Abstract
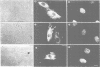
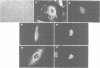
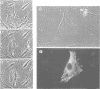
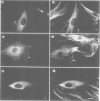
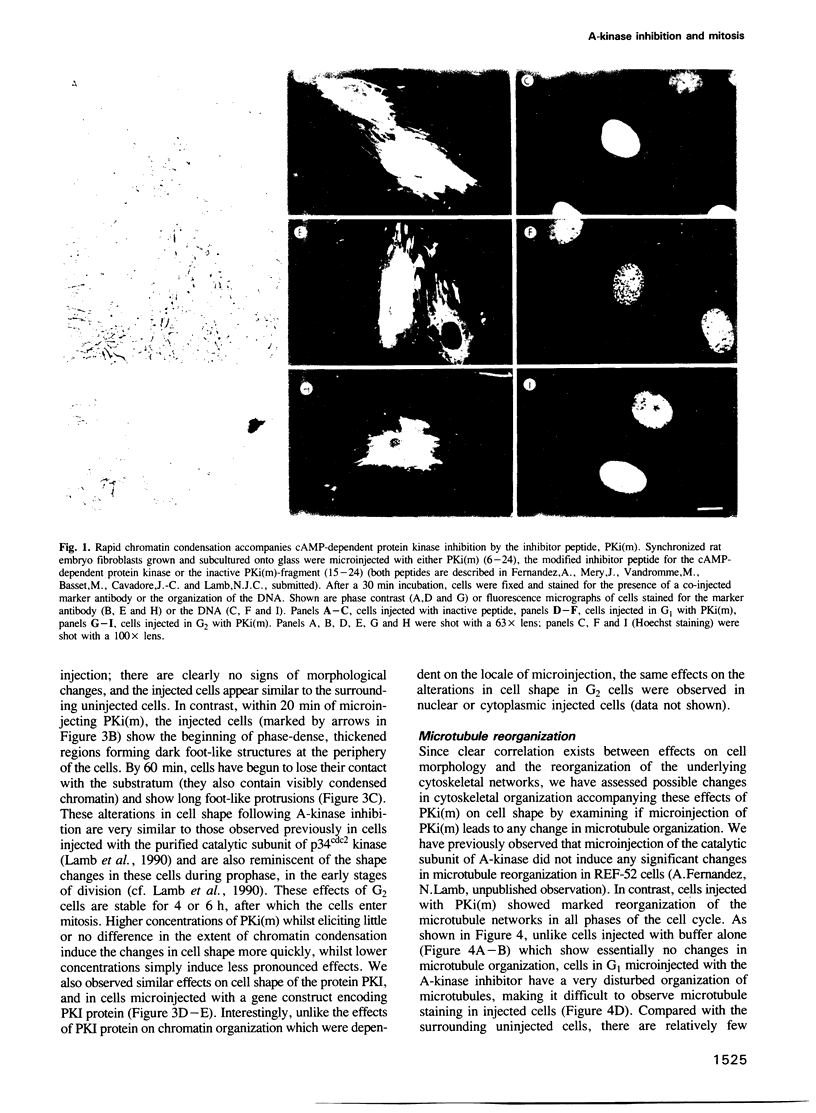
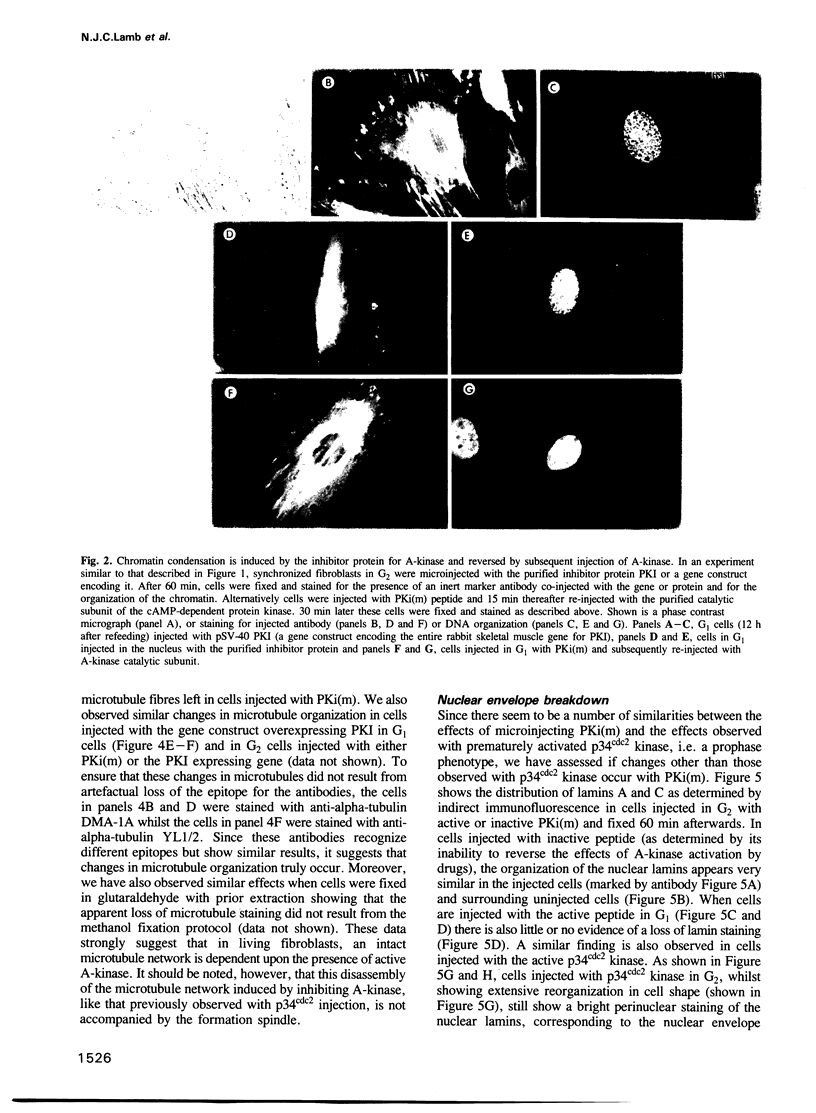
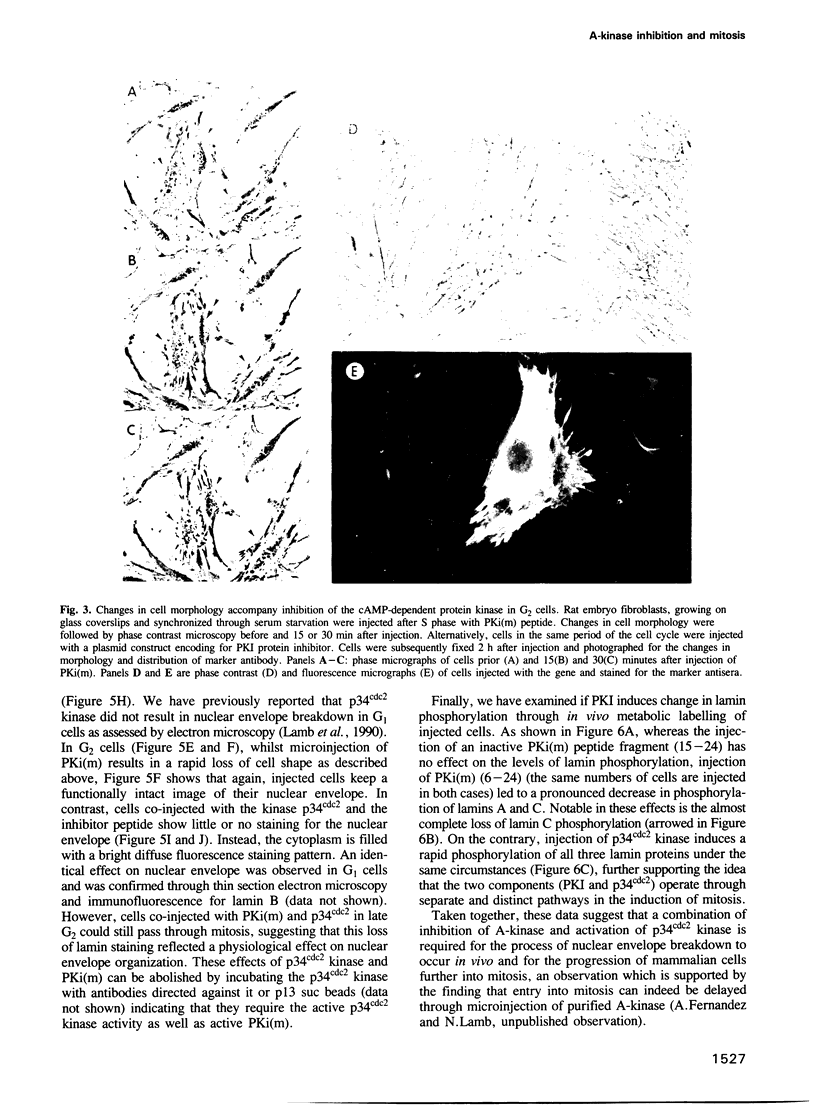
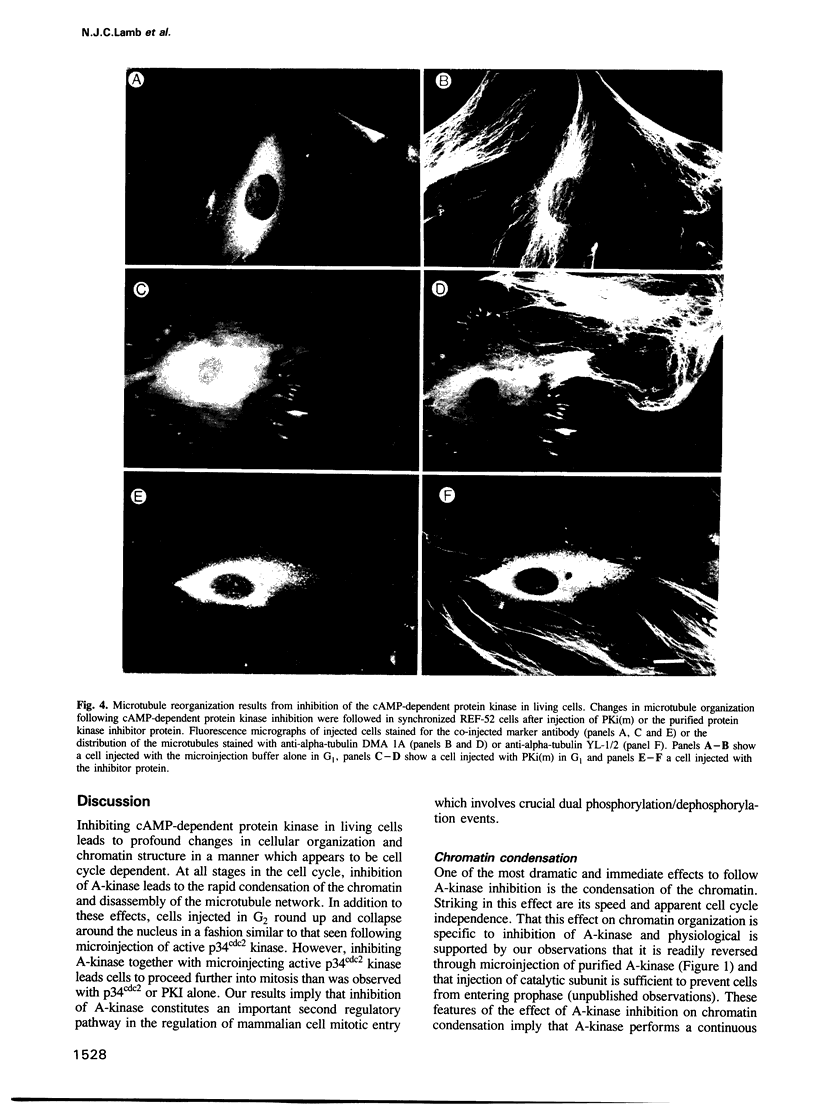
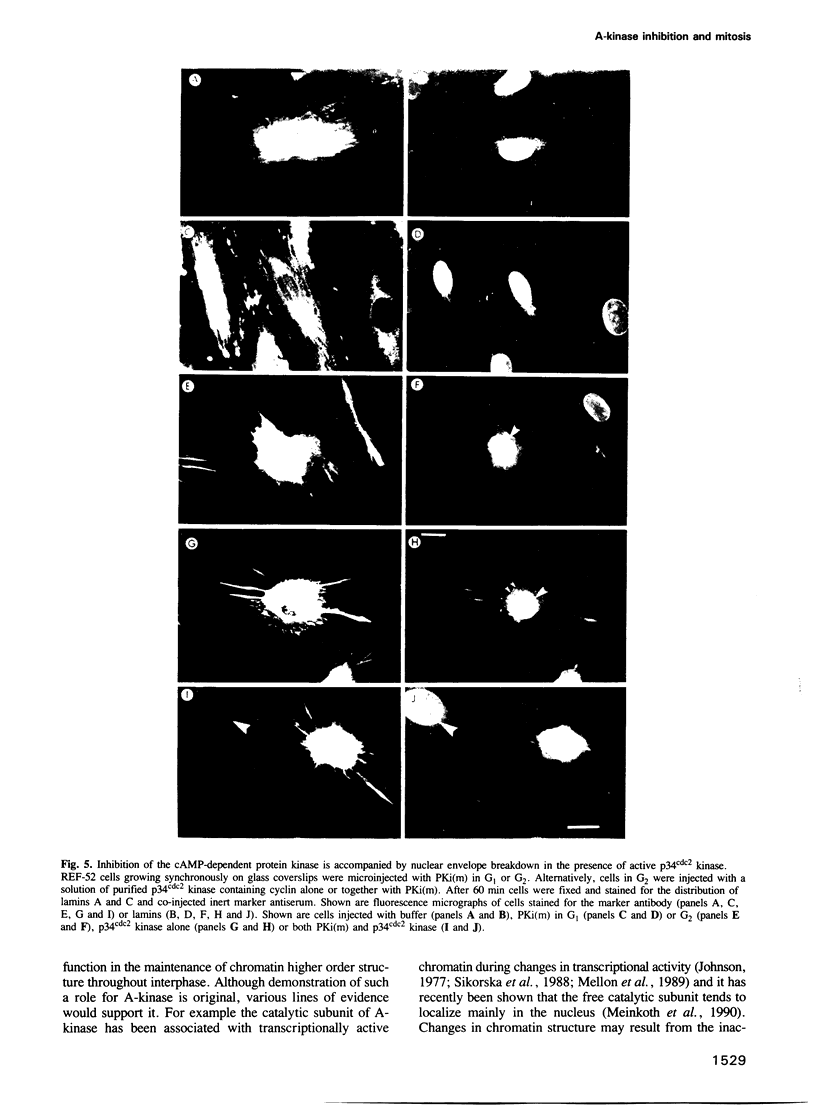
Inhibiting cAMP-dependent protein kinase (A-kinase) in mammalian fibroblasts through microinjection of a modified specific inhibitor peptide, PKi(m) or the purified inhibitor protein, PKI, resulted in rapid and pronounced chromatin condensation at all phases of the cell cycle. Together with these changes in chromatin, a marked reorganization of microtubule network occurred, accompanied in G2 cells by extensive alterations in cell shape which have many similarities to the premitotic phenotype previously observed after activation of p34cdc2 kinase, including the lack of spindle formation and the persistence of a nuclear envelope. In order to examine whether A-kinase inhibition and p34cdc2 kinase form part of the same or different inductive pathways, PKI and p34cdc2 kinase were injected together. Co-injection of both components resulted in nuclear envelope disassembly, an event not observed with injection of either component alone. This result implies that p34cdc2 and A-kinase inhibition have complementary and additive effects on the process of nuclear envelope breakdown in living fibroblasts, a conclusion further supported by our observation of a pronounced dephosphorylation of lamins A and C in cells after injection of PKi(m). Taken together, these data suggest that down-regulation of A-kinase is a distinct and essential event in the induction of mammalian cell mitosis which co-operates with the p34cdc2 pathway.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornslaeger E. A., Mattei P., Schultz R. M. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev Biol. 1986 Apr;114(2):453–462. doi: 10.1016/0012-1606(86)90209-5. [DOI] [PubMed] [Google Scholar]
- Browne C. L., Bird M. L., Bower W. Effect of inhibition of the catalytic activity of cyclic AMP-dependent protein kinase on mitosis in PtK1 cells. Cell Motil Cytoskeleton. 1987;7(3):248–257. doi: 10.1002/cm.970070307. [DOI] [PubMed] [Google Scholar]
- Browne C. L., Lockwood A. H., Su J. L., Beavo J. A., Steiner A. L. Immunofluorescent localization of cyclic nucleotide-dependent protein kinases on the mitotic apparatus of cultured cells. J Cell Biol. 1980 Nov;87(2 Pt 1):336–345. doi: 10.1083/jcb.87.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Büchler W., Meinecke M., Chakraborty T., Jahnsen T., Walter U., Lohmann S. M. Regulation of gene expression by transfected subunits of cAMP-dependent protein kinase. Eur J Biochem. 1990 Mar 10;188(2):253–259. doi: 10.1111/j.1432-1033.1990.tb15397.x. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Cicirelli M. F., Smith L. D. Cyclic AMP levels during the maturation of Xenopus oocytes. Dev Biol. 1985 Mar;108(1):254–258. doi: 10.1016/0012-1606(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Costa M., Gerner E. W., Russell D. H. Cell cycle-specific activity of type I and type II cyclic adenosine 3':5'-monophosphate-dependent protein kinases in Chinese hamster ovary cells. J Biol Chem. 1976 Jun 10;251(11):3313–3319. [PubMed] [Google Scholar]
- Day R. N., Walder J. A., Maurer R. A. A protein kinase inhibitor gene reduces both basal and multihormone-stimulated prolactin gene transcription. J Biol Chem. 1989 Jan 5;264(1):431–436. [PubMed] [Google Scholar]
- DeManno D. A., Goetz F. W. Steroid-induced final maturation in brook trout (Salvelinus fontinalis) oocytes in vitro: the effects of forskolin and phosphodiesterase inhibitors. Biol Reprod. 1987 Jun;36(5):1321–1332. doi: 10.1095/biolreprod36.5.1321. [DOI] [PubMed] [Google Scholar]
- Dorée M., Kishimoto T., Le Peuch C. J., Demaille J. G., Kanatani H. Calcium-mediated transduction of the hormonal message in meiosis reinitiation of starfish oocytes: modulation following injection of cholera toxin and cAMP-dependent protein kinase. Exp Cell Res. 1981 Oct;135(2):237–249. doi: 10.1016/0014-4827(81)90159-2. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G. Cell cycle control in eukaryotes: molecular mechanisms of cdc2 activation. Trends Biochem Sci. 1990 Oct;15(10):378–383. doi: 10.1016/0968-0004(90)90235-4. [DOI] [PubMed] [Google Scholar]
- Grove J. R., Price D. J., Goodman H. M., Avruch J. Recombinant fragment of protein kinase inhibitor blocks cyclic AMP-dependent gene transcription. Science. 1987 Oct 23;238(4826):530–533. doi: 10.1126/science.2821622. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Cell cycle-specific changes in histone phosphorylation associated with cell proliferation and chromosome condensation. J Cell Biol. 1974 Feb;60(2):356–364. doi: 10.1083/jcb.60.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990 May 18;61(4):579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Huchon D., Ozon R., Fischer E. H., Demaille J. G. The pure inhibitor of cAMP-dependent protein kinase initiates Xenopus laevis meiotic maturation. A 4-step scheme for meiotic maturation. Mol Cell Endocrinol. 1981 May;22(2):211–222. doi: 10.1016/0303-7207(81)90092-7. [DOI] [PubMed] [Google Scholar]
- Johnson E. M. Cyclic AMP-dependent protein kinase and its nuclear substrate proteins. Adv Cyclic Nucleotide Res. 1977;8:267–309. [PubMed] [Google Scholar]
- Lake R. S., Salzman N. P. Occurrence and properties of a chromatin-associated F1-histone phosphokinase in mitotic Chinese hamster cells. Biochemistry. 1972 Dec 5;11(25):4817–4826. doi: 10.1021/bi00775a027. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Watrin A., Labbé J. C., Cavadore J. C. Microinjection of p34cdc2 kinase induces marked changes in cell shape, cytoskeletal organization, and chromatin structure in mammalian fibroblasts. Cell. 1990 Jan 12;60(1):151–165. doi: 10.1016/0092-8674(90)90725-t. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Characterization of highly phosphorylated subcomponents of rat thymus H1 histone. J Biol Chem. 1982 Dec 25;257(24):14835–14846. [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., DeCamilli P., Einig I., Walter U. High-affinity binding of the regulatory subunit (RII) of cAMP-dependent protein kinase to microtubule-associated and other cellular proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6723–6727. doi: 10.1073/pnas.81.21.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L., Butcher F. R., Krebs E. G. Early effect of progesterone on levels of cyclic adenosine 3':5'-monophosphate in Xenopus oocytes. J Biol Chem. 1979 Feb 10;254(3):579–582. [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Mar 10;252(5):1712–1718. [PubMed] [Google Scholar]
- Maller J. L. OOcyte maturation in amphibians. Dev Biol (N Y 1985) 1985;1:289–311. doi: 10.1007/978-1-4615-6814-8_6. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Smith D. S. Two-dimensional polyacrylamide gel analysis of changes in protein phosphorylation during maturation of Xenopus oocytes. Dev Biol. 1985 May;109(1):150–156. doi: 10.1016/0012-1606(85)90355-0. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Both isoforms of the cAMP-dependent protein kinase catalytic subunit can activate transcription of the prolactin gene. J Biol Chem. 1989 Apr 25;264(12):6870–6873. [PubMed] [Google Scholar]
- McPherson J. M., Whitehouse S., Walsh D. A. Possibility of shape conformers of the protein inhibitor of the cyclic adenosine monophosphate dependent protein kinase. Biochemistry. 1979 Oct 30;18(22):4835–4845. doi: 10.1021/bi00589a011. [DOI] [PubMed] [Google Scholar]
- Meijer L., Dostmann W., Genieser H. G., Butt E., Jastorff B. Starfish oocyte maturation: evidence for a cyclic AMP-dependent inhibitory pathway. Dev Biol. 1989 May;133(1):58–66. doi: 10.1016/0012-1606(89)90296-0. [DOI] [PubMed] [Google Scholar]
- Meijer L., Zarutskie P. Starfish oocyte maturation: 1-methyladenine triggers a drop of cAMP concentration related to the hormone-dependent period. Dev Biol. 1987 Jun;121(2):306–315. doi: 10.1016/0012-1606(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Meinkoth J. L., Ji Y., Taylor S. S., Feramisco J. R. Dynamics of the distribution of cyclic AMP-dependent protein kinase in living cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9595–9599. doi: 10.1073/pnas.87.24.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P. L., Clegg C. H., Correll L. A., McKnight G. S. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4887–4891. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh M. P., Steiner A. L., Davies P. J. Localization of the catalytic subunit of cyclic AMP-dependent. Protein kinase in cultured cells using a specific antibody. J Cell Biol. 1982 Oct;95(1):64–72. doi: 10.1083/jcb.95.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Hilz H., Eppenberger H. M., Dutly F. Rapid and reversible translocation of the catalytic subunit of cAMP-dependent protein kinase type II from the Golgi complex to the nucleus. EMBO J. 1985 Nov;4(11):2801–2806. doi: 10.1002/j.1460-2075.1985.tb04006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. p34cdc2: the S and M kinase? New Biol. 1990 May;2(5):389–401. [PubMed] [Google Scholar]
- Riabowol K. T., Fink J. S., Gilman M. Z., Walsh D. A., Goodman R. H., Feramisco J. R. The catalytic subunit of cAMP-dependent protein kinase induces expression of genes containing cAMP-responsive enhancer elements. Nature. 1988 Nov 3;336(6194):83–86. doi: 10.1038/336083a0. [DOI] [PubMed] [Google Scholar]
- Roberge M., Th'ng J., Hamaguchi J., Bradbury E. M. The topoisomerase II inhibitor VM-26 induces marked changes in histone H1 kinase activity, histones H1 and H3 phosphorylation, and chromosome condensation in G2 phase and mitotic BHK cells. J Cell Biol. 1990 Nov;111(5 Pt 1):1753–1762. doi: 10.1083/jcb.111.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. Y., Schulman I. G., Richman R., Cook R. G., Allis C. D. Characterization of phosphorylation sites in histone H1 in the amitotic macronucleus of Tetrahymena during different physiological states. J Cell Biol. 1988 Dec;107(6 Pt 2):2473–2482. doi: 10.1083/jcb.107.6.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Baulieu E. E. Forskolin increases cAMP and inhibits progesterone induced meiosis reinitiation in Xenopus laevis oocytes. Endocrinology. 1982 Oct;111(4):1385–1387. doi: 10.1210/endo-111-4-1385. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. E. Cyclic AMP-mediated control of meiosis: effects of progesterone, cholera toxin, and membrane-active drugs in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):850–854. doi: 10.1073/pnas.79.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Montgomery R. R., Belanoff J. R. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983 Jun;97(2):264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Sikorska M., Whitfield J. F., Walker P. R. The regulatory and catalytic subunits of cAMP-dependent protein kinases are associated with transcriptionally active chromatin during changes in gene expression. J Biol Chem. 1988 Feb 25;263(6):3005–3011. [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem. 1982 Mar 25;257(6):3284–3290. [PubMed] [Google Scholar]
- Urner F., Herrmann W. L., Baulieu E. E., Schorderet-Slatkine S. Inhibition of denuded mouse oocyte meiotic maturation by forskolin, an activator of adenylate cyclase. Endocrinology. 1983 Sep;113(3):1170–1172. doi: 10.1210/endo-113-3-1170. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Bloom G. S., Theurkauf W. E. Microtubule-associated proteins: subunits of the cytomatrix. J Cell Biol. 1984 Jul;99(1 Pt 2):38s–44s. doi: 10.1083/jcb.99.1.38s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Patten S. M., Heisermann G. J., Cheng H. C., Walsh D. A. Tyrosine kinase catalyzed phosphorylation and inactivation of the inhibitor protein of the cAMP-dependent protein kinase. J Biol Chem. 1987 Mar 5;262(7):3398–3403. [PubMed] [Google Scholar]
- Ward G. E., Kirschner M. W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990 May 18;61(4):561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- Ward G. E., Kirschner M. W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990 May 18;61(4):561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- West M. H., Pantazis P., Bonner W. M. Studies on nuclease digestion of chromatin phosphorylated in vivo. J Biol Chem. 1985 Apr 25;260(8):4558–4560. [PubMed] [Google Scholar]
- Westwood J. T., Wagenaar E. B., Church R. B. Synthesis of unique low molecular weight proteins during late G2 and mitosis. J Biol Chem. 1985 Jan 25;260(2):695–698. [PubMed] [Google Scholar]
- Woodford T. A., Pardee A. B. Histone H1 kinase in exponential and synchronous populations of Chinese hamster fibroblasts. J Biol Chem. 1986 Apr 5;261(10):4669–4676. [PubMed] [Google Scholar]
- Wu R. S., Panusz H. T., Hatch C. L., Bonner W. M. Histones and their modifications. CRC Crit Rev Biochem. 1986;20(2):201–263. doi: 10.3109/10409238609083735. [DOI] [PubMed] [Google Scholar]