direct predictors of fluke exposure but are likely to indicate effects of different herd management, and further investigation of these factors might help to better explain why some farms are more affected by fluke than others.
The importance of both environmental and management factors in the control of fluke is logical as although climate and environmental conditions determine snail populations (Rapsch et al., 2008), contact between infective metacercariae and cattle depends on other factors. However only recently has the possibility of controlling fluke through grazing management been investigated with a view to creating farm-specific plans (Charlier et al., 2011).
The amount of variation explained by both management factors and climate is comparable with two recent studies comparing herd level variation in the UK and Europe (Bennema et al., 2011; McCann, personal communication). Other studies are in agreement that rainfall, slope, stocking density and temperature can have an effect on the prevalence of fluke and other snail borne diseases (Bennema et al., 2011; Charlier et al., 2011; Gonzalez-Warleta et al., 2013). However, the size and direction of the effect varies between studies, indicating the importance of localised effects on these variables. Only one previous study was able to explain most of the variation at farm level (85%), and this was achieved by visiting each farm and performing a snail survey, on 39 carefully selected farms (Charlier et al., 2011).
Although it is possible to use climate and environmental data to accurately predict F. hepatica exposure either averaged across regions (Ollerenshaw and Rowlands, 1959; McCann et al., 2010a; Bennema et al., 2011;), or when using statistically smoothed individual farm results (Claridge et al., 2012), when using raw results for individual herds the prediction becomes much less accurate (Bennema et al., 2011). This is likely to be due to a combination of localised variations in G. truncatula habitat and low spatial resolution of the climate and environmental variables.
In terms of controlling subclinical infections, these models show that management factors are equally as important as most climate and environmental factors. This is an important finding, because farmers may be able to mitigate some of the management risk factors. This was a conclusion also reached by Morgan and Wall (2009). Approaches that have been previously suggested include limiting access to pastures that are likely to be suitable as snail habitats, and grazing rotation to minimise pasture contamination in combination with flukicide treatment (Roberts and Suhardono, 1996; Knubben-Schweizer et al., 2010). However, to be certain that these measures are effective, they would need to be planned with a view to the individual farm situation, and the veterinary surgeon has an important role in advising on holistic fluke control (Parr and Gray, 2000; Knubben-Schweizer et al., 2010). Not all risk factors have been fully quantified, for example, how large an area of snail habitat is required for maintenance of F. hepatica infection within a herd, and the exact nature of snail habitats on farms.
This study showed a strong association between exposure to F. hepatica and reduced milk yield in this group of high yielding dairy herds. Several other studies have reported an association between fluke infection and reduced milk yield, both at the cow and the herd level. In these studies, the magnitude of the effect was smaller at around 8% (Charlier et al., 2007; Mezo et al., 2011; Charlier et al., 2012). The current study was an observational study and it was not possible to control for other factors affecting yield such as lactation stage, parity and nutritional status; therefore we demonstrate association rather than causation. The use of an estimated yield would have introduced a degree of error due to variation in lactation stage. However this should not have introduced bias, and the effect size was similar for both yield and estimated yield. The prevalence of fluke infection found in this study was higher than in other studies, which ranged from 31 to 67% (Mezo et al., 2008; Bennema et al., 2009; Kuerpick et al., 2012b). A higher BMT result is likely to mean higher fluke burdens in individual animals, which in turn may lead to a greater effect on health and production. This could be a reason why the magnitude of effect in this study is higher than others. At the time of the study, the milk price was £0.32 per kg, thus a reduction in annual yield of 1042 kg represents a financial loss of £333 per cow per annum, a very significant economic loss (Farmers Weekly, 2014).
Butterfat and protein content were not significantly associated with fluke exposure. Other studies vary in their findings (Black and Froyd, 1972; Lopez-Diaz et al., 1998; Khan et al., 2011; Charlier et al., 2012). Small variations that depend on lactation stage may be masked in whole herd data, and changes to butterfat and protein content may be more marked in a breed with higher butterfat percentages than the Holstein–Friesian. Effects on fertility were not significant in this group of herds when yield was controlled for, and this is in agreement with some previous studies (Simsek et al., 2007; Mezo et al., 2011) although others did find an effect (Dargie, 1987; Charlier et al., 2007). Again, these effects are likely to be small and depend on the breed and management system.
There is evidence that fluke infection can affect the susceptibility of the host to bacterial diseases including bovine tuberculosis, Bordetella bronchiseptica and Salmonella dublin (Aitken et al., 1978; Flynn et al., 2007; Jolles et al., 2008; Claridge et al., 2012), although no significant relationships were found with any other diseases in the current study. However, given the high prevalence of fluke infection and the small number of herds testing positive for any of the other diseases included, these findings are not conclusive. The relationship found between fluke infection and bactoscan or somatic cell count was not clinically relevant, which was in agreement with others (Mezo et al., 2011).
The herds in this study were not randomly selected, and as high yielding herds they may be managed differently to other UK herds. Therefore caution should be applied in generalising the findings to other herds. However, F. hepatica had a significant economic effect on this group of UK dairy farms, specifically through a substantial reduction in milk yield. Farm management factors were identified that had an effect on exposure to F. hepatica, suggesting that efforts to control fluke should focus on reducing contact between cattle and snail habitats.
Acknowledgements
This study was partly funded by Tesco in support of the Tesco Dairy Centre of Excellence, University of Liverpool, but they had no involvement in the planning, execution or writing up of this report. AH was funded by a BBSRC DTP. We would like to thank NML, Arla and Müller Wiseman for facilitating collection of milk samples and questionnaires. AH would also like to thank Peter Cripps for statistical advice. The study was conceived and designed by AH and DW. Questionnaire and statistics support was provided by GP. RS provided practical and logistical assistance. The manuscript was written by AH and commented on by all authors.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.prevetmed.2015.05.013.
Supplementary data
The following are Supplementary data to this article:
Scatterplot of observed versus predicted F. hepatica ELISA results for the holdout sample of 55 UK dairy farms.
Getis-Ord clustering of variables included in the final model for F. hepatica in UK dairy herds (n = 485). Each dot represents one farm. Red dots indicate more high value farms located near each other than would be expected by chance, whereas blue dots indicate more low value farms located nearby than would be expected by chance. Yellow dots indicate no particular pattern i.e. low and high values randomly scattered in the area. The variables Rainfall, Slope and Beef show the strongest clustering patterns. Individual farm results are not shown for confidentiality reasons.
Getis-Ord clustering ofF. hepatica ELISA results (left) and model residuals (right)in UK dairy herds (n = 485). Each dot represents one farm. Red dots indicate more high value farms located near each other than would be expected by chance, whereas blue dots indicate more low value farms located nearby than would be expected by chance. High ELISA values are clustered in regions 1 (Scotland) and 2 (Lancashire). Low values are clustered in 3 (Cheshire/Staffordshire/Shropshire) and 4 (East Midlands). Yellow dots indicate no particular pattern i.e. low and high values randomly scattered in the area. The right hand map shows that some weak clustering remains in the variation unexplained by the model. The predicted values were lower than the observed values in region 5 (Derbyshire) and higher than observed in 6, 7 and 8 (East Midlands, South Wales and Devon). Individual farm results are not shown for confidentiality reasons.
Variograms of the F. hepaticain UK dairy herds (n = 485) model residuals at different distances: globally, (left) and up to 10 km (right), showing 95% confidence envelopes. The points fall within the envelopes indicating that there is no statistically significant spatial dependence unaccounted for by the model.
References
- Aitken M.M., Jones P.W., Hall G.A., Hughes D.L., Collis K.A. Effects of experimental salmonella dublin infection in cattle given fasciola hepatica 13 weeks previously. J. Comp. Pathol. 1978;88:75–84. doi: 10.1016/0021-9975(78)90063-4. [DOI] [PubMed] [Google Scholar]
- Bennema S., Vercruysse J., Claerebout E., Schnieder T., Strube C., Ducheyne E., Hendrickx G., Charlier J. The use of bulk-tank milk elisas to assess the spatial distribution of fasciola hepatica, ostertagia ostertagi and dictyocaulus viviparus in dairy cattle in flanders (belgium) Vet. Parasitol. 2009;165:51–57. doi: 10.1016/j.vetpar.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Bennema S.C., Ducheyne E., Vercruysse J., Claerebout E., Hendrickx G., Charlier J. Relative importance of management, meteorological and environmental factors in the spatial distribution of fasciola hepatica in dairy cattle in a temperate climate zone. Int. J. Parasitol. 2011;41:225–233. doi: 10.1016/j.ijpara.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Bennema S.C., Vercruysse J., Morgan E., Stafford K., Hoglund J., Demeler J., von Samson-Himmelstjerna G., Charlier J. Epidemiology and risk factors for exposure to gastrointestinal nematodes in dairy herds in northwestern europe. Vet. Parasitol. 2010;173:247–254. doi: 10.1016/j.vetpar.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Black N.M., Froyd G. Possible influence of liver fluke infestation on milk quality. Vet. Rec. 1972;90:71–75. doi: 10.1136/vr.90.3.71. [DOI] [PubMed] [Google Scholar]
- Charlier J., Bennema S.C., Caron Y., Counotte M., Ducheyne E., Hendrickx G., Vercruysse J. Towards assessing fine-scale indicators for the spatial transmission risk of Fasciola hepatica in cattle. Geospat. Health. 2011;5:239–245. doi: 10.4081/gh.2011.176. [DOI] [PubMed] [Google Scholar]
- Charlier J., Duchateau L., Claerebout E., Williams D., Vercruysse J. Associations between anti-fasciola hepatica antibody levels in bulk-tank milk samples and production parameters in dairy herds. Prev. Vet. Med. 2007;78:57–66. doi: 10.1016/j.prevetmed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Charlier J., Hostens M., Jacobs J., Van Ranst B., Duchateau L., Vercruysse J. Integrating fasciolosis control in the dry cow management: the effect of closantel treatment on milk production. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier J., Vercruysse J., Morgan E., van Dijk J., Williams D.J.L. Recent advances in the diagnosis, impact on production and prediction of Fasciola hepatica in cattle. Parasitology. 2014;141:326–335. doi: 10.1017/S0031182013001662. [DOI] [PubMed] [Google Scholar]
- Claridge J., Diggle P., McCann C.M., Mulcahy G., Flynn R., McNair J., Strain S., Welsh M., Baylis M., Williams D.J.L. Fasciola hepatica is associated with the failure to detect bovine tuberculosis in dairy cattle. Nat. Commun. 2012;3:853. doi: 10.1038/ncomms1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R., van Dijk J., Jenkins T., Akca A., Mearns R., Williams D.J.L., A Composite faecal egg count reduction test to detect resistance to triclabendazole in fasciola hepatica. Vet. Rec. 2012;171:153. doi: 10.1136/vr.100588. 158. [DOI] [PubMed] [Google Scholar]
- Dargie J.D. The impact on production and mechanisms of pathogenesis of trematode infections in cattle and sheep. Int. J. Parasitol. 1987;17:453–463. doi: 10.1016/0020-7519(87)90121-4. [DOI] [PubMed] [Google Scholar]
- Dohoo I., Martin W., Stryhn H. 2nd ed. AVC; Canada: 2009. Veterinary Epidemiologic Research. [Google Scholar]
- Donker K. Fascioliasis in dairy cattle. Results of a specific treatment with hexachlorophene. Wien. Tierarztl. Monat. 1970;57:149–155. [PubMed] [Google Scholar]
- Farmers Weekly, 2014. Available at: http://www.fwi.co.uk/gr/markets/FWMP_Dairyco_Datum.pdf (accessed 11.08.14.).
- Flynn R.J., Mannion C., Golden O., Hacariz O., Mulcahy G. Experimental Fasciola hepatica infection alters responses to tests used for diagnosis of bovine tuberculosis. Infect. Immun. 2007;75:1373–1381. doi: 10.1128/IAI.01445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.J., White P.C.L., McClean C.J., Marion G., Evans A., Hutchings M.R. Predicting impacts of climate change on Fasciola hepatica risk. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getis A., Ord J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992;24:189–206. [Google Scholar]
- Gonzalez-Warleta M., Lladosa S., Antonio Castro-Hermida J., Maria Martinez-Ibeas A., Conesa D., Munoz F., Lopez-Quilez A., Manga-Gonzalez Y., Mezo M. Bovine paramphistomosis in galicia (spain): prevalence, intensity, aetiology and geospatial distribution of the infection. Vet. Parasitol. 2013;191:252–263. doi: 10.1016/j.vetpar.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Gordon D., Zadoks R., Skuce P., Sargison N. Confirmation of triclabendazole resistance in liver fluke in the uk. Vet. Rec. 2012;171:159–160. doi: 10.1136/vr.e5381. [DOI] [PubMed] [Google Scholar]
- Hartigan P.M. Computation of the dip statistic to test for unimodality. Appl. Stat. J. Roy. St. C. 1985;34:320–325. [Google Scholar]
- Hodgkinson J., Cwiklinski K., Beesley N.J., Paterson S., Williams D.J.L. Identification of putative markers of triclabendazole resistance by a genome-wide analysis of genetically recombinant Fasciola hepatica. Parasitology. 2013;140:1523–1533. doi: 10.1017/S0031182013000528. [DOI] [PubMed] [Google Scholar]
- Horchner F., Hennings R., Verspohl F., Averbeck W., Boch J. Medicinal control of fasciolosis in cattle in the steinfurt area: part 2 results after 3 years treatment. Berl. Münch. Tieraerztl. 1970;83:21–26. [PubMed] [Google Scholar]
- Jolles A.E., Ezenwa V.O., Etienne R.S., Turner W.C., Olff H. Interactions between macroparasites and microparasites drive infection patterns in free-ranging african buffalo. Ecology. 2008;89:2239–2250. doi: 10.1890/07-0995.1. [DOI] [PubMed] [Google Scholar]
- Kenyon F., Sargison N.D., Skuce P.J., Jackson F. Sheep helminth parasitic disease in south eastern scotland arising as a possible consequence of climate change. Vet. Parasitol. 2009;163:293–297. doi: 10.1016/j.vetpar.2009.03.027. [DOI] [PubMed] [Google Scholar]
- Khan M.K., Sajid M.S., Khan M.N., Iqbal Z., Arshad M., Hussain A. Point prevalence of bovine fascioliasis and the influence of chemotherapy on the milk yield in a lactating bovine population from the district of Toba Tek Singh, Pakistan. J. Helminthol. 2011;85:334–338. doi: 10.1017/S0022149X10000659. 2011. [DOI] [PubMed] [Google Scholar]
- Khan M.K., Sajid M.S., Khan M.N., Iqbal Z., Iqbal M.U. Bovine fasciolosis: prevalence, effects of treatment on productivity and cost benefit analysis in five districts of Punjab, Pakistan. Res. Vet. Sci. 2009;87:70–75. doi: 10.1016/j.rvsc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Knubben-Schweizer G., Ruegg S., Torgerson P.R., Rapsch C., Grimm F., Hassig M., Deplazes P., Braun U. Control of bovine fasciolosis in dairy cattle in switzerland with emphasis on pasture management. Vet. J. 2010;186:188–191. doi: 10.1016/j.tvjl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kuerpick B., Fiedor C., von Samson-Himmelstjerna G., Schnieder T., Strube C. Bulk milk-estimated seroprevalence of Fasciola hepatica in dairy herds and collecting of risk factor data in East Frisia, Northern Germany. Berl. Münch. Tierarztl. 2012;125:345–350. [PubMed] [Google Scholar]
- Kuerpick B., Schnieder T., Strube C. Seasonal pattern of Fasciola hepatica antibodies in dairy herds in Northern Germany. Parasitol. Res. 2012;111:1085–1092. doi: 10.1007/s00436-012-2935-5. [DOI] [PubMed] [Google Scholar]
- Lang Z., Reiczigel J. Confidence limits for prevalence of disease adjusted for estimated sensitivity and specificity. Prev. Vet. Med. 2014;113:13–22. doi: 10.1016/j.prevetmed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Lopez-Diaz M.C., Carro M.C., Cadorniga C., Diez-Banos P., Mezo M. Puberty and serum concentrations of ovarian steroids during prepuberal period in Friesian heifers artificially infected with Fasciola hepatica. Theriogenology. 1998;50:587–593. doi: 10.1016/s0093-691x(98)00163-0. [DOI] [PubMed] [Google Scholar]
- McCann C.M., Baylis M., Williams D.J.L. The development of linear regression models using environmental variables to explain the spatial distribution of Fasciola hepatica infection in dairy herds in England and Wales. Int. J. For. Parasitol. 2010;40:1021–1028. doi: 10.1016/j.ijpara.2010.02.009. [DOI] [PubMed] [Google Scholar]
- McCann C.M., Baylis M., Williams D.J.L. Seroprevalence and spatial distribution of fasciola hepatica-infected dairy herds in England and Wales. Vet. Rec. 2010;166:612–617. doi: 10.1136/vr.b4836. [DOI] [PubMed] [Google Scholar]
- Mezo M., Gonzalez-Warleta M., Antonio Castro-Hermida J., Muino L., Ubeira F.M. Association between anti-F. hepatica antibody levels in milk and production losses in dairy cows. Vet. Parasitol. 2011;180:237–242. doi: 10.1016/j.vetpar.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Mezo M., Gonzalez-Warleta M., Antonio Castro-Hermida J., Ubeira F.M. Evaluation of the flukicide treatment policy for dairy cattle in Galicia (NW Spain) Vet. Parasitol. 2008;157:235–243. doi: 10.1016/j.vetpar.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Mitchell G. Update on fasciolosis in cattle and sheep. In Pract. 2002;24:378. 382. [Google Scholar]
- Morgan E.R., Wall R. Climate change and parasitic disease: farmer mitigation. Trends Parasitol. 2009;25:308–313. doi: 10.1016/j.pt.2009.03.012. [DOI] [PubMed] [Google Scholar]
- NADIS (National Animal Disease Information Service), 2014. Available at: http://www.nadis.org.uk/parasite-forecast.aspx (accessed 11.08.14.).
- Natural England, 2011. Lowland water level management and drainage. Available at: http://publications.naturalengland.org.uk/publication/30026 (accessed 22.01.15.).
- Natural England, 2011. Drainage and burning management on moorlands. Available at: http://publications.naturalengland.org.uk/publication/30026 (accessed 22.01.15.).
- Nebel R.L., McGilliard M.L. Interactions of high milk yield and reproductive performance in dairy cows. J. Dairy Sci. 1993;76:3257–3268. doi: 10.3168/jds.S0022-0302(93)77662-6. [DOI] [PubMed] [Google Scholar]
- Ollerenshaw C.B., Rowlands W.T. A method of forecasting the incidence of fascioliasis in Angelsey. Vet. Rec. 1959;71:591–598. [Google Scholar]
- Parr S.L., Gray J.S. A strategic dosing scheme for the control of fasciolosis in cattle and sheep in ireland. Vet. Parasitol. 2000;88:187–197. doi: 10.1016/s0304-4017(99)00210-1. [DOI] [PubMed] [Google Scholar]
- Pritchard G.C., Forbes A.B., Williams D.J.L., Salimi-Bejestani M.R., Daniel R.G. Emergence of fasciolosis in cattle in east Anglia. Vet. Rec. 2005;157:578–582. doi: 10.1136/vr.157.19.578. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2011. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ ISBN 3-900051-07-0, URL. [Google Scholar]
- Randell W.F., Bradley R.E. Effects of hexachlorethane on the milk yields of dairy-cows in north florida infected with Fasciola hepatica. Am. J. Vet. Res. 1980;41:262–263. [PubMed] [Google Scholar]
- Rapsch C., Dahinden T., Heinzmann D., Torgerson P.R., Braun U., Deplazes P., Hurni L., Baer H., Knubben-Schweizer G. An interactive map to assess the potential spread of Lymnaea truncatula and the free-living stages of Fasciola hepatica in switzerland. Vet. Parasitol. 2008;154:242–249. doi: 10.1016/j.vetpar.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Ribeiro, P.J., Jr, Diggle, P.J., 2001. geoR: A Package for Geostatistical Analysis. R-NEWS, Vol. 1, No 2, 15-18. ISSN 1609-3631.
- Roberts, Suhardono J.A. Approaches to the control of fasciolosis in ruminants. Int. J. Parasitol. 1996;26:971–981. doi: 10.1016/s0020-7519(96)80074-9. [DOI] [PubMed] [Google Scholar]
- Rogan, Gladen Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 1978;107:71–76. doi: 10.1093/oxfordjournals.aje.a112510. [DOI] [PubMed] [Google Scholar]
- Ross J.G. Economics of Fasciola hepatica infections in cattle. Brit. Vet. J. 1970;126:8–15. [PubMed] [Google Scholar]
- Salimi-Bejestani M.R., Daniel R., Cripps P., Felstead S., Williams D.J.L. Evaluation of an enzyme-linked immunosorbent assay for detection of antibodies to Fasciola hepatica in milk. Vet. Parasitol. 2007;149:290–293. doi: 10.1016/j.vetpar.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Salimi-Bejestani M.R., Daniel R.G., Felstead S.M., Cripps P.J., Mahmoody H., Williams D.J.L. Prevalence of Fasciola hepatica in dairy herds in england and wales measured with an elisa applied to bulk-tank milk. Vet. Rec. 2005;156:729–731. doi: 10.1136/vr.156.23.729. [DOI] [PubMed] [Google Scholar]
- Salimi-Bejestani M.R., McGarry J.W., Felstead S., Ortiz P., Akca A., Williams D.J.L. Development of an antibody-detection elisa for fasciola hepatica and its evaluation against a commercially available test. Res. Vet. Sci. 2005;78:177–181. doi: 10.1016/j.rvsc.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Sargison N. Diagnosis of triclabendazole resistance in Fasciola hepatica. Vet. Rec. 2012;171:151–152. doi: 10.1136/vr.e5357. [DOI] [PubMed] [Google Scholar]
- Sargison N.D., Scott P.R. Diagnosis and economic consequences of triclabendazole resistance in Fasciola hepatica in a sheep flock in south-east Scotland. Vet. Rec. 2011;168:159–164. doi: 10.1136/vr.c5332. [DOI] [PubMed] [Google Scholar]
- Schweizer G., Braun U., Deplazes P., Torgerson P.R. Estimating the financial losses due to bovine fasciolosis in Switzerland. Vet. Rec. 2005;157:188–193. doi: 10.1136/vr.157.7.188. [DOI] [PubMed] [Google Scholar]
- Simsek S., Risvanli A., Utuk A.E., Yuksel M., Saat N., Koroglu E. Evaluation of relationship between repeat breeding and Fasciola hepatica and hydatid cyst infections in cows in elazig district of eastern Turkey. Res. Vet. Sci. 2007;83:102–104. doi: 10.1016/j.rvsc.2006.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplot of observed versus predicted F. hepatica ELISA results for the holdout sample of 55 UK dairy farms.
Getis-Ord clustering of variables included in the final model for F. hepatica in UK dairy herds (n = 485). Each dot represents one farm. Red dots indicate more high value farms located near each other than would be expected by chance, whereas blue dots indicate more low value farms located nearby than would be expected by chance. Yellow dots indicate no particular pattern i.e. low and high values randomly scattered in the area. The variables Rainfall, Slope and Beef show the strongest clustering patterns. Individual farm results are not shown for confidentiality reasons.
Getis-Ord clustering ofF. hepatica ELISA results (left) and model residuals (right)in UK dairy herds (n = 485). Each dot represents one farm. Red dots indicate more high value farms located near each other than would be expected by chance, whereas blue dots indicate more low value farms located nearby than would be expected by chance. High ELISA values are clustered in regions 1 (Scotland) and 2 (Lancashire). Low values are clustered in 3 (Cheshire/Staffordshire/Shropshire) and 4 (East Midlands). Yellow dots indicate no particular pattern i.e. low and high values randomly scattered in the area. The right hand map shows that some weak clustering remains in the variation unexplained by the model. The predicted values were lower than the observed values in region 5 (Derbyshire) and higher than observed in 6, 7 and 8 (East Midlands, South Wales and Devon). Individual farm results are not shown for confidentiality reasons.
Variograms of the F. hepaticain UK dairy herds (n = 485) model residuals at different distances: globally, (left) and up to 10 km (right), showing 95% confidence envelopes. The points fall within the envelopes indicating that there is no statistically significant spatial dependence unaccounted for by the model.