Abstract
Mucosa resident dendritic cells (DCs) may represent one of the first immune cells that HIV-1 encounters during sexual transmission. The virions in body fluids can be opsonized with complement factors because of HIV-mediated triggering of the complement cascade, and this appears to influence numerous aspects of the immune defense targeting the virus. One key attribute of host defense is the ability to attract immune cells to the site of infection. In this study, we investigated whether the opsonization of HIV with complement (C-HIV) or a mixture of complement and Abs (CI-HIV) affected the cytokine and chemokine responses generated by DCs, as well as their ability to attract other immune cells. We found that the expression levels of CXCL8, CXCL10, CCL3, and CCL17 were lowered after exposure to either C-HIV or CI-HIV relative to free HIV (F-HIV). DCs exposed to F-HIV induced higher cell migration, consisting mainly of NK cells, compared with opsonized virus, and the chemotaxis of NK cells was dependent on CCL3 and CXCL10. NK cell exposure to supernatants derived from HIV-exposed DCs showed that F-HIV induced phenotypic activation (e.g., increased levels of TIM3, CD69, and CD25) and effector function (e.g., production of IFNγ and killing of target cells) in NK cells, whereas C-HIV and CI-HIV did not. The impairment of NK cell recruitment by DCs exposed to complement-opsonized HIV and the lack of NK activation may contribute to the failure of innate immune responses to control HIV at the site of initial mucosa infection.
Introduction
Dendritic cells (DCs) are one of the first cell types that have the opportunity to interact with HIV at the site of infection in the genital or rectal mucosa (1). DCs play an important role in the induction of HIV-specific responses (2). However, they also have the capability to amplify infection by coordinately activating CD4+ T cells and transferring virus to them (3) and to induce regulatory–suppressor T cells that suppress HIV-specific responses (4–6).
The complement system is one of the key innate defense systems against infections (7) and is present in all body fluids implicated in HIV transmission, including semen, cervicovaginal secretions, and breast milk (8). Although the presence of complement generally protects the body from pathogens, it increases both direct HIV infection of immature DCs and DC mediated HIV trans infection of T cells (9–12). We recently found that the elevated infection of DCs induced by complement-opsonized HIV is due to complement-mediated suppression of antiviral and inflammatory responses (13).
The responses induced by HIV in DCs can influence the outcome of infection via secretion of various cytokines and chemokines into the microenvironment. Recruitment of immune cells, particularly NK cells, to the site of infection by the production of chemoattractants can restrict the spread of viruses such as HSV type 2 (HSV2) (14). Besides, NK cells can be important in antiviral host defense by killing infected cells (15) and have been shown to secrete factors such as CCL3, CCL4, and CCL5 that can restrict HIV replication in vitro (16). NK cell activity has been correlated with protection in exposed uninfected individuals (17). Furthermore, the preservation of NK functions is associated with improved disease outcome (18), indicating that these cells might have an important role in HIV pathogenesis.
In this study, we examined the ability of immature DCs to produce chemotactic factors and induce the migration of immune cells in response to free HIV (F-HIV), complement-opsonized HIV (C-HIV), and complement- and Ab-opsonized HIV (CI-HIV). We found that HIV induced the secretion of CCL3, CXCL8, and CXCL10 in DCs with F-HIV giving rise to significantly higher levels of CCL3 and CXCL10 than C-HIV and CI-HIV. The supernatants from DCs exposed to F-HIV induced the migration of immune cells, and the majority of these were NK cells. The migration of NK cells was dependent on CCL3 and CXCL10 and was severely decreased when the virus was opsonized with complement. In addition, we found a low but significantly increased level of activation markers TIM3, CD25, CD69, and HLADR when NK cells were exposed to supernatants from DCs exposed to F-HIV but not to C-HIV or CI-HIV. Moreover, the exposure to F-HIV supernatants enhanced the production of IFN-γ and the ability by NK cells to kill target cells, whereas these effector functions were not induced by C-HIV or CI-HIV.
Our results demonstrated that DC interaction with C-HIV impaired the recruitment of NK cells, as well as the NK cell activation, which may contribute to the failure of innate immune responses to control HIV at the site of initial mucosa infection.
Materials and Methods
Preparation and culturing of DCs
Monocyte-derived DCs were prepared and cultured as described previously (19). In brief, PBMCs were separated from whole blood from healthy volunteers (ethical permit EPN 173-07). DC progenitors were enriched by adhesion of PBMCs to plastic tissue culture plates. The cells were cultured in RPMI 1640 with l-glutamine supplemented with 10 mM HEPES, 20 μg/ml gentamicin (all from Fisher Scientific, Leicestershire, U.K.), 100 IU/ml recombinant human GM-CSF (Immunex, Seattle, WA), 300 U/ml recombinant human IL-4 (R&D Systems, Minneapolis, MN) and 1% human plasma for 5 d.
Virus generation and exposure assay
Infectious HIV-1BaL (lot nos. 4143, 4238, 4235), aldrithiol-2 (AT2)-inactivated HIV-1BaL (lot no. 4243) and infectious HIVMN (lot no. 4091) were produced from SUP-T 1/CCR5 cells and purified by sucrose gradient banding, essentially as described previously (20). In addition, a primary isolate (lot no. 4293) was used. This virus was derived from the limiting dilution clone HIV-1 THRO/A66-R5 CL.29, cell line number 506 (A.K.A. [CLN506]). The clone was sequenced and found to be largely identical to the original sequence, and it was confirmed that it retained its R5 tropism.
The virus was incubated with either an equal volume of RPMI 1640 (Sigma-Aldrich, Stockholm, Sweden) to generate (F-HIV) single-donor human serum supplemented with 25% veronal buffer, to generate complement-opsonized HIV (C-HIV), or single-donor human serum supplemented with 25% veronal buffer and 2 μg/ml HIV-specific IgG and 20 μg/ml γ globulins, to generate CI-HIV. DCs were resuspended in culture media and incubated with the multiplicity of infection (MOI) of 8, corresponding to 2,4μg/ml p24, of F-HIV, C-HIV, CI-HIV, or mock. After 6 to 24 h, the supernatants were harvested, and the cells were prepared for quantitative PCR (qPCR) analysis.
Cytometric bead array
Protein levels of CXCL8, CXCL10, and CCL3 in cell supernatants were assessed using a commercial Cytometric Bead Array (BD Biosciences, Stockholm, Sweden) performed on a BD FACSCanto II flow cytometer (BD Biosciences) and analyzed using FCAP Array version 3 software (BD Biosciences) according to the manufacturer’s protocols.
Immune cell migration assay
Cell-free supernatants collected from DCs 24 h after exposure to HIV or mock were placed in the bottom wells of a Transwell migration plate (Costar Corning, Lowell, MA). PBMCs were added to the upper wells, and the plates were incubated for 90 min to allow the cells to migrate. The migrated cells were collected, counted, and immunostained using the following Abs: VioBlue-CD19 (Miltenyi Biotec, Stockholm, Sweden) and FITC-CD56, PeCy5-CD3, PE-CD8, and APC-CD4 (BD, Stockholm, Sweden). The cells were run on a BD FACSCanto II flow cytometer and analyzed using FlowJo Software (TreeStar, Ashland, OR). CD3+CD4+ cells were defined as CD4+ T cells, CD3+CD8+ as CD8+ T cells, CD56+ cells as NK cells, CD19+ cells as B cells, and CD14+ cells as monocytes. To examine the factors responsible for the chemotaxis of immune cells, the assay was performed as above with the exception that neutralizing Abs targeting CCL3, CXCL8, CXCL10 or an isotype control (R&D Systems) were added to the DC supernatants before allowing the PBMCs to migrate toward them.
NK cell purification and flow cytometry
Human NK cells were purified from PBMCs using negative selection (NK Cell Isolation Kit; Miltenyi Biotec, Stockholm, Sweden). The NK cells were incubated with supernatants derived from DCs exposed to mock, F-HIV, C-HIV, or CI-HIV for 24 h and subsequently stained with FITC-CD25, PeCy5-CD69, APC-TIM3, PerCP-HLADR, and Alexa Fluor 647-CCR7 mAbs (BD, Stockholm, Sweden). Samples were acquired on a BD FACSCanto II flow cytometer and analyzed using FlowJo software.
Total RNA extraction, reverse transcription, and qPCR
Total RNA was extracted using Mini or Micro RNA purification kits from Bioline (London, U.K.). Reverse transcription was performed using the SuperScript III Reverse Transcriptase First Strand cDNA Synthesis Kit (Life Technologies, Stockholm, Sweden) according to the manufacturer’s protocols. qPCR was performed with SYBR Green master mix (Bioline) using a CFX96 Real-Time System (Bio-Rad, Solna, Sweden) according to manufacturer’s protocols. Primers targeting β-actin and GAPDH were used as housekeeping genes for reference as described by Vandesompele et al. (21). Primers targeting IL-2, IL-10, IL-15, CCL3, CCL13, CCL17, CCL18, CCL22, CXCL8, CXCL10, granzyme B, and perforin were used (Supplemental Table I). All primers were purchased from CyberGene AB (Stockholm, Sweden). Samples were run in triplicate and compared with both housekeeping and target genes on the same plate.
Killing assay
K562 cells (ATCC, Manassas, VA), a cell line negative for MHC class 1, were cultured in RPMI 1640 with l-glutamine supplemented with 10 mM HEPES, 20 μg/ml gentamicin, and 10% FBS (all from Fisher Scientific, Leicestershire, U.K.) and used as target cells for NK killing. DCs were exposed to F-HIV, C-HIV, CI-HIV, or mock for 24 h and the supernatants harvested as described above. Purified NK cells from the same donor were exposed to these supernatants for 24 h and then cocultured with K526 cells stained with CFSE (Molecular Probes, Life Technologies, Stockholm, Sweden) for 6 h at a 1:8 ratio. NK cells were treated with RPMI or 15 μg/ml PHA and 300 U/ml IL-2 as negative and positive controls, respectively. The frequency of CFSE-labeled K562 cells undergoing cell death was determined using a BD FACSCanto II flow cytometer and analyzed using FlowJo software (TreeStar).
Statistical analysis
The −ΔΔCT2 method, as described by Rieu and Powers (22), was used to normalize the variations between qPCR plates. Later, the samples were normalized to have a mock treated control of 1. All results were analyzed using GraphPad 5.0 software (GraphPad Software, La Jolla, CA) with repeated measures ANOVA followed by Bonferroni post test; p < 0.05 was considered statistically significant, n denotes the number of independent experiments performed with cells derived from a different healthy individual.
Results
Complement opsonization of HIV results in decreased production of HIV-induced chemokines by DCs
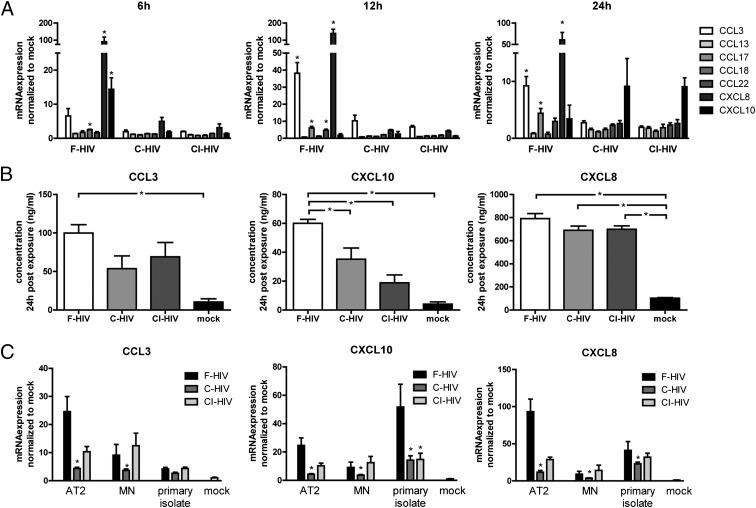
Complement opsonization of HIV has been shown by several studies, including our own, to increase virus uptake and infection of immature DCs in a CR3-dependent manner while suppressing inflammatory responses (9–12, 23). In this study, we extended these studies to examine the effects of complement opsonization on the ability of HIV to activate DC expression of chemotactic factors. The expression of an array of CC- and CXC-chemokine ligands associated with leukocyte migration—namely CCL3, CCL13, CCL17, CCL18, CCL22, CXCL8, and CXCL10—were examined by qPCR in DCs exposed to 8 MOI HIV-1BaL, either as F-HIV, C-HIV, or CI-HIV virions. CXCL10 expression peaked 6 h after exposure, while CCL3, CCL17, CCL22, and CXCL8 peaked 12 h after exposure (Fig. 1A). The levels of all these factors were significantly higher for F-HIV compared with C-HIV and CI-HIV (Fig. 1A). The HIV-induced upregulation of CXCL10, CCL3, and CXCL8 was considerable and visible for all virus groups, whereas the upregulation of CCL17 and CCL22 was less pronounced and visible only for F-HIV (Fig. 1A). Expression of CCL13 or CCL18 did not rise significantly above mock levels for any of the virus-exposed groups at any time point (Fig. 1A).
FIGURE 1.
Complement opsonization of HIV altered expression of HIV-induced chemokines in DCs. DCs were exposed to MOI 8 of HIVBaL in the form of F-HIV, C-HIV, CI-HIV, or mock treated. (A) Gene expression of CCL3, CCL13, CCL17, CCL22, CCL18, CXCL8, and CXCL10 at 6, 12, and 24 h after exposure was evaluated with qPCR. *p < 0.05, F-HIV compared with C-HIV and CI-HIV. (B) CCL3, CXCL10, and CXCL8 secretion was determined by measuring protein concentrations in DC supernatants 24 h after exposure using a cytometric bead array. *p < 0.05. (C) DCs were exposed to MOI 8 of free or opsonized AT2-inactivated HIVBaL, HIVMN, or a R5-tropic primary isolate, and the gene levels of CCL3, CXCL8, and CXCL10 at 6 h after exposure were determined using qPCR. Results were tested for statistical significance using repeated measures ANOVA followed by Bonferroni post test. (n = 3–6). *p < 0.05, compared with F-HIV.
Cytometric Bead Array analysis showed that F-HIV induced significantly higher levels of CXCL10 compared with C-HIV and CI-HIV (Fig. 1B). In addition, F-HIV induced significantly higher expression of CCL3 compared with mock treated samples, while C-HIV and CI-HIV did not (Fig. 1B). Expression of CXCL8 was significantly upregulated for all virus-exposed groups (Fig. 1B).
When lower viral titers were used, expression of CCL3, CXCL8, and CXCL10 decreased, but the profiles remained the same, with F-HIV giving rise to a higher expression than C-HIV or CI-HIV (Supplemental Fig. 1A).
The expression of CCL3, CXCL8, and CXL10 were also examined when AT2- HIVBaL, a chemically inactivated virus with the ability to bind and fuse with a target cell but not to replicate (24), the X4-virus HIVMN or a primary R5-tropic isolate were used instead of infectious HIVBaL. AT2-HIVBaL resulted in an almost identical profile as the infectious virus, indicating that infection of the DCs was not necessary to induce the cytokine expression (Fig. 1C). HIVMN also displayed a similar profile, with a lower cytokine expression after exposure to C-HIV compared with F-HIV, although the expression levels were somewhat lower and the difference was less pronounced compared with HIVBaL (Fig. 1C). The primary isolate gave rise to lower CCL3, but higher CXCL10 responses compared with HIVBaL, with F-HIV giving rise to higher expression compared with the complement-opsonized virus (Fig. 1C).
DCs exposed to F-HIV had the ability to recruit immune cells, especially NK cells, whereas DCs exposed to complement-opsonized HIV did not
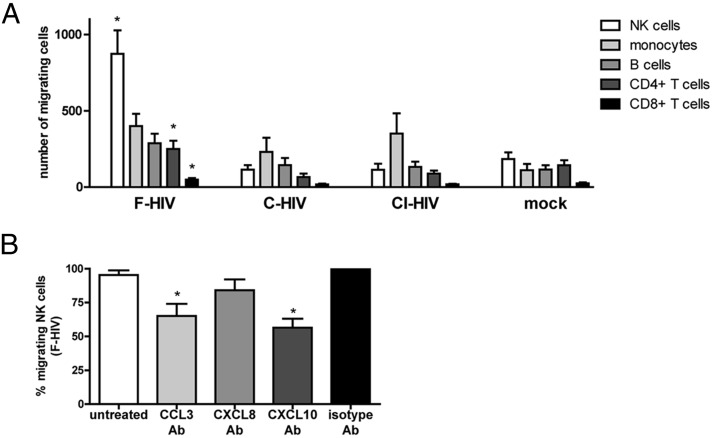
We next examined whether the differences in chemotactic ligand expression between DCs exposed to F-HIV and DCs exposed to opsonized HIV affected their ability to attract other immune cells. The ability of supernatants collected from DCs exposed to F-HIV, C-HIV, CI-HIV, or mock for 24 h to induce chemotaxis of PBMCs was examined using a transwell migration assay. The amount and type of immune cells migrating were determined by counting and staining the cells with markers for CD4+ T cells, CD8+ T cells, NK cells, monocytes, and B cells and performing flow cytometry. The ability to induce immune cell chemotaxis differed greatly between supernatants derived from immature DCs exposed to F-HIV, C-HIV, and CI-HIV (Fig. 2A). NK cells were the cell type most affected by HIV opsonization status, with a large number of cells migrating toward supernatants derived from DCs exposed to F-HIV, whereas no migration was induced by supernatants from cultures exposed to C-HIV and CI-HIV (Fig. 2A). CD4+ and CD8+ T cell migration was increased for F-HIV compared with groups treated with C-HIV and CI-HIV, although the levels of migrating CD8+ T cells were very low (Fig. 2A). No significant difference was found between free and opsonized virus in regard to the number of migrating monocytes and B cells (Fig. 2A).
FIGURE 2.
Complement opsonization of HIV decreased the migration of NK cells toward HIV-exposed DCs. PBMCs were allowed to migrate for 90 min toward supernatants collected from DCs exposed to F-HIV, C-HIV, CI-HIV, or mock for 24 h. (A) The type of cells migrating (i.e., NK cells [CD56+], monocytes [CD14+], B cells [CD19+], CD4+ T cells [CD3+CD4+], and CD8+ T cells [CD3+CD8+]) were determined with flow cytometry. (B) To determine which cytokines were responsible for the migration of NK cells toward supernatants from DCs exposed to F-HIV for 24 h, the supernatants were pretreated with neutralizing Abs targeting CCL3, CXCL8, CXCL10, or an isotype control prior to the migration assay. Results were tested for statistical significance using repeated measures ANOVA followed by Bonferroni post test. *p < 0.05 (n = 4).
To determine which chemokines were responsible for inducing migration, neutralizing Abs targeting CCL3, CXCL8, and CXCL10 or a matching isotype control were added to the supernatants prior to the migration assay. Blocking CCL3 or CXCL10 inhibited the migration of NK cells, whereas blocking CXCL8 did not (Fig. 2B, Supplemental Fig. 2). Migration of monocytes was decreased when CCL3 or CXCL8 was neutralized, whereas migration of T cells and B cells was not significantly affected by any of the neutralizing Abs (Supplemental Fig. 2).
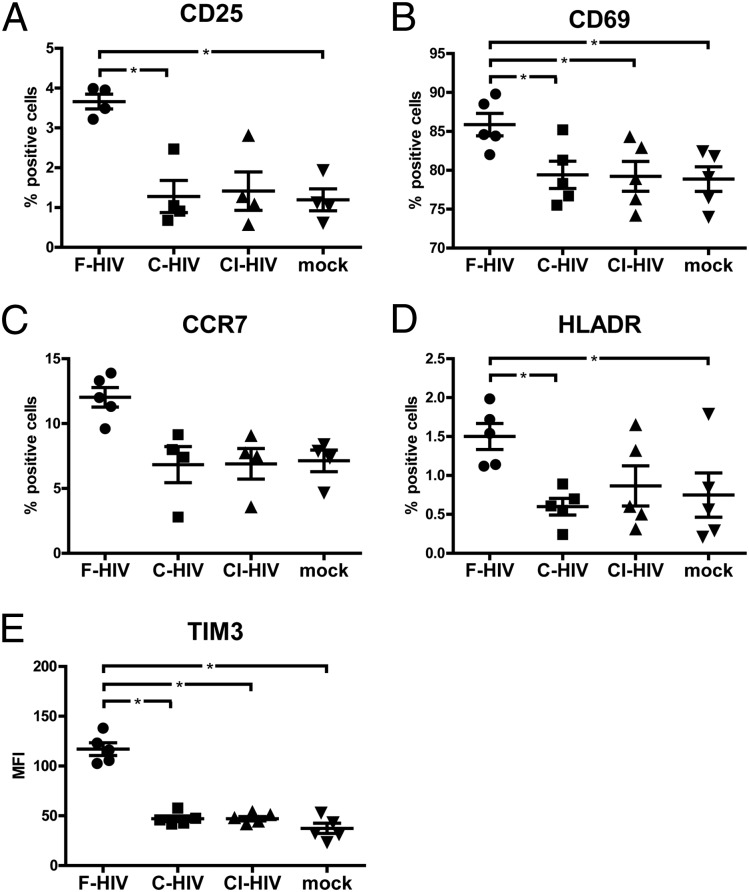
Supernatants from DCs exposed to F-HIV induced partial NK cell activation, whereas supernatants from DCs exposed to C-HIV did not
As the greatest effects on migration were seen for NK cells, we evaluated the effects of supernatants from F-HIV–, C-HIV–, and CI-HIV–exposed DCs on NK cell phenotype and activation status. The levels of NK cell activation markers CD69, CD25, TIM3, HLA DR, and CCR7 were assessed by staining the NK cells after 24 h of exposure to the DC supernatants followed by flow cytometry analysis. The expression of activation markers CD69, CD25, TIM3, and HLA DR were significantly increased in NK cells exposed to supernatants derived from DCs treated with F-HIV, whereas C-HIV and CI-HIV had minimal effects on their expression levels (Fig. 3).
FIGURE 3.
Complement opsonization of HIV decreased upregulation of activation markers on NK cells exposed to supernatants from HIV-exposed DCs. Supernatants were collected from DCs exposed to F-HIV, C-HIV, CI-HIV, or mock for 24 h. Purified NK cells were incubated in these supernatants for 24 h, after which the NK cells were stained and the expression of (A) CD25, (B) CD69, (C) CCR7, (D) HLADR, and (E) TIM3 was determined with flow cytometry. Results were tested for statistical significance using repeated measures ANOVA followed by Bonferroni post test. *p < 0.05 (n = 4).
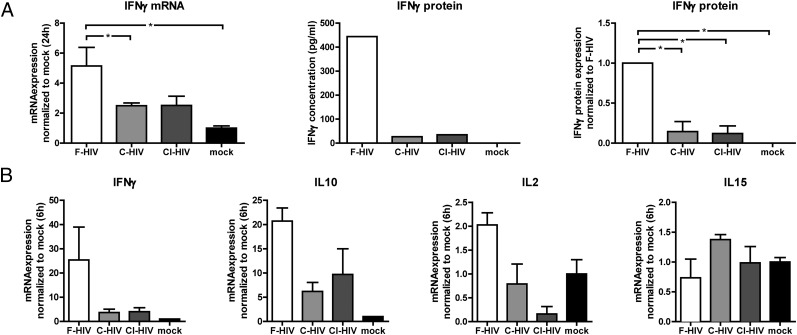
The gene expression levels of cytokines IFN-γ, IL-2, IL-15, and IL-10 in the NK cells were also examined 6 and 24 after exposure to the supernatants. We found that F-HIV significantly increased IFN-γ gene expression at both time points, whereas C-HIV and CI-HIV had no significant effects compared with supernatants from mock treated DCs (Fig. 4). This profile was also visible when the IFN-γ protein concentration in the NK cell supernatants 24 h after exposure was assessed with ELISA (Fig. 4A). Only relatively low IL-10 and IL-2, and no IL-15, gene upregulation was detected for NK cells exposed to F-HIV supernatants 6 h after exposure (Fig. 4B), and levels of all three factors were extremely low or borderline negative 24 h after exposure (data not shown).
FIGURE 4.
Complement opsonization of HIV-impaired IFN-γ expression in NK cells exposed to supernatants derived from HIV-exposed DCs. Supernatants were collected from DCs exposed to F-HIV, C-HIV, CI-HIV, or mock for 24 h. (A) Purified NK cells were incubated in these supernatants for 24 h, after which the NK cells were evaluated for IFN-γ gene expression using qPCR, and the release of IFN-γ protein was measured using ELISA. ELISA results are shown as one representative experiment and as all experiments normalized to F-HIV (n = 5). (B) NK cells were exposed to supernatants collected from HIV-exposed DCs for 6 h and gene expression of IFN-γ, IL-2, IL-10, and IL-15 were determined using qPCR (n = 3). Results were tested for statistical significance using repeated measures ANOVA followed by Bonferroni post test. *p < 0.05.
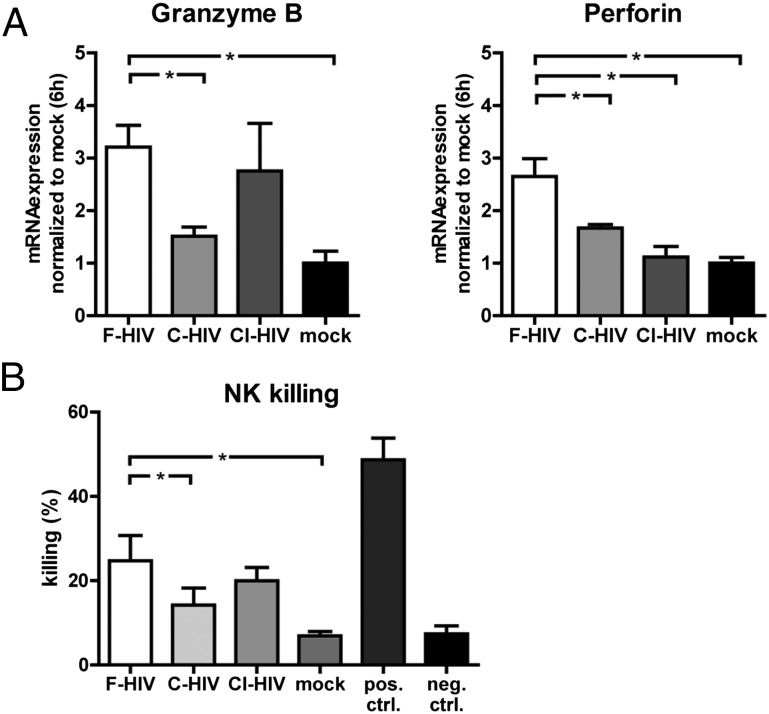
Complement opsonization of HIV resulted in decreased cytotoxicity in NK cells cultured in supernatants from HIV-exposed DCs
A few chemokines, such as CCL3, have been shown to enhance the cytolytic activity of NK cells (25, 26); therefore, we assessed the NK cell cytotoxicity after exposure to supernatants from HIV-exposed DCs. The gene expression of granzyme B and perforin and the ability to kill target cells were examined. NK cell gene expression levels of granzyme B and perforin were increased for all HIV groups, but the highest levels were seen for F-HIV (Fig. 5A). When assessing the ability to kill the cell line K562, commonly used as target cells to study NK cell killing, we found that the NK cells exposed to supernatants from DCs exposed to F-HIV increased their killing ability, whereas C-HIV and CI-HIV supernatants induced the same levels of killing that mock treatment did (Fig. 5B).
FIGURE 5.
Complement opsonization of HIV impaired the killing ability of NK cells exposed to supernatants from HIV-exposed DCs. Purified NK cells were cultured in supernatants collected from DCs exposed to F-HIV, C-HIV, CI-HIV, or mock for 24 h. (A) The NK cells were evaluated for Granzyme B and Perforin gene expression using qPCR (n = 4). (B) The NK cells were washed, and target cells (CFSE-labeled K562 cells) were added to the cultures for 6 h. The amount of killed target cells was determined using flow cytometry (n = 5). Results were tested for statistical significance using repeated measures ANOVA followed by Bonferroni post test. *p < 0.05 (n = 4).
Discussion
Complement opsonization of HIV increases viral internalization and infection of immature DCs (9–12, 23). In addition, we recently found that the inflammatory and antiviral responses induced by HIV in immature DCs are decreased when the virus is complement opsonized, resulting in enhanced infection (13). In this study, we show that the interaction between immature DCs and CI-HIV results in decreased production of chemokines and the ability to attract other immune cells, particularly NK cells. In addition, the activation and cytotoxic ability of the NK cells was reduced when they were cultured in supernatants from DCs exposed to CI-HIV compared with supernatants from DCs exposed to F-HIV. Seeing that HIV can be complement opsonized in vivo (8), this could adversely influence the body’s ability to fight the viral infection. NK cells are important because they kill virus-infected cells and enhance and modulate DC-induced immunity (15, 27), and the reduced ability of immature DCs exposed to CI-HIV to attract and activate these cells could favor viral establishment and persistence.
Inflammatory and chemotactic chemokines are normally produced during infection, and they determine the migration of immune cells to the site and exert direct antiviral properties (28, 29). The exposure of DCs to F-HIV activated the production of multiple chemotactic factors, whereas opsonization decreased these signals, affecting migration of CD4+ and CD8+ T cells, and especially NK cells. This can be explained in part by the decreased production of CCL3, CCL17, CXCL8, and CXCL10 produced by the DCs exposed to complement-opsonized HIV, cytokines that have chemotactic properties for both T cells and NK cells (25, 28, 30–32). A potential lack or suboptimal levels of these factors could be beneficial for HIV, because these chemokines can have other anti-HIV roles in addition to attracting cells with the ability to kill virus-infected cells (29, 33).
Most NK cells have the ability to migrate in response to several chemokines, such as CCL2, CCL3, CCL4, CCL5, CCL8, CXCL9, CXCL10, and CXCL11 (25, 34–36), because of the expression of their cognate receptors (e.g., CXCR1, CXCR2, CXCR3, CXCR4, CX3CR1) on these cells (34, 37). In our system, DCs exposed to F-HIV significantly upregulated gene expression of CCL3, CXCL8, and CXCL10, and to a lower degree CCL17 and CCL22, whereas C-HIV led to reduced expression of all these factors. HIV infection can be inhibited by CCL3 competition with the CCR5 tropic virus for binding to the HIV coreceptor CCR5 (38), and high genital mucosal levels of CCL3 have been linked to protection from HIV infection (39). High CCL17 levels also correlate with decreased susceptibility to HIV infection and slower disease progression (32, 40). As this cytokine is chemotactic for NK cells (31), it may help to recruit cells with the ability to kill virus-infected cells (41). CXCL10 attracts activated T cells and NK cells and stimulates monocytes (32). The impaired ability of DCs to respond to HIV exposure by upregulating CXCL10 when the virus was complement opsonized led to decreased NK cell migration, and this could aid viral establishment in the host.
All virus-exposed DCs secreted comparable amounts of CXCL8, whereas F-HIV induced a significantly higher expression of CCL3 and CXCL10 than complement-opsonized virus did. The NK cells in our system were able to migrate in response to the CCL3 and CXCL10 produced by DCs exposed to F-HIV, whereas the lower cytokine levels induced by complement-opsonized virus failed to induce a higher NK cell migration than the mock treated samples. Previous findings are inconsistent concerning the ability of CXCL8 to induce NK cell migration (25, 34). However, in our system, this cytokine did not appear to affect NK cell chemotaxis. The levels of B cell and monocyte migration were less affected by opsonization of the virions compared with NK cells and T cells. Chemokines such as CCL3 and CXCL10 have been reported to be involved in monocyte migration during HIV transmission (42, 43), and we found these factors to be involved in the monocyte migration in our system as well.
In addition to the number of cells migrating to the site of infection, NK-mediated protection is also dependent on the activation status and functionality of the recruited cells. Exposure of NK cells to factors produced by DCs at sites of inflammation and infection can lead to their activation with changes in phenotype and function (44–46) and affect NK cell–mediated destruction of infected cells (47). At the site of genital infection, NK cell migration and activity are an important defense against infections such as HSV2 (14) and NK cell cytotoxicity, and the ability to secrete antiviral cytokines has been correlated with protection in HIV-exposed noninfected individuals (17). NK cells may help to limit the initial HIV infection (41), whereas viremia leads to decreased numbers and impaired functionality of NK cells (48). Importantly, preservation of NK activity has been correlated with better disease outcome (49, 50). The activation status of the NK cells can affect interactions between NK cells and DCs and affect DC maturation with potential to modulate the adaptive immune response (51, 52), and NK cell–DC cross-talk in the periphery is crucial for the formation of optimal Th1 adaptive immunity (53).
We found that supernatants from DCs exposed to F-HIV induced a partial activation of NK cells with increased expression of TIM3, CD25, CD69, HLA DR, and CCR7, whereas supernatants from DCs exposed to complement-opsonized HIV failed to do so. In addition, the ability of the DCs to induce IFN-γ production in the NK cells was suppressed when HIV was complement opsonized.
The importance of NK cells is evident in many viral infections in which they help to clear the infection by killing infected cells and when impaired (i.e., low levels or dysfunctional NK cells), the host fails to clear the infection (41). Likewise, in vivo depletion of NK cells in mice infected with Citrobacter rodentium gave rise to a higher bacterial load and disseminated systemic infection (54, 55). NK cells control viral infections by killing virus-infected cells, an activity impacted by E:T ratio (56). The lower number of NK cells mobilized by complement-opsonized HIV should limit NK mediated–killing of infected cells at the site of infection. In addition, exposure to supernatant from DCs exposed to F-HIV enhanced the gene expression of granzyme B and perforin and the killing of target cells, whereas exposure to supernatants from DCs exposed to complement-opsonized virus did not. CCL3, CCL5, and CXCL10 are reported to be efficient at inducing the cytolytic activity of NK cells (25, 26); therefore, the NK cell exposure to CXCL10 and CCL3 produced by the DCs exposed to F-HIV should be responsible for their enhanced killing ability.
We have previously shown that C-HIV inhibits induction of inflammatory cytokines in DCs via CR3 interaction (13), and we show that this mechanism also affected the release of chemotactic chemokines and thereby the attraction of immune cells. In a recent SIV study, it was shown that a surprisingly low number of NK cells migrated to the site of infection after vaginal challenge, and the NK cells that did migrate lacked markers associated with activation and cytotoxicity (57). One explanation behind this could be the ability of HIV to exploit complement opsonization as a means to inhibit the secretion of chemokines and other factors by DCs that recruit and activate NK cells. The evasion of NK cell cytotoxicity and secretion of antiviral cytokines, such as IFN-γ, is likely beneficial for HIV and aids in the establishment of infection. This mechanism may also be important to consider when designing mucosal HIV vaccines, as the addition of an adjuvant that overrides the suppression of NK mobilization achieved by complement could prove beneficial for optimal host immune responses.
Supplementary Material
Acknowledgments
We thank Julian Bess and the Biological Products Core of the AIDS and Cancer Virus Program, Leidos Biomedical Research, Frederick National Laboratory (Frederick, MD), for providing HIV-1 virus preparations.
This work was supported by the Swedish Research Council and the Swedish Physicians against AIDS Research Foundation (AI52731); the Swedish International Development Cooperation Agency/Swedish Agency for Research Cooperation with Developing Countries-Special Assistant to the Resident Coordinator, VINNMER for Vinnova, Linköping University Hospital Research Fund, the central regional agreement on medical training and clinical research (CALF) between Östergötland County Council and Linköping University, and the Swedish Society of Medicine (to M.L.); and High Impact Research, University of Malaya (UM.C.625/1/HIR/139) (to E.M.S.).
The online version of this article contains supplemental material.
- AT2
- aldrithiol-2
- C-HIV
- complement-opsonized HIV
- CI-HIV
- complement- and Ab-opsonized HIV
- DC
- dendritic cell
- F-HIV
- free HIV
- MOI
- multiple of infection
- qPCR
- quantitative PCR.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Haase A. T. 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464: 217–223. [DOI] [PubMed] [Google Scholar]
- 2.Lubong Sabado R., Kavanagh D. G., Kaufmann D. E., Fru K., Babcock E., Rosenberg E., Walker B., Lifson J., Bhardwaj N., Larsson M. 2009. In vitro priming recapitulates in vivo HIV-1 specific T cell responses, revealing rapid loss of virus reactive CD4 T cells in acute HIV-1 infection. PLoS ONE 4: e4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson M. 2005. HIV-1 and the hijacking of dendritic cells: a tug of war. Springer Semin. Immunopathol. 26: 309–328. [DOI] [PubMed] [Google Scholar]
- 4.Shankar E. M., Che K. F., Messmer D., Lifson J. D., Larsson M. 2011. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Mol. Med. 17: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann D. E., Kavanagh D. G., Pereyra F., Zaunders J. J., Mackey E. W., Miura T., Palmer S., Brockman M., Rathod A., Piechocka-Trocha A., et al. 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 6.Day C. L., Kaufmann D. E., Kiepiela P., Brown J. A., Moodley E. S., Reddy S., Mackey E. W., Miller J. D., Leslie A. J., DePierres C., et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350–354. [DOI] [PubMed] [Google Scholar]
- 7.Cummings K. L., Waggoner S. N., Tacke R., Hahn Y. S. 2007. Role of complement in immune regulation and its exploitation by virus. Viral Immunol. 20: 505–524. [DOI] [PubMed] [Google Scholar]
- 8.Stoiber H., Banki Z., Wilflingseder D., Dierich M. P. 2008. Complement-HIV interactions during all steps of viral pathogenesis. Vaccine 26: 3046–3054. [DOI] [PubMed] [Google Scholar]
- 9.Bajtay Z., Speth C., Erdei A., Dierich M. P. 2004. Cutting edge: productive HIV-1 infection of dendritic cells via complement receptor type 3 (CR3, CD11b/CD18). J. Immunol. 173: 4775–4778. [DOI] [PubMed] [Google Scholar]
- 10.Bouhlal H., Chomont N., Réquena M., Nasreddine N., Saidi H., Legoff J., Kazatchkine M. D., Bélec L., Hocini H. 2007. Opsonization of HIV with complement enhances infection of dendritic cells and viral transfer to CD4 T cells in a CR3 and DC-SIGN-dependent manner. J. Immunol. 178: 1086–1095. [DOI] [PubMed] [Google Scholar]
- 11.Pruenster M., Wilflingseder D., Bánki Z., Ammann C. G., Muellauer B., Meyer M., Speth C., Dierich M. P., Stoiber H. 2005. C-type lectin-independent interaction of complement opsonized HIV with monocyte-derived dendritic cells. Eur. J. Immunol. 35: 2691–2698. [DOI] [PubMed] [Google Scholar]
- 12.Tjomsland V., Ellegård R., Kjölhede P., Wodlin N. B., Hinkula J., Lifson J. D., Larsson M. 2013. Blocking of integrins inhibits HIV-1 infection of human cervical mucosa immune cells with free and complement-opsonized virions. Eur. J. Immunol. 43: 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellegård R., Crisci E., Burgener A., Sjöwall C., Birse K., Westmacott G., Hinkula J., Lifson J. D., Larsson M. 2014. Complement opsonization of HIV-1 results in decreased antiviral and inflammatory responses in immature dendritic cells via CR3. J. Immunol. 193: 4590–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashkar A. A., Rosenthal K. L. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77: 10168–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fauci A. S., Mavilio D., Kottilil S. 2005. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat. Rev. Immunol. 5: 835–843. [DOI] [PubMed] [Google Scholar]
- 16.Alter G., Altfeld M. 2009. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J. Intern. Med. 265: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomescu C., Abdulhaqq S., Montaner L. J. 2011. Evidence for the innate immune response as a correlate of protection in human immunodeficiency virus (HIV)-1 highly exposed seronegative subjects (HESN). Clin. Exp. Immunol. 164: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieillard V., Fausther-Bovendo H., Samri A., Debré P., French Asymptomatiques à Long Terme (ALT) ANRS-CO15 Study Group 2010. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J. Acquir. Immune Defic. Syndr. 53: 564–573. [DOI] [PubMed] [Google Scholar]
- 19.Sabado R. L., Babcock E., Kavanagh D. G., Tjomsland V., Walker B. D., Lifson J. D., Bhardwaj N., Larsson M. 2007. Pathways utilized by dendritic cells for binding, uptake, processing and presentation of antigens derived from HIV-1. Eur. J. Immunol. 37: 1752–1763. [DOI] [PubMed] [Google Scholar]
- 20.Arthur L. O., Bess J. W., Jr., Chertova E. N., Rossio J. L., Esser M. T., Benveniste R. E., Henderson L. E., Lifson J. D. 1998. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res. Hum. Retroviruses 14(Suppl 3): S311–S319. [PubMed] [Google Scholar]
- 21.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieu I., Powers S. J. 2009. Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell 21: 1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tjomsland V., Ellegård R., Che K., Hinkula J., Lifson J. D., Larsson M. 2011. Complement opsonization of HIV-1 enhances the uptake by dendritic cells and involves the endocytic lectin and integrin receptor families. PLoS ONE 6: e23542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossio J. L., Esser M. T., Suryanarayana K., Schneider D. K., Bess J. W., Jr., Vasquez G. M., Wiltrout T. A., Chertova E., Grimes M. K., Sattentau Q., et al. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72: 7992–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taub D. D., Sayers T. J., Carter C. R., Ortaldo J. R. 1995. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J. Immunol. 155: 3877–3888. [PubMed] [Google Scholar]
- 26.Taub D. D., Ortaldo J. R., Turcovski-Corrales S. M., Key M. L., Longo D. L., Murphy W. J. 1996. Beta chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J. Leukoc. Biol. 59: 81–89. [DOI] [PubMed] [Google Scholar]
- 27.Pancino G., Saez-Cirion A., Scott-Algara D., Paul P. 2010. Natural resistance to HIV infection: lessons learned from HIV‐exposed uninfected individuals. J. Infect. Dis. 202(Suppl 3): S345–S350. [DOI] [PubMed] [Google Scholar]
- 28.Imai T., Baba M., Nishimura M., Kakizaki M., Takagi S., Yoshie O. 1997. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J. Biol. Chem. 272: 15036–15042. [DOI] [PubMed] [Google Scholar]
- 29.Robinson W. E., Jr., McDougall B., Tran D., Selsted M. E. 1998. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 63: 94–100. [DOI] [PubMed] [Google Scholar]
- 30.DiVietro J. A., Smith M. J., Smith B. R., Petruzzelli L., Larson R. S., Lawrence M. B. 2001. Immobilized IL-8 triggers progressive activation of neutrophils rolling in vitro on P-selectin and intercellular adhesion molecule-1. J. Immunol. 167: 4017–4025. [DOI] [PubMed] [Google Scholar]
- 31.Inngjerdingen M., Damaj B., Maghazachi A. A. 2000. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J. Immunol. 164: 4048–4054. [DOI] [PubMed] [Google Scholar]
- 32.Megjugorac N. J., Young H. A., Amrute S. B., Olshalsky S. L., Fitzgerald-Bocarsly P. 2004. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 75: 504–514. [DOI] [PubMed] [Google Scholar]
- 33.Mackewicz C. E., Yuan J., Tran P., Diaz L., Mack E., Selsted M. E., Levy J. A. 2003. alpha-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS 17: F23–F32. [DOI] [PubMed] [Google Scholar]
- 34.Campbell J. J., Qin S., Unutmaz D., Soler D., Murphy K. E., Hodge M. R., Wu L., Butcher E. C. 2001. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J. Immunol. 166: 6477–6482. [DOI] [PubMed] [Google Scholar]
- 35.Inngjerdingen M., Damaj B., Maghazachi A. A. 2001. Expression and regulation of chemokine receptors in human natural killer cells. Blood 97: 367–375. [DOI] [PubMed] [Google Scholar]
- 36.Allavena P., Bianchi G., Zhou D., van Damme J., Jílek P., Sozzani S., Mantovani A. 1994. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur. J. Immunol. 24: 3233–3236. [DOI] [PubMed] [Google Scholar]
- 37.Morohashi H., Miyawaki T., Nomura H., Kuno K., Murakami S., Matsushima K., Mukaida N. 1995. Expression of both types of human interleukin-8 receptors on mature neutrophils, monocytes, and natural killer cells. J. Leukoc. Biol. 57: 180–187. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Perez J., Rueda P., Staropoli I., Kellenberger E., Alcami J., Arenzana-Seisdedos F., Lagane B. 2011. New insights into the mechanisms whereby low molecular weight CCR5 ligands inhibit HIV-1 infection. J. Biol. Chem. 286: 4978–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lajoie J., Poudrier J., Massinga Loembe M., Guédou F., Leblond F., Labbé A. C., Alary M., Roger M. 2010. Chemokine expression patterns in the systemic and genital tract compartments are associated with HIV-1 infection in women from Benin. J. Clin. Immunol. 30: 90–98. [DOI] [PubMed] [Google Scholar]
- 40.Ullum H., Cozzi Lepri A., Victor J., Aladdin H., Phillips A. N., Gerstoft J., Skinhøj P., Pedersen B. K. 1998. Production of beta-chemokines in human immunodeficiency virus (HIV) infection: evidence that high levels of macrophage inflammatory protein-1beta are associated with a decreased risk of HIV disease progression. J. Infect. Dis. 177: 331–336. [DOI] [PubMed] [Google Scholar]
- 41.Altfeld M., Fadda L., Frleta D., Bhardwaj N. 2011. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat. Rev. Immunol. 11: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gouwy M., Struyf S., Berghmans N., Vanormelingen C., Schols D., Van Damme J. 2011. CXCR4 and CCR5 ligands cooperate in monocyte and lymphocyte migration and in inhibition of dual-tropic (R5/X4) HIV-1 infection. Eur. J. Immunol. 41: 963–973. [DOI] [PubMed] [Google Scholar]
- 43.Shin N., Solomon K., Zhou N., Wang K. H., Garlapati V., Thomas B., Li Y., Covington M., Baribaud F., Erickson-Viitanen S., et al. 2011. Identification and characterization of INCB9471, an allosteric noncompetitive small-molecule antagonist of C-C chemokine receptor 5 with potent inhibitory activity against monocyte migration and HIV-1 infection. J. Pharmacol. Exp. Ther. 338: 228–239. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri G. 1989. Biology of natural killer cells. Adv. Immunol. 47: 187–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitale M., Della Chiesa M., Carlomagno S., Romagnani C., Thiel A., Moretta L., Moretta A. 2004. The small subset of CD56brightCD16- natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur. J. Immunol. 34: 1715–1722. [DOI] [PubMed] [Google Scholar]
- 46.Gerosa F., Baldani-Guerra B., Nisii C., Marchesini V., Carra G., Trinchieri G. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melki M. T., Saïdi H., Dufour A., Olivo-Marin J. C., Gougeon M. L. 2010. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk—a pivotal role of HMGB1. PLoS Pathog. 6: e1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mavilio D., Lombardo G., Benjamin J., Kim D., Follman D., Marcenaro E., O’Shea M. A., Kinter A., Kovacs C., Moretta A., Fauci A. S. 2005. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. USA 102: 2886–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forthal D. N., Landucci G., Haubrich R., Keenan B., Kuppermann B. D., Tilles J. G., Kaplan J. 1999. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J. Infect. Dis. 180: 1338–1341. [DOI] [PubMed] [Google Scholar]
- 50.Ahmad R., Sindhu S. T., Toma E., Morisset R., Vincelette J., Menezes J., Ahmad A. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21: 227–233. [DOI] [PubMed] [Google Scholar]
- 51.Degli-Esposti M. A., Smyth M. J. 2005. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat. Rev. Immunol. 5: 112–124. [DOI] [PubMed] [Google Scholar]
- 52.Ferlazzo G., Tsang M. L., Moretta L., Melioli G., Steinman R. M., Münz C. 2002. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mailliard R. B., Son Y. I., Redlinger R., Coates P. T., Giermasz A., Morel P. A., Storkus W. J., Kalinski P. 2003. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 171: 2366–2373. [DOI] [PubMed] [Google Scholar]
- 54.Hall L. J., Murphy C. T., Quinlan A., Hurley G., Shanahan F., Nally K., Melgar S. 2013. Natural killer cells protect mice from DSS-induced colitis by regulating neutrophil function via the NKG2A receptor. Mucosal Immunol. 6: 1016–1026. [DOI] [PubMed] [Google Scholar]
- 55.Hall L. J., Murphy C. T., Hurley G., Quinlan A., Shanahan F., Nally K., Melgar S. 2013. Natural killer cells protect against mucosal and systemic infection with the enteric pathogen Citrobacter rodentium. Infect. Immun. 81: 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piccioli D., Sbrana S., Melandri E., Valiante N. M. 2002. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 195: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shang L., Smith A. J., Duan L., Perkey K. E., Qu L., Wietgrefe S., Zupancic M., Southern P. J., Masek-Hammerman K., Reeves R. K., et al. 2014. NK cell responses to simian immunodeficiency virus vaginal exposure in naive and vaccinated rhesus macaques. J. Immunol. 193: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.