Abstract
Cardiovascular autonomic imbalance, a cardinal phenotype of human heart failure, has adverse implications for symptoms during wakefulness and sleep; for cardiac, renal, and immune function; for exercise capacity; and for lifespan and mode of death. The objectives of this Clinical Review are to summarize current knowledge concerning mechanisms for disturbed parasympathetic and sympathetic circulatory control in heart failure with reduced ejection fraction and its clinical and prognostic implications; to demonstrate the patient-specific nature of abnormalities underlying this common phenotype; and to illustrate how such variation provides opportunities to improve or restore normal sympathetic/parasympathetic balance through personalized drug or device therapy.
Keywords: Baroreceptor reflex, Chemoreceptor reflex, Exercise, Heart failure, Human, Parasympathetic nervous system, Sleep apnoea, Sympathetic nervous system
Introduction
The balance between parasympathetic and sympathetic contributions to cardiovascular regulation shifts in response to increasing end-diastolic pressure or falling stroke volume at the very onset of left ventricular systolic dysfunction.1–3 Equilibrium may be re-established initially, but as heart failure advances, central neural networks transform and ordinarily quiescent autonomic reflexes are recruited. Irrespective of the aetiology of heart failure, the net result is vagal attenuation and, over time, progressively pathological augmentation of sympathetic outflow4 and neural and adrenal catecholamine release. Thus, heart failure should be considered a disorder of autonomic as well as myocardial function.2
The objectives of this Clinical Review are to summarize current knowledge concerning mechanisms for disturbed parasympathetic and sympathetic circulatory control in human heart failure with reduced ejection fraction (HFrEF) and its clinical and prognostic implications; to demonstrate the patient specificity of such abnormalities; and to propose how such variation provides opportunities for personalized heart failure therapy. The Journal's word and reference limits oblige us to eschew discussion of patients with heart failure due to preserved ejection fraction and also to limit the granularity of this review. Thus, we apologize, in advance, to those investigators whose important work we are able to cite only indirectly.
Evaluation of autonomic cardiovascular regulation
Parasympathetic and sympathetic activity can be estimated in conscious humans using a range of methods, as summarized in Table 1. Each has its particular advantages and limitations. In the time domain, tonic vagal heart rate modulation is appreciated as notable variation, particularly respiratory sinus variation, in cardiac cycle length. Within the frequency domain, tonic oscillations in parasympathetic drive generate the majority of high (0.15–0.5 Hz) spectral power and to a lesser extent harmonic and non-harmonic power spectral density at lower frequencies.5 Arterial baroreceptor reflex regulation of cardiac vagal tone (i.e. gain or sensitivity) can be quantified within the time domain as the extent to which spontaneous or drug-induced increases in systolic blood pressure prolong the subsequent pulse interval and in the frequency domain via transfer function analysis, with variability of blood pressure and pulse interval as the respective input and output variables.6
Table 1.
Methods to assess human autonomic cardiovascular regulation
| Vagal | Sympathetic | Ancillary data | Research utility | Clinical utility | |
|---|---|---|---|---|---|
| Heart rate (HR) at rest | Yes | Yes | + | ++ | |
| HR response to Valsalva's manoeuver | Yes | Yes | + | + | |
| HR variation: time domain | Yes | If with BP | Quantitation of spontaneous blood pressure (BP) → HR relationship to estimate arterial baroreflex regulation of sino-atrial deceleration (vagal) or acceleration (sympathetic) | ++ | +++ |
| HR variation: frequency domain | Yes | Yes | High-frequency spectral power reflects vagal HR modulation; spectral power at lower frequencies primarily sympathetic HR modulation; Concurrent BP → HR spectral power transfer function to estimate arterial baroreflex regulation of HR |
++ | + |
| HR responses to drug stimuli | Yes | Yes | Arterial baroreceptor stimulation by phenylephrine elicits immediate vagal response; unloading by vasodilators elicits reflex sympathetic response | +++ | ++ |
| HR response to mechanical stimuli | Yes | Yes | Responses to lower body negative or positive pressure primarily sympathetic; to negative or positive neck pressure primarily vagal | ++ | ? |
| BP variability | ? | ? | Time or frequency domain measures not specific to autonomic regulation; Principal utility when evaluated in conjunction with HRV spectrum |
? | ? |
| Arterial/venous norepinephrine (NE) | No | Yes | Global non-specific index | + | ++ |
| Urinary NE excretion | No | Yes | Global non-specific index | + | ++ |
| NE spillover to plasma | No | Yes | Total body or organ-specific (heart, kidney, limb muscle, brain) data | +++ | + |
| Sympathetic nerve recordings | No | Yes | Multi-unit or single-unit efferent muscle sympathetic nerve activity (MSNA); Sympathetic reflex response to baroreceptor, chemoreceptor, muscle mechano- and metabo-reflex and other reflex simulation or inhibition can be estimated in time domain; Concurrent BP → MSNA spectral power transfer function estimates arterial baroreflex efferent sympathetic regulation in frequency domain |
+++ | + |
| Sympathetic nerve imaging | No | Yes | Nuclear tracers or PET ligands to assess principally cardiac sympathetic innervation and NE uptake | ++ | ++ |
Clinicians still lack a reference biomarker of sympathetic activity in heart failure. Mean values for venous plasma norepinephrine concentrations will still anticipate the overall survival of cohorts, but their deficiencies with respect to the phenotypic characterization of individual patients have been well described and addressed only in part through the acquisition of arterial samples. Present insight into the mechanisms for and magnitude of sympathetic activation in individuals with heart failure derives from either radiotracer methodology, enabling calculation of total body or organ-specific norepinephrine release rates into plasma or uptake of norepinephrine into cardiac sympathetic nerves7 or by quantifying, using microelectrodes, multi- or single-unit discharge innervating skeletal muscle.8 Within the frequency domain, tonic oscillations in sympathetic drive have been demonstrated in healthy subjects to generate the majority of low (0.05–0.15 Hz) spectral power and some power below 0.05 Hz, and although not universally accepted, the ratio between low and high-frequency spectral power has been proposed as a crude estimate of sino-atrial sympathetic/parasympathetic balance.5 The principal limitation to the interpretation of spectral analyses in heart failure, however, is that its variability reflects the autonomic modulation of heart rate, not the magnitude of neurotransmitter input to the sino-atrial node.9 As central sympathetic outflow becomes intense and invariant, as occurs with advanced heart failure, low-frequency spectral power ebbs such that there is an inverse, rather than positive, relationship between muscle sympathetic nerve activity (MSNA) and low-frequency spectral power.10
Imbalance in heart failure: prognostic implications
The concept of autonomic reflex malfunction in human heart failure emerged from the demonstration by Eckberg et al.11 of attenuated bradycardia in response to a drug-induced rise in systolic arterial pressure. Over the last three decades, each of decreased tonic and reflex vagal heart rate modulation; increased heart rate, venous plasma norepinephrine, cardiac norepinephrine stores and cardiac norepinephrine spillover, renal norepinephrine spillover, and muscle sympathetic burst frequency; and decreased cardiac 123I-MIBG and 11C-HED uptake and low-frequency heart rate variability has been documented in HFrEF and each, reflecting abnormal autonomic control of the cardiovascular system (see Table 1) has been associated, in longitudinal cohort studies, with greater mortality risk.2,12–15
Neural reflex mechanisms
Arterial baroreceptor reflex
The blunted reflex vagal response to a rise in blood pressure in human heart failure was attributed initially to diminished carotid sinus and aortic baroreceptor afferent nerve firing in response to systolic stretch. It was assumed that as a consequence there would be, in parallel, less reflex restraint on efferent sympathetic discharge. It was inferred that the net result of such disinhibition would be an equivalent increase in sympathetic outflow to all innervated organs and vascular beds.16 However, data acquired using radiotracer methods or microneurography refuted that simplistic construct: in the majority of patients with mild to moderate heart failure, relative to control subjects, heart rate variation was attenuated17 and cardiac norepinephrine spillover was increased but renal norepinephrine spillover and muscle sympathetic nerve traffic were not.18 Group mean values for MSNA may increase progressively with mild to moderate to severe impairment of ventricular systolic function,4 but once left ventricular ejection fraction (LVEF) falls below 40%, there is no correlation within cohorts between LVEF and muscle sympathetic nerve discharge.19
Concurrently, the presumption that diminished arterial baroreceptor nerve input caused equal impairment of reflex parasympathetic and sympathetic responses was rendered untenable by the demonstrations that: (i) impaired ganglionic neurotransmission is the principal cause of diminished vagal heart rate modulation in canine heart failure;20 (ii) in an ovine heart failure model with impaired arterial baroreflex regulation of heart rate, baroreflex regulation of cardiac and renal sympathetic discharge is not attenuated,21,22 and (iii) there is no parallel alteration in the arterial baroreflex regulation of MSNA in other conditions, such as healthy ageing23 and primary hypertension,24 characterized by diminished reflex vagal heart rate modulation and increased efferent sympathetic discharge.
Nonetheless, the results of initial comparisons, in heart failure and control subjects, of changes in nerve firing rates (i.e. bursts/min) in response to drug-induced decreases and increases in blood pressure were interpreted as evidence for such impairment of arterial baroreceptor reflex regulation of muscle sympathetic outflow.4,25 However, control subjects were not always matched with heart failure patients for age,25 these differences were not observed consistently,26 and those frequency-based calculations did not consider heart rate-gated nature of sympathetic nerve firing and hence the simple arithmetical consequence of the attenuated vagally mediated chronotropic response to drug stimuli. When burst frequency was recalculated as burst incidence (i.e. bursts/100 cardiac cycles), a heart rate-independent variable, little or no difference in the MSNA response emerged between heart failure and control subjects.27
Additional evidence confirming intact arterial baroreflex control of sympathetic activity in human heart failure accrued from several experiments applying independent methods: preservation of pulse-synchronous MSNA discharge; synchronization, in end-stage heart failure, of muscle sympathetic alternans with pulsus alternans; instantaneous changes in muscle sympathetic burst amplitude and duration in response to the onset and off-set of ventricular bigeminy; and in comparisons of subjects with impaired and preserved systolic function, similar gains of the transfer function between blood pressure variability as input and MSNA variability as output, similar total body spillover responses to sodium nitroprusside-induced reductions in arterial pressure, and similar inhibition of MSNA by the Mueller manoeuver.2 From these observations, it could be concluded that the arterial baroreceptor reflex in human heart failure is responding appropriately to perceived decreases in stroke volume or diastolic blood pressure by eliciting sympathetic activation. Consequently, attention focused on establishing evidence for an upward shift, within the central nervous system, of the set point for adrenergic and vagal outflow,28 and on elucidating the contributions of other neural cardiovascular reflexes to the development of parasympathetic–sympathetic imbalance in heart failure.
Cardiopulmonary mechanosensitive reflexes
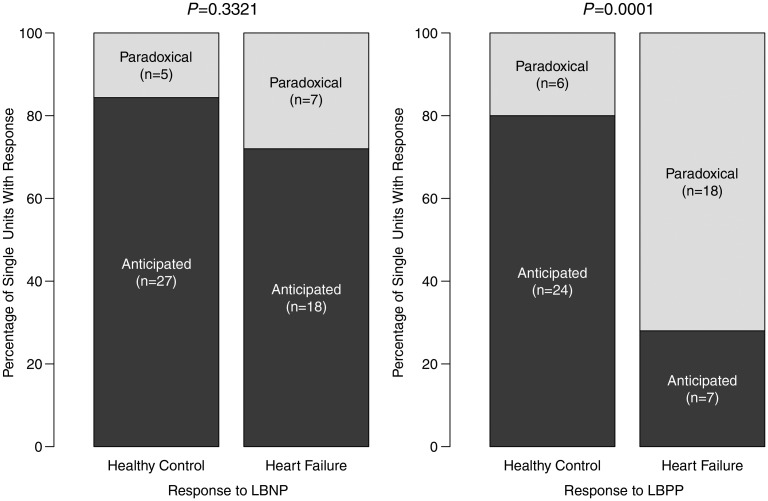
In contrast to these prior investigations concerning arterial baroreceptor reflexes, Dunlap et al.26 demonstrated significant impairment in the reflex sympatho-inhibition elicited normally by stretch of unmyelinated mechanoreceptor afferents sited within the heart and pulmonary veins. However, volume expansion in dogs has been shown to potentiate the cardiac sympathetic afferent reflex29 and in humans, a number of seemingly paradoxical clinical observations argue for the emergence in heart failure of an additional cardiac sympatho-excitatory reflex, arising potentially from stimulation of cardiac sympathetic afferents or cardiac myelinated afferents located primarily at the atrial-venous confluence. These include unanticipated inverse relationships between both left ventricular stroke volume index and left ventricular stroke work index and MSNA and positive relationships between pulmonary artery mean and pulmonary diastolic pressures and both MSNA and cardiac norepinephrine spillover and a paradoxical reduction in cardiac (but not total body) norepinephrine spillover when atrial pressures were reduced selectively without altering intra-arterial pressure.2 Recent evidence for paradoxical muscle sympathetic reflex activation by an increase in atrial pressure was established through the comparison, in middle-aged subjects with and without heart failure, of single-unit discharge in response to an increase in preload induced by non-hypertensive lower body positive pressure. Whereas discharge from the majority of single units diminished in response to this stimulus in control subjects, in those with heart failure the majority of single units exhibited increased firing rates (Figure 1).8
Figure 1.
Number and percentage of total identified efferent muscle sympathetic single units in healthy controls and heart failure that increase firing rate reflexively in response to non-hypotensive lower body negative pressure (LBNP; −10 mmHg) and decrease firing rate reflexively in response to non-hypertensive lower body positive pressure (LBPP; +10 mmHg): ‘anticipated’, or that decrease firing rate reflexively in response to non-hypotensive lower body negative pressure (LBNP; −10 mmHg) and increase firing rate reflexively in response to non-hypertensive lower body positive pressure (LBPP; +10 mmHg): ‘paradoxical’. P-values for proportion of anticipated : paradoxical responses observed in heart failure patients compared with healthy controls. Reproduced from Millar et al.8
Cardiac chemosensitive reflexes
Acute induction of both infero-posterior and anterior myocardial ischaemia also elicits reflex sympatho-excitation by stimulating cardiac sympathetic afferents.30
Zucker and his colleagues have established substantive evidence in experimental preparations for the contribution of an enhanced cardiac sympathetic afferent reflex to renal and cardiac sympatho-excitation in heart failure.28,31 In their hands, chemical ablation of cardiac sympathetic afferents resulted in sympatho-inhibiton and attenuation of adverse ventricular remodelling after myocardial infarction.32
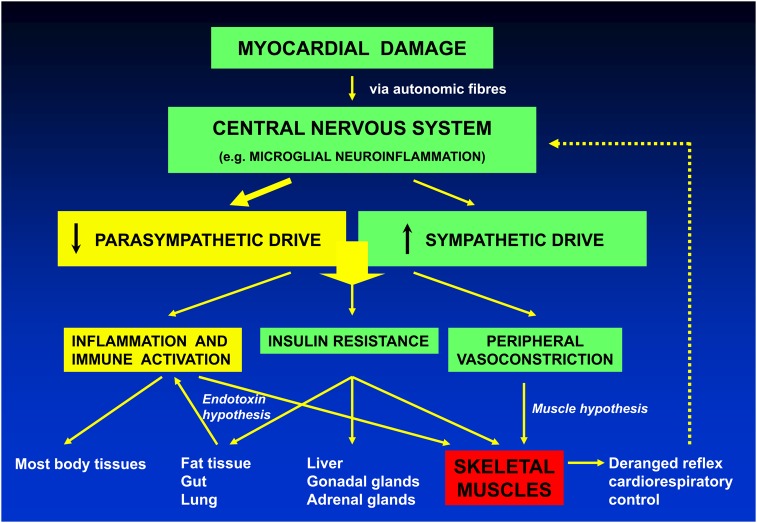
These intriguing studies in the experimental28,31,32 and clinical setting of heart failure8 confirm the presence of overactive cardiac afferent reflexes that operate as excitatory inputs to the central autonomic control system, shifting the balance towards sympathetic predominance. Interestingly, they are entirely in line with the hypothesis proposed by Poole-Wilson and colleagues33 that myocardial damage, whether caused by ischaemic or other pathological processes, elicited, through these cardiac afferent reflexes, functional and structural changes within the central nervous system triggering local and systemic inflammatory responses, parasympathetic depletion, and sympathetic activation. Disinhibition of immune systems by diminished vagal tone34 would, in turn, stimulate a broad range of pathophysiology, accelerate the progression of heart failure, and affect adversely survival (Figure 2).33 Autonomic dysfunction and deranged immune mechanisms in heart failure, traditionally viewed in isolation, may in fact interact closely.
Figure 2.
Hypothetical mechanisms linking altered myocardial function to central and peripheral disturbances of the immune and autonomic systems resulting in mutual facilitation of heart failure progression. Reproduced from Jankowska et al.33 with permission of the European Society of Cardiology.
Augmented cardiac reflex sympatho-excitation whether stimulated by transient impairment in ventricular systolic function as a consequence of myocardial damage with haemodynamic deterioration or by myocardial ischaemia or necrosis unrelated to any acute haemodynamic perturbation therefore should be included among pathophysiological mechanisms responsible for sympathetic over-activation in chronic heart failure syndromes. Augmented cardiac sympathetic excitatory reflexes also may contribute significantly to the development of acute heart failure. Transient increases in filling pressure ordinarily would redistribute fluid to capacitance vessels by inhibiting sympathetic outflow reflexively. However, in the setting of heart failure, ‘paradoxical’ sympathetic activation could shift blood volume from the venous (principally splanchnic) reservoir into the cardiopulmonary pool, causing congestion and eliciting dyspnoea. Thus, reflex-mediated fluid shifts rather than progressive renal sodium retention may underlie episodes of rapid clinical decompensation.35
Pulmonary stretch receptor reflex
With increased breathing rate and smaller tidal volumes, there is less stimulation of the sympatho-inhibitory reflex elicited by lung stretch. As a consequence heart failure patients with such breathing patterns exhibit greater muscle sympathetic burst frequency or amplitude.36
Reflexes arising from skeletal muscles
The concept placing skeletal muscle pathology as central to the pathophysiology of chronic heart failure and symptoms of exercise intolerance was proposed by Coats et al.37 as ‘the muscle hypothesis'. These investigators proposed that early metabolic distress augments afferent muscle ergo-receptor discharge. Sensitive to the metabolic products of muscle exercise, ergo-receptors modulate the haemodynamic, ventilatory, and autonomic responses during exercise to optimize muscle work.38 Amplification of this reflex in chronic HFrEF results in augmented efferent ventilatory and sympathoneural responses to exercise38 accompanied usually by evidence for increased arterial chemoreflex sensitivity and diminished arterial baroreflex regulation of heart rate all independent of left ventricular ejection fraction.39
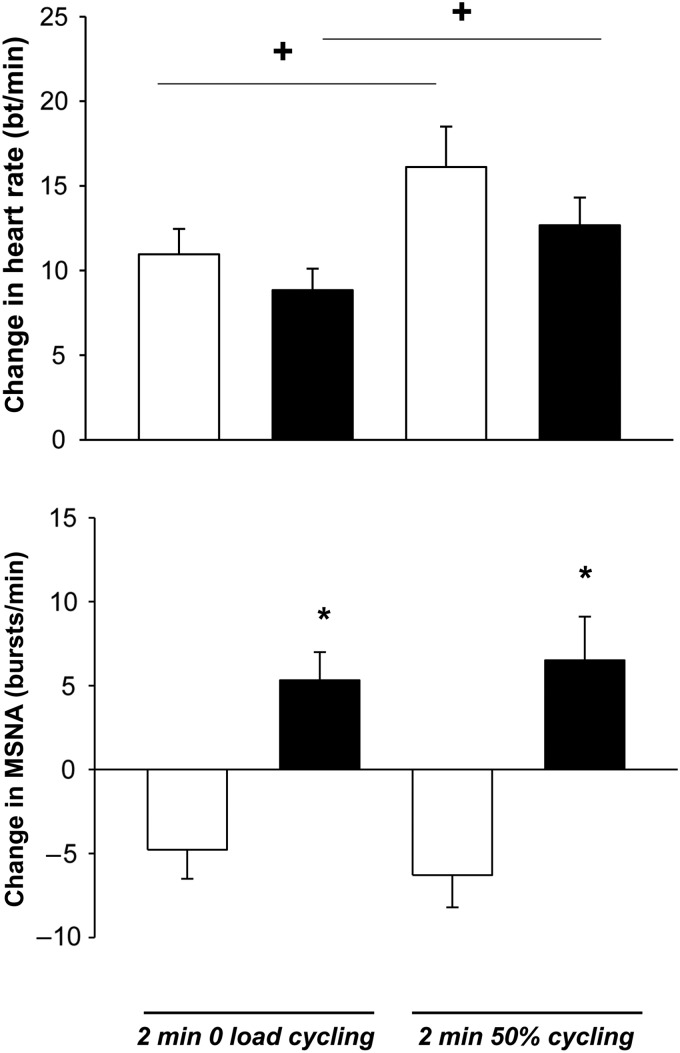
Most literature concerning sympathetic activity in heart failure reports data acquired at rest. Such information provides only limited insight into between-patient variation in the magnitude of sympathetic activity generated in the course of daily activity. For example, when a cohort whose mean left ventricular ejection fraction was 18% was divided on the basis of peak exercise performance into those with >56% (mean LVEF 17%) and those with <56% of that predicted on the basis of age, weight, and sex (mean LVEF 20%), in the former subgroup MSNA at rest was similar to that of matched control subjects, whereas in the latter subgroup resting MSNA was ∼50% greater. However, handgrip exercise increased MSNA significantly, relative to age-matched control subjects, in both subgroups. When post-handgrip ischaemia was applied to activate muscle the ergo- or metabo-reflex, MSNA remained elevated significantly in the subgroup with diminished exercise capacity.19 It is now possible to record MSNA from one leg while the contra-lateral leg propels a cycling ergometer. Applying this protocol, Notarius et al.40 detected both a significant inverse relationship between exercise-induced MSNA and VO2peak in subjects with and without heart failure, and a qualitatively different between-group response to this stimulus: a reduction in MSNA burst frequency during exercise in middle-aged healthy controls, but sympathetic excitation in those with heart failure (Figure 3).
Figure 3.
Similar heart rate (top panel) but divergent muscle sympathetic nerve activity burst frequency (lower panel) responses to graded one-legged dynamic exercise in subjects with and without heart failure. Heart rate increased significantly in response to increasing exercise intensity during the second minute of one-legged cycling in both healthy control (white bars) and heart failure (black bars) subjects (+, P < 0.003 for 50% vs. zero load cycling) with no between-group difference (P = 0.10). Muscle sympathetic nerve activity burst frequency (bursts/min) decreased significantly from baseline during both exercise intensities in healthy control subjects but decreased significantly during both exercise intensities in heart failure patients (*, P < 0.001 HFrEF vs. control). This divergence became more pronounced as exercise intensity increased (P = 0.01) with no between-group interaction. There was no significant difference in muscle sympathetic nerve activity between exercise intensities within groups (P = 0.78). Adapted from Notarius et al.40
Peripheral and central chemoreceptor reflexes
In humans, stimulation of chemoreceptors (peripheral, responding primarily to hypoxia, and central, responding primarily to hypercapnia) elicits reflex increases in ventilation and sympathetic outflow. Compared with healthy subjects, patients with heart failure and impaired ejection fraction demonstrate augmented peripheral and central chemosensitivity, which results in a disproportionate increase in MSNA responses elicited. At rest, a substantial minority of heart failure patients exhibit increased peripheral (hypoxic) or central (hypercapneic) chemoreflex-mediated increases in MSNA.41–43 This observation has been recently confirmed in a contemporary, optimally treated heart failure population, in whom nearly half exhibited increased peripheral chemosensitivity and augmented increases in systolic blood pressure and heart rate in response to transient hypoxia.44 In HFrEF patients, augmented tonic excitatory input from peripheral chemoreceptors can suppress arterial baroreflex inhibition of sympathetic outflow45 in addition to stimulating low-frequency periodic oscillations in breathing, an increased ventilatory response to exercise, and ventricular arrhythmias.41 Those who manifest peripheral and central chemoreflex-mediated sympatho-excitation are reported to suffer a 50% 4-year mortality rate.46 As with reflexes arising from skeletal muscle, a propensity to increased peripheral chemosensitivity may be unmasked by exercise47 and attenuated by the adenosine receptor antagonist, caffeine.48 In rat and rabbit models of HFrEF, carotid body denervation resulted in significant reductions in indices of sympathetic outflow,49 and in a heart failure patient, autonomic balance was restored after surgical removal of one carotid body.50
Renal afferent nerves
The development of percutaneous methods for renal denervation in humans has generated considerable interest in the sensory neural properties of the kidney.51 In experimental preparations, myelinated renal afferent nerves have been shown to increase discharge in response to haemodynamic stimuli, such as increased intra-renal pressure and systemic hypotension, and chemical stimuli commonly present in heart failure, such as renal ischaemia and increased bradykinin, adenosine and urea. The result is a reflex sympatho-excitatory response.52 Whether these renal afferents play a similar role in human heart failure has yet to be determined. However, in one heart failure cohort, renal insufficiency defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2, predicted independently patients' MSNA.53
Central neural mechanisms
Application of radiotracer methodology to studies of jugular venous catecholamine content has documented in human heart failure significant increases in spillover of epinephrine and in norepinephrine and serotonin metabolites, and a significant relationship between these indices of brain catecholamine turnover and cardiac norepinephrine spillover.54 Selective sampling of venous effluent from cortical and subcortical brain regions of treated heart failure and control subjects identified a four-fold increase in mean values for supra-bulbar subcortical norepinephrine turnover in patients, in whom there was as well a significant relationship between turnover and total body norepinephrine spillover.55
The parasympathetic/sympathetic imbalance in heart failure is more than a simple consequence of alterations in the absolute and relative magnitude of these several neural inputs to brainstem centres involved in the generation of autonomic outflow or of communication within the network of cortical regions participating in their modulation. These reflexes themselves interact and integrate centrally via involving additive summation, mutual facilitation, or mutual inhibition.56 Augmentation in heart failure of the gain of one reflex arc, such as the peripheral chemoreceptor reflex, often inhibits the responsiveness of another, such as the arterial baroreceptor reflex.45
There is now extensive experimental evidence that early in the course of heart failure neurohumoral and inflammatory disturbances within the central nervous system initiate sympatho-excitation and dampen parasympathetic outflow. These changes in turn initiate a positive feedback loop stimulating further adverse immune responses.28,33,57 Up-regulation of the brain and plasma renin-angiotensin-aldosterone pathways plays a key role in this shift.28 Angiotensin II interacts with the arterial baroreflex at several sites within and outside the blood–brain barrier to inhibit vagal discharge and increase central sympathetic outflow. Adrenally produced aldosterone has been shown to increase angiotensin AT1 receptor mRNA, protein, and NAD(P)H subunit gene expression in the paraventricular nucleus and at the same time plasma norepinephrine concentrations.58 In animal heart failure models, the cardiac sympathetic afferent reflex, which is potentiated by chronic central infusion of angiotensin II, is augmented centrally as a consequence of increases in brain angiotensin II, decreased neuronal nitric oxide synthesis, generation of reactive oxygen species, and activation of rho kinase.59 The efferent sympathetic response elicited can be normalized by central infusion of antisense oligodeoxynucleotides to AT1 receptor mRNA.60 Sympatho-excitation in a rabbit model of heart failure is characterized by increased rostral ventrolateral medullary angiotensin AT1 receptor and NAD(P)H oxidase subunit gene expression with consequent up-regulation of NADPH-dependent superoxide anion production, resulting in a state of intense oxidative stress.61 Sympatho-excitatory responses to intracerebroventrical infusion of angiotensin II were abolished by central infusion of the angiotensin AT1 receptor antagonist, losartan; by the superoxide dismutase mimetic, tempol; and by the inhibitor of NAD(P)H, apocyanin.61
In rat models, intracerebroventricular infusion of either Fab fragments, inhibiting endogenous ouabain,62 or the mineralocorticoid antagonist, spironolactone,63 reduced efferent renal sympathetic discharge and enhanced its arterial baroreflex regulation. In ovine heart failure, central infusion of the angiotensin AT1 receptor antagonist, losartan, reduced cardiac but not renal sympathetic nerve activity.64 Collectively, these findings indicate that heart failure begets a positive feedback loop, with angiotensin II at its core, which propels relentless progression of these immune, inflammatory, and autonomic disturbances and foreshortens survival.
Synthesis
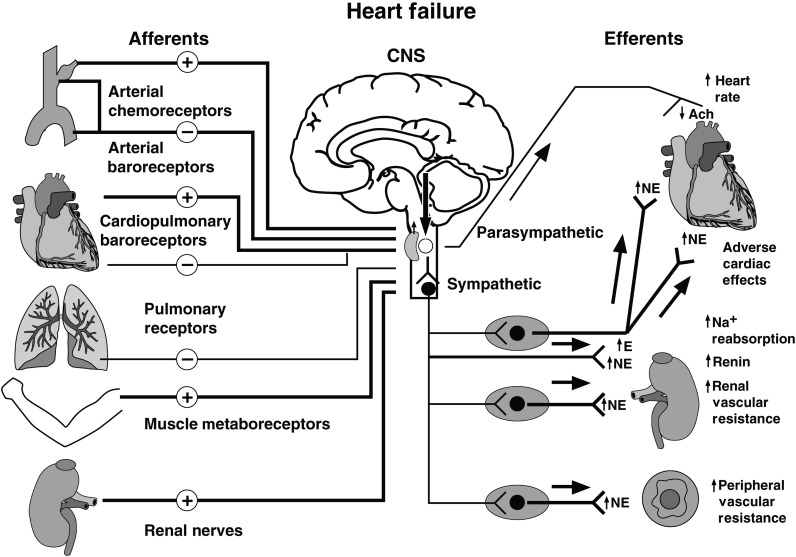
Autonomic imbalance characterized by sympathetic predominance is a classical feature of HFrEF with clinically relevant consequences. These include disease progression, development or deterioration of exercise intolerance, ventricular remodelling and arrhthymias, and premature death. The mechanisms underlying these processes and their relative time courses have yet to be elucidated fully. However, it is now evident, from a diverse range of animal experiments, that the set points for sympathetic and vagal efferent discharge are altered within the central nervous system by several mechanisms including inflammation, perturbations in the brain renin-angiotensin-aldosterone system and endogenous ouabain, generation of reactive oxygen species, and chemoreceptor sensitization. Our present understanding of the peripheral neural reflex interactions that conspire to alter parasympathetic/sympathetic equilibrium in human heart failure also has evolved considerably over the last decade. It is now appreciated that there is an upward shift in the central set point for sympathetic outflow; that altered ganglionic neurotransmission impairs vagal heart rate modulation; that conventional inhibitory cardiopulmonary reflexes are less potent; that arterial and pulmonary stretch reflex gains are not compromised, but that the stimulus to these mechanoreceptors is attenuated; that sympatho-excitatory muscle mechanoreflexes are augmented; that a paradoxical sympatho-excitatory low-pressure reflex surfaces; and that in a substantial number of patients the chemoreceptor reflex, the muscle metabo-reflex, and renal afferent sympatho-excitatory reflex all are augmented.2 These findings have resulted in a more nuanced and complex model of mechanisms responsible for neural cardiovascular disequilibrium in human heart failure (Figure 4).
Figure 4.
Mechanisms involved in the autonomic disturbances of HFrEF. Input from arterial and cardiac mechano- and chemoreceptor afferents, arterial chemoreceptor, pulmonary stretch receptor, muscle metabo- and mechanoreceptor, and renal afferent nerves converse to modulate sympathetic outflow about a centrally mediated set point increase, involving an angiotensin II-AT1 receptor-NADPH-superoxide pathway. As systolic dysfunction progresses, input effecting sympatho-inhibition (−) by stimulating ventricular and a population of atrial mechanoreceptor nerve afferents decreases (thin line), whereas inhibitory modulation of efferent sympathetic nerve traffic by arterial baroreceptors (thick line) is preserved. Efferent vagal heart rate responses to arterial baroreflex perturbations are attenuated (thin line). Excitatory (+) afferent input arises from: a normally quiescent atrial reflex, activated by increases in cardiac filling pressures; chemically sensitive ventricular afferent nerve endings, triggered by ischaemia; augmented sympatho-excitatory input from arterial chemoreceptors; exercising skeletal muscle in heart failure; and renal afferent nerves (thick lines). The central set point for sympathetic outflow (arrow pointing down) is raised further by central chemoreceptor sensitization, by sleep apnoeas, and possibly by obesity. Efferent mechanisms for increased NE spillover include pre-junctional facilitation of its release and impaired NE uptake. The time course through which these mechanisms are engaged differs between individuals. Relatively asymptomatic systolic dysfunction is characterized by a selective increase in cardiac NE release, and a reduction in tonic and reflex vagal heart rate modulation; as heart failure advances there is a generalized increase in sympathetic nerve traffic to the heart, adrenal, kidney, skeletal muscle, and other vascular beds (thick arrow shafts, thick lines). Ach, acetylcholine; CNS, central nervous system; E, epinephrine; Na+, sodium; NE, norepinephrine. (Reproduced with the first author's permission).
Sleep
A fundamental limitation of contemporary literature, and in particular clinical trial literature, concerning the role and prognostic significance of autonomic disturbances in heart failure is that it focuses almost exclusively upon measurements obtained with patients awake. The impact of sleep, and particularly disturbed sleep, on these inter-relationships in heart failure has only recently been appreciated. This is not a trivial consideration. Heart failure patients sleep on average 78 min less than individuals without heart failure and hence spend a greater proportion of the day in a state of wakefulness that is itself accompanied by increased sympathetic and decreased vagal tone.65 Even with contemporary heart failure therapy, including evidence-based drug and device treatment, clinically significant sleep apnoea (an apnoea–hypopnoea index >15 events/h) is evident in ∼50% of heart failure patients. Of these, half will have primarily obstructive and half primarily central sleep apnoea.66
Although these two entities differ with respect to aetiology, with the former caused by the collapse of the pharynx and the latter by withdrawal of central drive to muscles of respiration during sleep, these two conditions share a common nocturnal phenotype, involving repetitive cycles of apnoea, hypoxia, hypercapnia, arousal from sleep, and clustered bursts of efferent sympathetic nerve discharge elicited and entrained by those stimuli. Moreover, the acute autonomic consequences of sleep-related breathing disorders have after effects that carry over into wakefulness: in heart failure patients with sleep apnoea muscle sympathetic activity is increased by 15 bursts/100 cardiac cycles on average, relative to patients without sleep apnoea67 and in a randomized controlled trial abolition of obstructive sleep apnoea with treatment resulted in an almost equivalent reduction in MSNA burst incidence.68 Indeed, the majority of patients in whom each cardiac cycle is accompanied by an efferent sympathetic burst have either obstructive or central sleep apnoea. If directed at the myocardium, increased sympathetic traffic increases the risk of ventricular arrhythmia;69 that directed at resistance vessels increases afterload and compromises exercise capacity; and an increase in efferent renal sympathetic nerve traffic will increase renal release, renal sodium reabsorption, renal vascular release, and resistance to the action of loop diuretics. Importantly, the co-existence of sleep and heart failure has prognostic implications: in a recent series examining specifically patients with ischaemic cardiomyopathy the adjusted hazard ratio for mortality if sleep apnoea was present was 3.03.70
The concept that sodium retention consequent to increased efferent sympathetic outflow in heart failure with co-existing sleep apnoea will worsen both conditions has received particular recent attention.71 Peripheral oedema that accumulates over the course of the day shifts rostrally during supine sleep. Relative to heart failure patients without sleep apnoea, the volume of fluid displaced from the legs is greatest in those with central sleep apnoea and intermediate, but still increased significantly in patients with obstructive sleep apnoea. Fluid that accumulates overnight in the neck causes peri-pharyngeal oedema and increase both neck circumference and pharyngeal resistance; these changes, in turn, will intensify obstructive apnoea acutely and over time increase the severity of heart failure. Fluid that accumulates primarily in the lungs causes pulmonary congestion which in turn irritates pulmonary afferents that elicit reflexively hyperventilation, inducing hypocapnea and initiating acutely the cycles of apnoea and hyperpnoea characteristic of Cheyne–Stokes respiration and over time worsening ventricular systolic function. Thus, there is a bidirectional relationship between heart failure and sleep apnoea in which the sympathetic nervous system participates as an important mechanistic intermediary.71
Summary and therapeutic implications
The autonomic phenotype of a heart failure patient reflects the integration, within that individual of central nervous system resetting plus diminished inhibitory and augmented excitatory neural reflex influences, some being appropriate homeostatic responses to the haemodynamic chemical and metabolic consequences of heart failure, but others pathological. Consequently, the dynamics, magnitude, and time course of parasympathetic and sympathetic derangements elicited by heart failure will vary from patient to patient. This diversity provides the opportunity to improve the morbidity and survival of such patients by addressing patient-specific pathophysiology, i.e. by adding tailored patient-specific interventions to the foundation of evidence-based drug and device therapy.
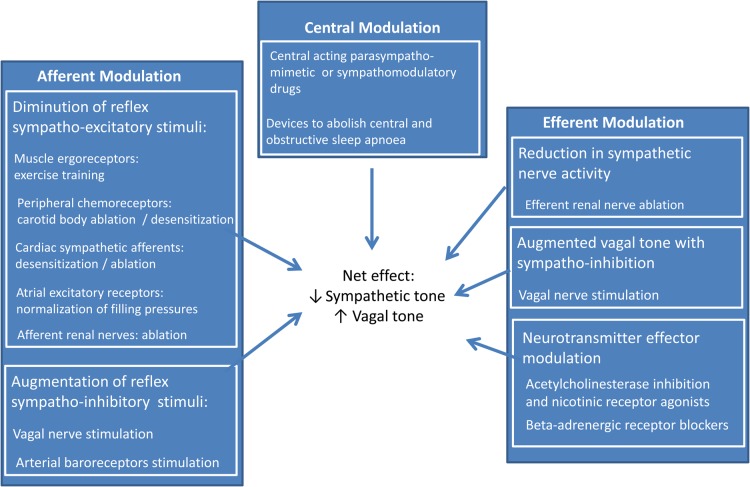
Thus, recognition and individual characterization of the magnitude and mechanisms of parasympathetic/sympathetic imbalance in heart failure provides a range of therapeutic opportunities that may be categorized broadly under the themes of afferent, central, and efferent neural modulation72 (Figure 5). Indeed, contemporary evidence-based therapy for HFrEF73 exploits several of these opportunities and as a consequence alters many of the autonomic measures itemized in Table 1. Beta-adrenoceptor antagonists increase arterial baroreflex heart rate modulation but have no effect on MSNA or its reflex modulation.74,75 Carvedilol, but not metoprolol, reduces cardiac and total body norepinephrine spillover likely due to its specific antagonism of peripheral sympathetic pre-junctional beta2-adrenergic receptors74 and restoration of norepinephrine uptake as assessed using cardiac 123I-MIBG imaging.76 In contrast, both angiotensin-converting enzyme inhibition and angiotensin receptor antagonism have been reported to inhibit MSNA,77–79 in addition to increasing arterial baroreflex heart rate modulation78,80 and reducing cardiac 123I-MIBG release.81,82 Aside from the demonstration that spironolactone increases 123I-MIBG uptake,83 there is little at present in the human HFrEF randomized trial literature concerning the impact of mineralocorticoid antagonism on sympathetic discharge or vagal heart rate modulation. Digoxin84 and cardiac resynchronization therapy85 have been reported to increase HRV, presumably by enhancing vagal HR modulation. In a recent trial, HFrEF patients with OSA who were randomized to CPAP were found after 6–8 weeks to have greater myocardial 11C-hydroxyephredrine retention, a presynaptic marker of sympathetic nerve function.86
Figure 5.
Present and future therapeutic opportunities to restore autonomic balance in heart failure.
Examples of afferent neural modulation to restore autonomic equilibrium currently under investigation include: vagal nerve stimulation; electrical carotid baroreceptor reflex activation (for which demonstration of preserved arterial baroreflex modulation of MSNA was pivotal); reduction of a sympatho-excitatory atrial reflex by normalizing cardiac filling pressure; reduction of MSNA and augmentation of cardiac vagal tone through exercise training; desensitization of cardiac sympathetic afferents; destruction of afferent renal nerves; and pharmacological or device-based desensitization, inhibition, or abolition of carotid chemoreceptors. Upwardly reset efferent sympathetic traffic and diminished vagal outflow may be addressed at cortical or brainstem sites by interventions that, in conjunction with centrally acting angiotensin antagonists or angiotensin-converting enzyme inhibitors, increase neuronal NOS expression or synthesis, antagonize the generation of reactive oxygen species, inhibit Rho kinase and associated enzymes, increase angiotensin-converting enzyme 2 expression, or stimulate angiotensin AT2 receptors;87,88 by exercise training;88 by other centrally acting drugs (including lipophilic statins89); or by devices that abolish obstructive or central sleep apnoea. Examples of efferent neural modulation include: vagal nerve stimulation; acetylcholinesterase inhibition and nicotinic receptor agonists (to increase vagal pre-ganglionic discharge, ganglionic neurotransmission, and neurotransmitter bio-availability); restoration of neuronal re-uptake of norepinephrine by cardiac and sympathetic nerve terminals; pre-junctional inhibition of norepinephrine release (a property of carvedilol, but not β1-selective antagonists); targeting G-protein-coupled receptor kinase 2 signalling; and efferent renal denervation. The risk is that without prior autonomic phenotyping, these interventions may be applied indiscriminately or inappropriately.90 The opportunity is to improve substantially symptoms and survival through application on the basis of evidence derived from appropriately designed randomized controlled trials.
Funding
J.S.F. holds the Tier 1 Canada Research Chair in Integrative Cardiovascular Biology. His research has been supported by Operating Grants from the Heart and Stroke Foundation of Ontario and the Canadian Institutes of Health Research. P.P.'s research has been supported by grants from the Polish National Science Centre, Wroclaw Medical University and Cibiem.
Conflict of interest: J.S.F. has no disclosures. P.P. received consultancies from Cibiem and Respicardia and honoraria for lectures from Respicardia.
References
- 1.Amorim DS, Dargie HJ, Heer K, Brown M, Jenner D, Olsen EG, Richardson P, Goodwin JF. Is there autonomic impairment in congestive (dilated) cardiomyopathy? Lancet 1981;1:525–527. [DOI] [PubMed] [Google Scholar]
- 2.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Card 2009;54:375–385. [DOI] [PubMed] [Google Scholar]
- 3.Benedict CR, Shelton B, Johnstone DE, Francis G, Greenberg B, Konstam M, Probsfield JL, Yusuf S. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventriculardysfunction. SOLVD Investigators. Circ 1996;94:690–697. [DOI] [PubMed] [Google Scholar]
- 4.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circ 1995;92:3206–3211. [DOI] [PubMed] [Google Scholar]
- 5.Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation and clinical use. Circ 1996;93:1043–1065. [PubMed] [Google Scholar]
- 6.Ando S, Dajani HR, Floras JS. Frequency domain characteristics of muscle sympathetic nerve activity in heart failure and healthy humans. Am J Physiol 1997;273(1 Pt 2):R205–R212. [DOI] [PubMed] [Google Scholar]
- 7.Esler M. The 2009 Carl Ludwig Lecture: pathophysiology of the human sympathetic nervous system in cardiovascular disease: the transition from mechanisms to medical management. J Appl Physiol 2010;108:227–237. [DOI] [PubMed] [Google Scholar]
- 8.Millar PJ, Murai H, Floras JS. Paradoxical muscle sympathetic reflex activation in human heart failure. Circ 2015;131:459–468. [DOI] [PubMed] [Google Scholar]
- 9.Notarius CF, Floras JS. Limitations of the use of spectral analysis of heart rate variability for the estimation of cardiac sympathetic activity in heart failure. Europace 2001;3:29–38. [DOI] [PubMed] [Google Scholar]
- 10.Notarius CF, Butler GC, Ando S, Pollard MJ, Senn BL, Floras JS. Dissociation between microneurographic and heart rate variability estimates of sympathetic tone in normal and heart failure subjects. Clin Sci 1999;96:557–565. [PubMed] [Google Scholar]
- 11.Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med 1971;285:877–883. [DOI] [PubMed] [Google Scholar]
- 12.Fallavollita JA, Heavey BM, Luisi AJ, Michalek SM, Baldwa S, Mashtare TL, Jr, Hutson AD, deKemp RA, Haka MS, Sajjad M, Cimato TR, Curtis AB, Cain ME, Canty JM., Jr Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol 2014;63:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J, ADMIRE-HF Investigators. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Card 2010;55:2212–2221. [DOI] [PubMed] [Google Scholar]
- 14.Benedict CR, Weiner DH, Johnson DE, Bourassa MG, Ghali JK, Nicklas J, Kirlin P, Greenberg B, Quinones MA, Yusuf S. Comparative neurohormonal responses in patients with preserved and impaired left ventricular function. Results of the Studies of Left Ventricular Dysfunction (SOLVD) Registry. J Am Coll Cardiol 1993;22:146A–153A. [DOI] [PubMed] [Google Scholar]
- 15.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon A. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311:819–824. [DOI] [PubMed] [Google Scholar]
- 16.Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal in heart failure. JACC 1993;22:72A–84A. [DOI] [PubMed] [Google Scholar]
- 17.Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF, Jr, Mohanty PK. Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. J Clin Invest 1990;85:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rundqvist B, Elam M, Bergman-Sverrisdottir Y, Eisenhofer G, Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circ 1997;95:169–175. [DOI] [PubMed] [Google Scholar]
- 19.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol 2001;280:H969–H976. [DOI] [PubMed] [Google Scholar]
- 20.Bibevski S, Dunlap ME. Ganglionic mechanisms contribute to diminshed vagal control in heart failure. Circ 1999;99:2958–2963. [DOI] [PubMed] [Google Scholar]
- 21.Ramchandra R, Hood SG, Denton DA, Woods RL, McKinley MJ, McAllen RM, May CN. Basis for the preferential activation of cardiac sympathetic nerve activity in heart failure. Proc Natl Acad Sci USA 2009;106:924–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson AM, Hood SG, Ramchandra R, McAllen RM, May CN. Increased cardiac sympathetic nerve activity in heart failure is not due to desentization of the arterial baroreflex. Am J Physiol 2007;293:H798–H804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol 1992;263:H798–H803. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 1998;31:68–72. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol 1992;69:523–531. [DOI] [PubMed] [Google Scholar]
- 26.Dibner-Dunlap ME, Smith ML, Kinugawa T, Thames MD. Enalaprilat augments arterial and cardiopulmonary baroreflex control of sympathetic nerve activity in patients with heart failure. J Am Coll Cardiol 1996;27:358–364. [DOI] [PubMed] [Google Scholar]
- 27.Floras JS. Arterial baroreceptor and cardiopulmonary reflex control of sympathetic outflow in human heart failure. Ann NY Acad Sci 2001;940:500–513. [DOI] [PubMed] [Google Scholar]
- 28.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension 2006;48:1005–1011. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Schultz HD, Ma R. Volume expansion potentiates cardiac sympathetic afferent reflex in dogs. Am J Physiol 2001;280:H576–H581. [DOI] [PubMed] [Google Scholar]
- 30.Minisi AJ, Thames MD. Distribution of left ventricular sympathetic afferents demonstrated by reflex responses to transmural myocardial ischemia and to intracoronary and epicardial bradykinin. Circ 1993;87:240–246. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Zucker IH. Cardiac sympathetic afferent reflex in dogs with congestive heart failure. Am J Physiol 1996;271:R751–R756. [DOI] [PubMed] [Google Scholar]
- 32.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 2014;64:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure - pathophysiological links. Cardiovasc Res 2006;70:434–445. [DOI] [PubMed] [Google Scholar]
- 34.Tracey KJ. The inflammatory reflex. Nature 2002;420:853–859. [DOI] [PubMed] [Google Scholar]
- 35.Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail 2011;4:669–675. [DOI] [PubMed] [Google Scholar]
- 36.Goso Y, Asanoi H, Ishise H, Kameyama T, Hirai T, Nozawa T, Takashima A, Umeno K. Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circ 2001;104:418–423. [DOI] [PubMed] [Google Scholar]
- 37.Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J 1994;72:S36–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the ‘muscle hypothesis’ of exercise intolerance in chronic heart failure. Am Heart J 1999;137:1050–1056. [DOI] [PubMed] [Google Scholar]
- 39.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circ 2001;104:2324–2330. [DOI] [PubMed] [Google Scholar]
- 40.Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P, Floras JS. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol 2015;593:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponikowski PP, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circ 2001;104:544–549. [DOI] [PubMed] [Google Scholar]
- 42.Di Vanna A, Braga AM, Laterza MC, Ueno LM, Rondon MU, Barretto AC, Middlekauff HR, Negrao CE. Blunted muscle vasodilatation during chemoreceptor stimulation in patients with heart failure. Am J Physiol 2007;293:H846–H852. [DOI] [PubMed] [Google Scholar]
- 43.Kara T, Narkiewicz K, Somers VK. Chemoreflexes - physiology and clinical implications. Acta Physiol Scand 2003;177:377–384. [DOI] [PubMed] [Google Scholar]
- 44.Niewinski P, Engelman ZJ, Fudim M, Tubek S, Paleczny B, Jankowska EA, Banasiak W, Sobotka PA, Ponikowski P. Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. J Card Fail 2013;19:408–415. [DOI] [PubMed] [Google Scholar]
- 45.Ponikowski P, Chua TP, Piepoli M, Ondusova D, Webb-Peploe K, Harrington D, Anker SD, Volterrani M, Colombo R, Massuero G, Giordano A, Coats AJ. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circ 1997;96:2586–2594. [DOI] [PubMed] [Google Scholar]
- 46.Giannoni A, Emdin M, Poletti R, Bramanti F, Prontera C, Piepoli M, Passino C. Clinical significance of chemosensitivity in chronic heart failure: influence on neurohormonal derangement, Cheyne-Stokes respiration and arrhythmias. Clin Sci 2008;114:489–497. [DOI] [PubMed] [Google Scholar]
- 47.Stickland MK, Miller JD, Smith CA, Dempsey JA. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res 2007;100:1371–1378. [DOI] [PubMed] [Google Scholar]
- 48.Notarius CF, Atchison DJ, Rongen GA, Floras JS. Effect of adenosine receptor blockade with caffeine on sympathetic response to handgrip exercise in heart failure. Am J Physiol 2001;281:H1312–H1318. [DOI] [PubMed] [Google Scholar]
- 49.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 2013;62:2422–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niewinski P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W, Hart EC, Paton JF, Ponikowski P. Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol 2013;168:2506–2509. [DOI] [PubMed] [Google Scholar]
- 51.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol 2010;298:R245–R253. [DOI] [PubMed] [Google Scholar]
- 52.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 1997;77:75–197. [DOI] [PubMed] [Google Scholar]
- 53.Oda Y, Joho S, Harada D, Hirai T, Asanoi H, Inoue H. Renal insufficiency coexisting with heart failure is related to elevated sympathetic nerve activity. Auton Neurosci 2010;155:104–108. [DOI] [PubMed] [Google Scholar]
- 54.Lambert G, Kaye DM, Lefkovits J, Jennings G, Turner AG, Cox HS, Esler M. Increased central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circ 1995;92:1813–1818. [DOI] [PubMed] [Google Scholar]
- 55.Aggarwal A, Esler M, Lambert GW, Hastings J, Johnston L, Kaye DM. Norepinephrine turnover is increased in suprabulbar subcortical brain regions and is related to whole-body sympathetic activity in human heart failure. Circ 2002;105:1031–1033. [DOI] [PubMed] [Google Scholar]
- 56.Abboud FM, Thames MD. Interaction of cardiovascular reflexes in circulatory control. In: Shepherd JT, Abboud FM. (eds). Handbook of Physiology, Section 2: The Cardiovascular System; Volume III: Peripheral Circulation and Organ Blood Flow, Part 2. Bethesda: American Physiological Society; 1983. p.675–753. [Google Scholar]
- 57.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension 2012;59:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 2008;51:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haack KK, Gao L, Schiller AM, Curry PL, Pellegrino PR, Zucker IH. Central Rho kinase inhibition restores baroreflex sensitivity and angiotensin II type 1 receptor protein imbalance in conscious rabbits with chronic heart failure. Hypertension 2013;61:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu GQ, Gao L, Li Y, Patel KP, Zucker IH, Wang W. AT1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Am J Physiol 2004;287:H1828–H1835. [DOI] [PubMed] [Google Scholar]
- 61.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 2004;95:937–944. [DOI] [PubMed] [Google Scholar]
- 62.Huang BS, Yuan B, Leenen FH. Chronic blockade of brain ‘ouabain’ prevents sympathetic hyper-reactivity and impairment of acute baroreflex resetting in rats with congestive heart failure. Can J Physiol Pharmacol 2000;78:45–53. [DOI] [PubMed] [Google Scholar]
- 63.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol 2001;281:H2241–H2251. [DOI] [PubMed] [Google Scholar]
- 64.Ramchandra R, Hood SG, Watson AM, Allen AM, May CN. Central angiotensin type 1 receptor blockade decreases cardiac but not renal sympathetic nerve activity in heart failure. Hypertension 2012;59:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arzt M, Young T, Finn L, Skatrud JB, Ryan CM, Newton GE, Mak S, Parker JD, Floras JS, Bradley TD. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med 2006;166:1716–1722. [DOI] [PubMed] [Google Scholar]
- 66.Yumino D, Wang H, Floras JS, Newton GE, Mak S, Ruttanaumpawan P, Parker JD, Bradley TD. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Cardiac Fail 2009;15:279–285. [DOI] [PubMed] [Google Scholar]
- 67.Spaak J, Egri ZJ, Kubo T, Yu E, Ando S, Kaneko Y, Usui K, Bradley TD, Floras JS. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension 2005;46:1327–1332. [DOI] [PubMed] [Google Scholar]
- 68.Usui K, Bradley TD, Spaak J, Kubo T, Kaneko Y, Floras JS. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Card 2005;45:2008–2011. [DOI] [PubMed] [Google Scholar]
- 69.Bitter T, Westerheide N, Prinz C, Hassain MS, Vogt J, Langer C, Horstkotte D, Oldenburg O. Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J 2011;32:61–74. [DOI] [PubMed] [Google Scholar]
- 70.Yumino D, Wang H, Floras JS, Newton GE, Mak S, Ruttanaumpawan P, Parker JD, Bradley TD. Relationship between sleep apnoea and mortality in patients with ischaemic heart failure. Heart 2009;95:819–824. [DOI] [PubMed] [Google Scholar]
- 71.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circ 2012;126:1495–1510. [DOI] [PubMed] [Google Scholar]
- 72.Floras JS. Alterations in the sympathetic and parasympathetic nervous systems in heart failure. In: Mann DL, Felker GM. (eds). Heart Failure: A Companion to Braunwald's Heart Disease. 3rd ed Philadelphia: Harcourt; 2015. [Google Scholar]
- 73.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology, ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;15:361–362. [DOI] [PubMed] [Google Scholar]
- 74.Azevedo ER, Kubo T, Mak S, Al-Hesayen A, Schofield A, Allan R, Kelly S, Newton GE, Floras JS, Parker JD. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure differential effects on sympathetic activity. Circ 2001;104:2194–2199. [DOI] [PubMed] [Google Scholar]
- 75.Kubo T, Parker JD, Azevedo ER, Atchison DJ, Newton GE, Picton P, Floras JS. Vagal heart rate responses to chronic beta-blockade in human heart failure relate to cardiac norepinephrine spillover. Eur J Heart Fail 2005;7:878–881. [DOI] [PubMed] [Google Scholar]
- 76.Cohen-Solal A, Rouzet F, Berdeaux A, Le Guludec D, Abergel E, Syrota A, Merlet P. Effects of carvedilol on myocardial sympathetic innervation in patients with chronic heart failure. J Nucl Med 2005;46:1796–1803. [PubMed] [Google Scholar]
- 77.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Pozzi M, Morganti A, Carugo S, Mancia G. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circ 1997;96:1173–1179. [DOI] [PubMed] [Google Scholar]
- 78.Hikosaka M, Yuasa F, Mimura J, Kawamura A, Motohiro M, Iwasaki M, Sugiura T, Iwasaka T. Candesartan and arterial baroreflex sensitivity and sympathetic nerve activity in patients with mild heart failure. Cardiovasc Pharmacol 2002;40:875–880. [DOI] [PubMed] [Google Scholar]
- 79.Ruzicka M, Floras JS, McReynolds AG, Coletta E, Haddad H, Davies R, Lennen FHH. Do high doses of AT1-receptor blockers attenuate central sympathetic outflow in humans with chronic heart failure? Clin Sci 2013;124:589–595. [DOI] [PubMed] [Google Scholar]
- 80.De Tommasi E, Iacoviello M, Romito R, Ceconi C, Guida P, Massari F, Francolini G, Bertocchi F, Ferrari R, Rizzon P, Pitzalis MV. Comparison of the effect of valsartan and lisinopril on autonomic nervous system activity in chronic heart failure. Am Heart J 2003;146:E17. [DOI] [PubMed] [Google Scholar]
- 81.Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 2005;45:661–667. [DOI] [PubMed] [Google Scholar]
- 82.Takeishi Y, Atsumi H, Fujiwara S, Takahashi K, Tomoike H. ACE inhibition reduces cardiac iodine-123-MIBG release in heart failure. J Nucl Med 1997;38:1085–1089. [PubMed] [Google Scholar]
- 83.Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol 1995;76:1259–1265. [DOI] [PubMed] [Google Scholar]
- 84.Brouwer J, van Veldhuisen DJ, Man in‘t Veld AJ, Dunselman PH, Boomsma F, Haaksma J, Lie KI. Heart rate variability in patients with mild to moderate heart failure: effects of neurohormonal modulation by digoxin and ibopamine. The Dutch Ibopamine Multicenter Trial (DIMT) Study Group. J Am Coll Cardiol 1995;26:983–990. [DOI] [PubMed] [Google Scholar]
- 85.Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circ 2003;108:266–269. [DOI] [PubMed] [Google Scholar]
- 86.Hall AB, Ziadi MC, Leech JA, Chen SY, Burwash IG, Renaud J, deKemp RA, Haddad H, Mielniczuk LM, Yoshinaga K, Guo A, Chen L, Walter O, Garrard L, DaSilva JN, Floras JS, Beanlands RS. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation 2014;130:892–901. [DOI] [PubMed] [Google Scholar]
- 87.Gao J, Zucker IH, Gao L. Activation of central angiotensin type 2 receptors by compound 21 improves arterial baroreflex sensitivity in rats with heart failure. Am J Hypertens 2014;27:1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 2014;126:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Millar PJ, Floras JS. Statins and the autonomic nervous system. Clin Sci (Lond) 2014;126:401–415. [DOI] [PubMed] [Google Scholar]
- 90.Floras JS. The ‘unsympathetic’ nervous system of heart failure. Circ 2002;105:1753–1755. [DOI] [PubMed] [Google Scholar]