Abstract
The mucosa of alimentary tract heals more rapidly than cutaneous wounds. The underlying mechanisms of this enhanced healing have not been completely elucidated. Constant exposure to salivary growth factors has been shown to play a critical role in mucosal homeostasis and tissue repair. Angiogenesis also has an essential role in successful wound repair. One of the main angiogenic growth factors, vascular endothelial growth factor (VEGF), has a pleiotropic role in tissue repair via neovascularization, reepithelialization, and regulation of extracellular matrix. We have previously reported a critical role for salivary VEGF in bowel adaptation after small bowel resection. We hypothesize that salivary VEGF is an essential stimulus for oral mucosal tissue repair, and use the murine palatal wound model to test our hypothesis. In a loss-of-function experiment, we removed the primary source of VEGF production through selective submandibular gland (SMG) sialoadenectomy in a murine model and observed the effects on wound closure and neovascularization. We then performed a selective loss-of-function experiment using the protein VEGF-Trap to inhibit salivary VEGF. In a gain-of-function experiment, we supplemented oral VEGF following SMG sialoadenectomy. After SMG sialoadenectomy, there was significant reduction in salivary VEGF level, wound closure, and vessel density. Lower levels of salivary VEGF were correlated with impaired neovascularization and reepithelialization. The selective blockade of VEGF using VEGF-Trap resulted in a similar impairment in wound healing and neovascularization. The sole supplementation of oral VEGF after SMG sialoadenectomy rescued the impaired wound healing phenotype and restored neovascularization to normal levels. These data show a novel role for salivary-VEGF in mucosal wound healing, and provide a basis for the development of novel therapeutics aimed at augmenting wound repair of the oral mucosa, as well as wounds at other sites in the alimentary tract.
The mucosa of the alimentary tract is endowed with a remarkable capacity to rapidly heal despite continual environmental insult. The relative accessibility of the oral mucosa, as compared with other more invasive models of alimentary mucosal wounds, makes a palate wound model cost-effective and easily reproducible to study the mechanisms underlying the enhanced mucosal repair. Oral mucosal tissue repair proceeds through the classic phases of wound healing including hemostasis, inflammation, proliferation, and tissue regeneration, similar to cutaneous wound healing. This orchestrated sequence of events results in reepithelialization and restoration of tissue homeostasis. However, in contrast to cutaneous wound healing, wounds in the oral mucosa have been shown to heal more rapidly, in a regenerative manner and with diminished inflammation.1,2 The mechanisms of this enhanced wound healing phenotype have not been fully elucidated, although saliva is known to be a critical determinant of oral homeostasis.3,4 This importance is shown in clinically deficient disease states such as xerostomia due to medications, head and neck radiation, or autoimmune diseases such as Sjögren’s syndrome, which all result in an impaired wound healing phenotype.5
Growth factors are known to play a significant role in wound repair. A number of vulnerary growth factors are secreted into saliva, including vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), transforming growth factor-α and -β, acidic and basic fibroblast growth factors, and insulin-like growth factors (IGF-1, IGF-2).4,6 The expression profile of these growth factors is significantly different between skin and oral mucosa, and may account for differences in the wound healing phenotype between the two tissues.7,8 We have previously shown a novel role for salivary VEGF in the small bowel response to intestinal resection. The lack of salivary VEGF significantly attenuated gastrointestinal adaptation following small bowel resection. This deficit was partially corrected with oral VEGF supplementation, suggesting that VEGF-driven angiogenesis plays a critical role in maintaining the integrity of gastrointestinal mucosa.9
Angiogenesis is a critical factor in successful wound repair and regeneration and is known to be tightly regulated in a complex interplay of angiogenic and angiostatic growth factors. VEGF is a multifunctional vasoactive peptide with both “direct” and “indirect” angiogenic potential. VEGF is known to act through two receptors, VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1). VEGFR-2 is generally agreed to be the more potent mediator of mitogenic, angiogenic, and vascular permeability effects of VEGF. VEGF is noted to be present in both human and murine saliva at high concentrations. In humans, VEGF is primarily derived from the parotid glands.3,10,11 Whereas in mice, VEGF is secreted from the submandibular glands (SMGs). This finding provides the unique opportunity to selectively deplete salivary VEGF by removing the SMG and study its role in mucosal wound repair.9
Taken together, we hypothesized that salivary VEGF is an essential stimulus for oral mucosal tissue repair. To test this hypothesis, we used the murine palate mucosa model, and then determined the effects of salivary VEGF on mucosal wound repair. In a loss-of-function experiment, we removed the primary source of VEGF production through SMG sialoadenectomy in a murine model and observed the effects on wound closure and neovascularization. To further investigate the role of salivary VEGF in the palatal wound healing phenotype, we performed a selective loss-of-function experiment through inhibition of salivary VEGF using a novel protein, VEGF-Trap, and assessed palatal wound healing and neovascularization. Lastly, in a gain-of-function experiment, we determined if oral VEGF supplementation could rescue the impaired wound healing phenotype following depletion of all SMG-derived salivary growth factors by SMG sialoadenectomy.
MATERIALS AND METHODS
Animals
C57BL/6 female mice, 8–10 weeks old (Jackson Laboratories, Bar Harbor, ME), were housed in a temperature- and light-controlled facility. The mice were acclimated to the new environment for at least 5 days before using them in any experiments. All the protocols for this study were approved by the Cincinnati Children’s Hospital Research Foundation and Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee.
Sialoadenectomy
Sialoadenectomy was performed as previously described.12 Briefly, under inhalational isoflurane anesthesia, bilateral SMGs were dissected free of surrounding tissue through a midline cervical incision. The vascular pedicles supplying the SMG were cauterized with a fine-tip heat-cautery probe, and the glands were excised. Skin was closed with 4-0 Vicryl suture (Ethicon Inc., Somerville, NJ). The sham operation control group received a midline cervical incision with suture closure similar to the sialoadenectomy group. All mice were resuscitated with subcutaneous injections of normal saline (1 mL). Mice were fed a liquid chow diet (Land O’Lakes Purina Mills, St. Paul, MN) and allowed to recover for at least 3 days.
Palatal wounding model
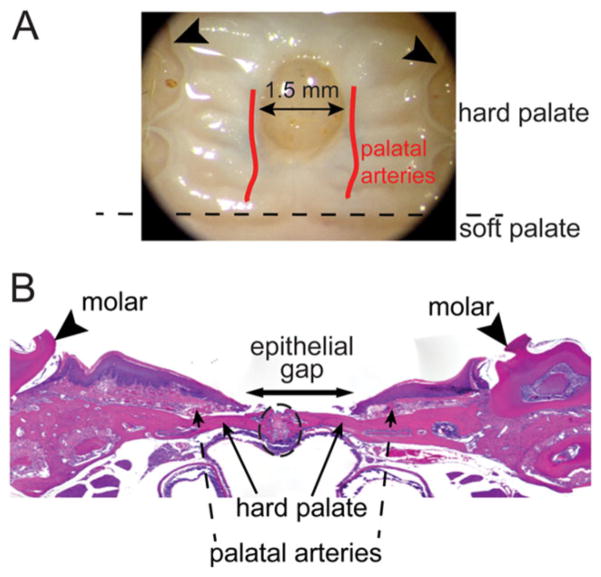
Following recovery, palatal wounds were created in sialoadenectomized and sham-operated mice. Mice were anesthetized with inhalational isoflurane (0.5 mL titrated) and subsequently administered intramuscular injections of ketamine (100 mg/kg) and xylazine (10 mg/kg). Under aseptic conditions, a single midline excisional wound was made using a 1.5 mm punch biopsy (Miltex, Inc., York, PA) in the mucosa overlying the hard palate. The wound was marked with India ink diluted 1:10 in phosphate-buffered saline solution (PBS). Wound location was standardized with the anterior edge of the wound aligned with the first molars as an anatomic reference. A 10× objective on the surgical microscope (Leica Microsystems, Heerbrugg, Switzerland) was used to strategically place the wounds and make sure that only the epithelial and submucosal tissues were excised, with extreme care taken to minimize injury to the underlying periosteum and adjacent palatal arteries (Figure 1A).
Figure 1.
Schematic of palatal wounding model and quantification of wound healing. (A) Palate wounds were created in the hard palate. The wound site was standardized such that the anterior edge of the wound abutts an imaginary line drawn between the 1st molars (arrowheads) as anatomic reference. Wounds (1.5 mm) are created using punch biopsy dipped in India ink to mark the wound. Both epithelial and submucosal tissues are excised and care is taken to minimize injury to the underlying periosteum and the palatal arteries. (B) H&E stained plate wound sections are used to quantify wound healing phenotype. The inclusion criterion of the wounds in analysis was to make sure that the palate suture line (enclosed within the black dotted oval) is intact with no significant presence of polymorphonuclear leukocytes. Epithelial gap, measured as distance between the encroaching epithelial margins (arrow), is measured to quantify wound closure.
Selective inhibition of VEGF
Selective inhibition of VEGF in the salivary glands was performed using an adenoviral vector that encodes for the protein VEGF-Trap (provided by John Rudge, PhD, Regeneron Inc., Tarrytown, NY). Ad-VEGF-Trap inhibits VEGF by producing VEGF-receptors Flt-1 and Flk-1 bound to the Fc portion of an IgG1. This vector is an E1, E3-deleted incompetent adenoviral construct driven by a CMV reporter. This inhibitor binds VEGF with high affinity and has a prolonged in vivo half-life.13 Ad-VEGF-Trap was administered through retrograde injection into bilateral salivary ducts at 1 × 108 plaque forming units (PFU) in 50 μL of PBS as previously described.11,14 To control for the effects of the adenovirus, 1 × 108 PFU of Ad-LacZ in 50 μL of PBS was administered in a separate group of animals. Another group of animals received retrograde injection of 50 μL of PBS alone. Palatal wounds were created 12 hours following Ad-VEGF-Trap, Ad-LacZ, or PBS administration.
Oral VEGF supplementation
In a gain-of-function experiment, oral VEGF protein (recombinant murine VEGF, Peprotech, Rocky Hill, NJ) was administered at a dose of 750 pg/mL in drinking water and liquid rodent chow ad libitum following palatal wounding. This dose is one and one half-fold the normal concentration of salivary VEGF and was arbitrarily chosen to compensate for the absence of continuous bathing of the wound with VEGF-rich saliva.
Protein quantification of VEGF using enzyme linked immunosorbent assay (ELISA)
Pre- and postoperative salivary samples were collected after subcutaneous injection of pilocarpine (0.5 mg/kg). Salivary VEGF level was determined using a Quantikine mouse VEGF ELISA kit (R&D Systems Inc, Minneapolis, MN) according to manufacturer’s instructions. Mice were allowed to recover for 3 days following saliva collection to allow for normalization of saliva output prior to further experimentation.
Wound harvesting and processing
Animals were euthanized by CO2 inhalation 3, 5, 7, and 10 days after wounding, followed by cervical dislocation. Palatal tissue was harvested en bloc and fixed in 10% neutral buffered formalin for 24 hours at room temperature and then decalcified in Immunocal solution (Decal Chemical Corporation, Tallman, NY) for 48 hours at 4 °C. Excess tissue was sharply removed and the hard palate was isolated using the molars as an anatomic marker. The samples were mechanically processed and paraffin embedded.
Histology and immunohistochemistry
Five-micrometer sections were cut, deparaffinized, serially hydrated and stained with hematoxylin and eosin (H&E) as previously described.15 From the H&E stained sections of the palate wounds, we made sure that the local environment is viable and the palate suture is intact with no persistent inflammatory cell infiltrate or necrosis, as the criterion for inclusion of wounds in the study analysis. The epithelial gap, defined as the distance between encroaching epidermal elements, was measured in micrometers using computer-assisted morphometric analysis (Metamorph, Sunnyvale, CA) (Figure 1B).
Immunohistochemistry was performed to evaluate neovascularization (anti-CD31 antibody) and the role of VEGFR-2 (Flk-1).9 A rat anti-mouse CD31 monoclonal antibody (1:100, Pharmingen, San Diego, CA) was added for 2 hours at room temperature. A biotinylated rabbit anti-rat (mouse-adsorbed) antibody (1:200, Vector Laboratories, Burlingame, CA) was then added for 30 minutes at room temperature. The slides were rinsed, developed using DAB, counterstained with hematoxylin, and mounted. Capillary lumen density was measured by counting CD31-positive vessels in four high-power fields (HPFs; 40×) of the mucosa. The sections were taken in the healing connective tissue internal to the advancing wound edges on either side. The area of measurement was standardized. The first section on either side bordered the leading edge of the epithelium, and three adjacent sections were taken sweeping outward.
Tissue sections were rehydrated with distilled water and antigen retrieval was performed using target retrieval solution per manufacturer’s protocol (Dako Cytomation, Carpentine, CA). Sections were then blocked in 5% normal goat serum and 1% bovine serum albumin for 90 minutes at room temperature. Rabbit anti-mouse Flk-1 antibody (1:100, Abcam, Cambridge, MA) was added for 1 hour at room temperature. Slides were rinsed and biotinylated goat anti-rabbit antibody (1:200, Vector Laboratories) was then added for 30 minutes at room temperature. The slides were rinsed, developed using DAB, and counterstained with hematoxylin. Flk-1 positive cells were counted in the mucosa of the wounds in four HPFs (40×) chosen near the healing edge of each wound. All histologic assessments were blinded.
Statistical analysis
Results are reported as mean ± SEM. Data were analyzed by one-way analysis of variance (ANOVA). Individual group differences were analyzed by Tukey’s post hoc testing. A p-value of <0.05 was considered statistically significant. The strength of the linear relationship between two variables was tested using the Pearson’s correlation coefficient.
RESULTS
SMG sialoadenectomy attenuates palatal mucosal healing and neovascularization following palatal wounding
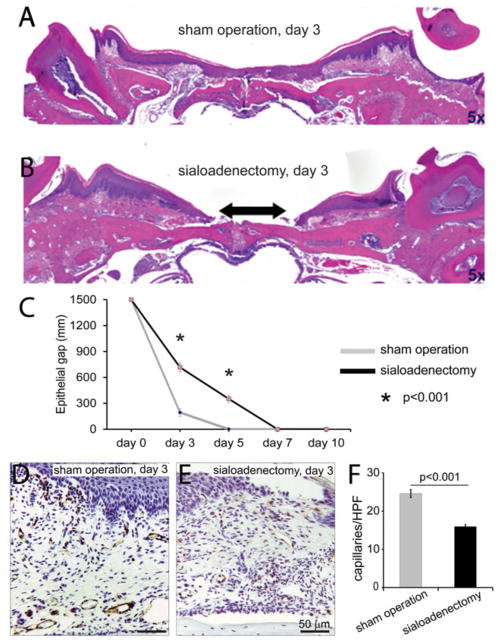
To determine if the removal of the SMG impairs palatal mucosal wound healing, C57BL/6 mice (n = 6) underwent SMG sialoadenectomy followed by palatal mucosal wounding. SMG sialoadenectomized mice had similar connective tissue architecture as the sham controls, with no apparent difference in the epithelial height of the wounds. At 3 days postwounding, SMG sialoadenectomized mice had a significant impairment in wound healing, shown by a deficit in epithelial gap closure as compared with those that underwent sham operation (Day 3: 716 ± 50 μm epithelial gap vs. 194 ± 43 μm, p < 0.0001, Figure 2A and B). At days 5 and 7 postinjury, the palate wounds of sham-operated mice are completely reepithelialized. In contrast, in the SMG sialoadenectomized mice, there is a persistent delay in epithelial closure (Day 5: 352.1 ± 40.1 μm epithelial gap) and they required up to 7 days to reepithelialize (Figure 2C). Palatal wounds of mice that underwent SMG sialoadenectomy also had a significant reduction in capillary density compared with mice that underwent sham operation (Day 3: 17.3 ± 0.6 caps/hpf vs. 24.0 ± 1.4 caps/hpf, p < 0.0001, Figure 2D–F). SMG sialoadenectomy results in impaired wound healing and neovascularization compared with sham operated controls.
Figure 2.
Submandibular gland (SMG) sialoadenectomy significantly impairs palatal mucosal wound healing and neovascularization. Staining was performed on control mice and SMG sialoadenectomized mice palate sections to compare epithelial gap closure (H&E) and neovascularization (CD31-immunohistochemistry). Representative photomicrographs (×5) of H&E stained palate wound sections of sham-operated mice (A) and SMG sialoadenectomied mice (B) show no apparent differences in the connective tissue architecture or epithelial height of the wounds at day 3 postwounding. In submandibular sialoadenectomized animals, there is a significant deficit in epithelial gap closure (gap in epithelium shown by arrow in B) as compared with sham-operated control animals. Epithelial gap was measured as the length (in μm) between the two encroaching wound epithelial ridges. Rate of wound closure was measured at days 3, 5, 7, and 10 post-wounding, quantitatively represented in (C). Most of the sham-operated mice completely healed their palate wounds by day 3 and all wounds in this group were completely healed by day 5. SMG sialoadenectomy results in delayed healing and these wounds required up to 7 days to completely heal (C). SMG sialoadenectomy also results in a significant reduction in capillary density in mice as compared with sham operation at day 3 (D, E), quantitatively shown in (F). All bars represent average ± SEM, n = 5, p-values by ANOVA.
Decreased salivary VEGF levels following sialoadenectomy is correlated with impaired wound healing
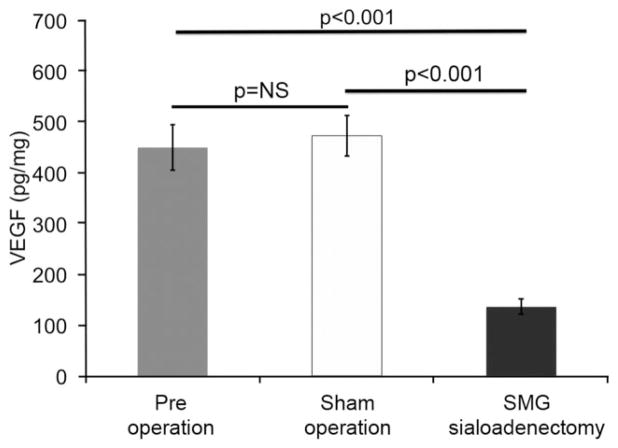
Salivary VEGF levels were significantly decreased in mice that underwent SMG sialoadenectomy (n = 6) compared with sham operation mice (n = 5) (136 ± 15.9 pg/mg vs. 472 ± 40 pg/mg, p < 0.001, Figure 3). To determine if there was a correlation between salivary VEGF levels, wound healing, and neovascularization, we analyzed our data using Pearson’s correlation coefficient. Salivary VEGF level is inversely correlated to epithelial gap (Pearson’s correlation coefficient = −0.6, p < 0.05, Figure 4A) and is positively correlated with the number of vessels (Pearson’s correlation coefficient = 0.87, p < 0.01, Figure 4B).
Figure 3.
Sialoadenectomy significantly decreases salivary VEGF levels. To quantify salivary VEGF pre- and post-sialoadenectomy, VEGF protein levels are measured by obtaining saliva after pilocarpine treatment. The levels of VEGF are normalized to the total protein. VEGF levels significantly decrease in sialoadenectomized mice at day 5 post-SMG removal, compared with pre- and sham-operated mice. The bars represent average ± SEM, n = 5, p-values by ANOVA. SMG, submandibular gland; VEGF, vascular endothelial growth factor.
Figure 4.
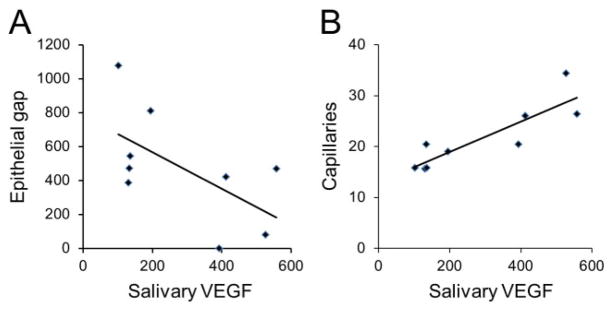
Salivary VEGF level is inversely correlated to epithelial gap and positively correlated to neovascularization. Pear-son’s correlation coefficient was used to correlate salivary VEGF levels to epithelial gap and neovascularization. Same group of mice (from sham and SMG sialoadenectomy groups) are used in the analysis in both A and B. At day 3 postpalatal mucosal wounding, salivary VEGF levels are inversely correlated to epithelial gap or impaired wound healing (A, Pearson’s correlation coefficient = −0.6, p < 0.05) and positively correlated to the number of vessels per HPF (B, Pearson’s correlation coefficient = 0.87, p < 0.01). HPF, high-power field; SMG, submandibular gland; VEGF, vascular endothelial growth factor.
Selective inhibition of VEGF decreases salivary VEGF levels and attenuates mucosal healing and neovascularization following palatal wounding
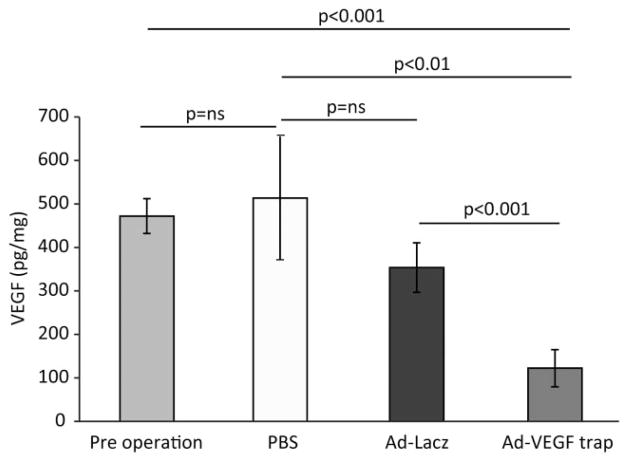
To examine the contribution of salivary-derived VEGF to palatal mucosal wound healing and neovascularization, we selectively inhibited VEGF by retrograde injection of Ad-VEGF-Trap in mice with intact salivary glands. We determined if Ad-VEGF-Trap reduced VEGF levels by measuring VEGF in saliva pre- and postinjection of mice with Ad-VEGF-Trap, Ad-LacZ, or PBS. Ad-VEGF-Trap significantly decreases salivary VEGF levels compared with controls (Ad-VEGF-Trap 122 ± 42 pg/mg vs. Ad-LacZ 353 ± 56 pg/mg vs. PBS 513 ± 141 pg/mg, p < 0.01, ANOVA, Figure 5). There was no significant difference in VEGF levels between Ad-LacZ or PBS-treated controls (353 ± 56 pg/mg vs. 513 ± 141 pg/mg, p = ns, ANOVA Figure 5).
Figure 5.
Ad-VEGF-Trap significantly decreases salivary VEGF levels. A novel adenoviral construct (Ad-VEGF-Trap) was used to selectively inhibit salivary VEGF. AD-VEGF-Trap results in a significant reduction in salivary VEGF levels compared with baseline or PBS controls at day 5. There was no significant difference in VEGF levels in Ad-LacZ control when compared with PBS or preoperation controls. The levels of VEGF are normalized to the total protein. The bars represent average ± SEM, n = 4–6, p-values by ANOVA. PBS, phosphate-buffered saline solution; VEGF, vascular endothelial growth factor.
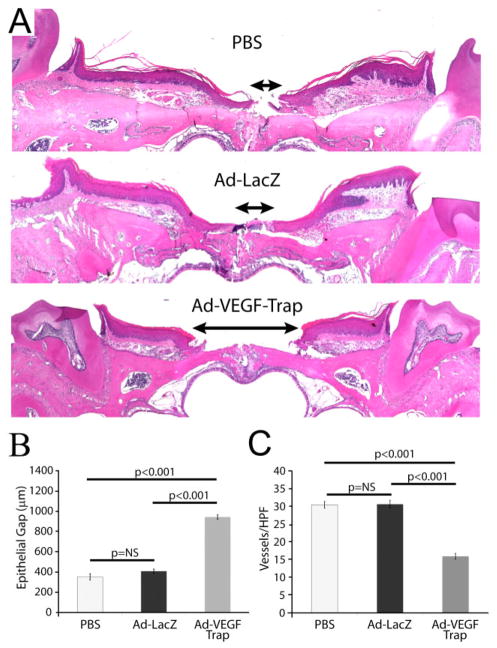
To determine the effect of selective inhibition of salivary VEGF on palatal mucosal wound healing, we measured epithelial gap and neovascularization. Following selective inhibition of VEGF, there was a significant increase in the epithelial gap or impaired wound healing as compared with Ad-LacZ or PBS-treated controls (Ad-VEGF-Trap 947 ± 22 μm vs. Ad-LacZ 411 ± 21 μm vs. PBS 355 ± 31 μm, p < 0.001, ANOVA, Figure 6A and B). There was, however, no significant difference in the epithelial gap between Ad-LacZ or PBS control-treated animals.
Figure 6.
Selective inhibition of salivary VEGF using Ad-VEGF-Trap impairs palatal mucosal wound healing and neovascularization and recapitulate the SMG sialoadenectomy phenotype. (A) At day 3 postwounding, in mice that had selective inhibition of VEGF (by VEGF trap), there was a significant deficit in epithelial gap closure (indicated by the length of the arrow between the two encroaching epithelial margins) as compared with Ad-LacZ or PBS treated. (B) Quantitative representation of the epithelial closure deficit (in μm) in the three groups is shown. (C) VEGF-Trap also results in a significant decrease in capillary lumen density as compared with Ad-LacZ and PBS-treated controls. There was no significant difference in the neovascularization between Ad-LacZ or PBS control animals. The bars represent average ± SEM, n = 4–6, p-values by ANOVA. SMG, submandibular gland; VEGF, vascular endothelial growth factor.
To assess neovascularization, CD31-positive lumens were counted per HPF (40×). Ad-VEGF-Trap results in a significant decrease in capillary lumen density compared with Ad-LacZ and PBS-treated controls (Ad-VEGF-Trap 15.95 ± 0.83 caps/HPF vs. Ad-LazZ 30.87 ± 1.1 caps/HPF vs. PBS 30.5 ± 0.95 caps/HPF, p < 0.001, ANOVA, Figure 6C). There was no significant difference in the neovascularization between Ad-LacZ or PBS-treated control animals.
Oral supplementation of VEGF after SMG sialoadenectomy restores normal wound healing after palatal wounding
In a gain-of-function experiment, recombinant VEGF was orally supplemented to determine if VEGF only can rescue the wound healing impairment of SMG sialoadenectomized mice (n = 4). Oral VEGF supplementation after SMG sialoadenectomy rescued the impairment in wound healing in the SMG sialoadenectomized group and restored the epithelial gap (sham operation 194 ± 43 μm vs. SMG SAL + oral VEGF 350 ± 46 μm, p = ns, ANOVA, Figure 7A) and neovascularization (sham operation 24.0 ± 1.4 caps/HPF vs. SMG SAL + oral VEGF 18.6 ± 2.1 caps/HPF, p = ns, Figure 7B) to the normal levels.
Figure 7.
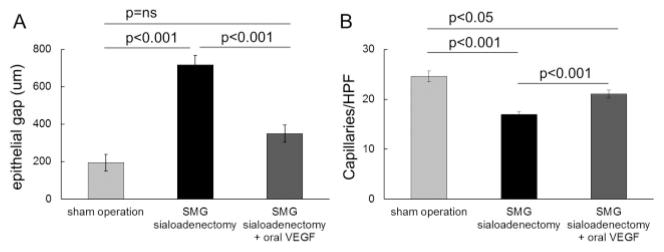
VEGF supplementation rescues palatal wound healing and neovascularization deficit in SMG sialoadenectomized mice. Sole supplementation of VEGF via water and liquid diet after wounding in submandibular gland sialoadenectomy mice (n = 4), rescued the wound healing impairment and restored it to the levels of sham-operated control mice. (A) SMG sialoadenectomized mice have a significant impairment in wound epithelial closure at day 3 postwounding as compared with sham-operated mice, which is reversed by oral VEGF supplementation. (B) Similarly, impaired wound neovascularization in the SMG sialoadenectomized mice at day 3 is restored to sham by oral VEGF supplementation. The bars represent average ± SEM, n = 4–6, p-values by ANOVA. SMG, submandibular gland; VEGF, vascular endothelial growth factor.
SMG sialoadenectomy results in impaired VEGF Receptor 2 (Flk-1) expression in palate wounds, which is restored by oral VEGF supplementation after palatal wounding
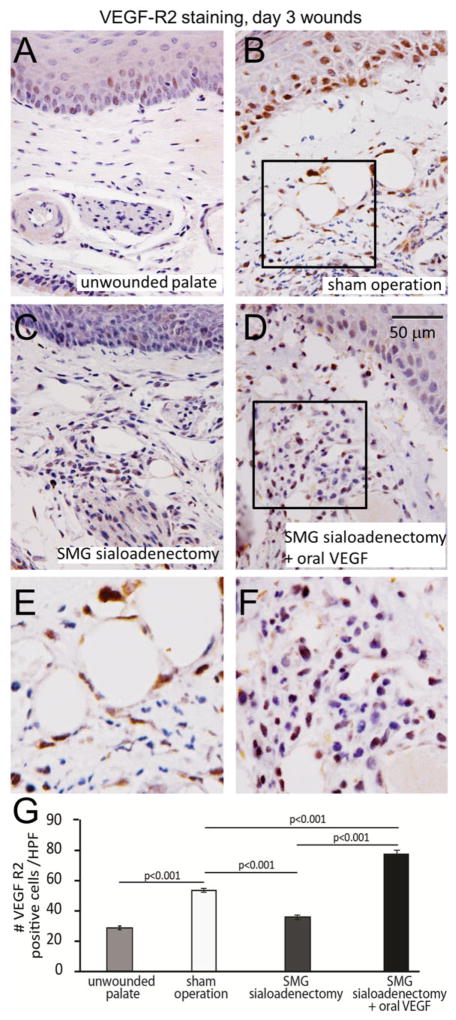
The effects of VEGF depletion through SMG sialoadenectomy and subsequent VEGF supplementation on Flk-1 expression profile in mucosa were evaluated. Palatal wounding results in a significant increase in Flk-1 positive cells, as compared with the unwounded palate (53.5 ± 1.3 cells/HPF vs. 28.5 ± 1.2 cells/HPF, p < 0.001, ANOVA, Figure 8A and B, E). SMG sialoadenectomy significantly reduces Flk-1 positive cells at day 3 following wounding, as compared with the sham operation controls (36 ± 1.5 cells/HPF vs. 53.5 ± 1.3 cells/HPF, p < 0.001, ANOVA, Figure 8B and C, E). Oral VEGF supplementation after sialoadenectomy results in a significant increase in the Flk-1 positive cells, compared with both sialoadenectomy and sham operation wounds (SMG SAL + oral VEGF 77.5 ± 2.0 cells/HPF vs. SMG SAL 36 ± 1.5 cells/HPF, sham operation 53.5 ± 1.3 cells/HPF, p < 0.001, ANOVA, Figure 8B–E). Interestingly, the density of the Flk-1 positive cells in the epithelium is lower in SMG sialoadenectomy wounds, compared with the sham-operated wounds. Oral VEGF supplementation results in an increase in Flk-1 positive cells in SMG sialoadenectomy wounds.
Figure 8.
VEGF Receptor 2 (Flk-1) expression in palate wounds is impaired by SMG sialoadenectomy, and restored by oral VEGF supplementation. (A, B) Palatal wounding results in a significant increase in VEGFR-2 positive cells compared with unwounded controls. SMG sialoadenectomy abrogates the increase in VEGFR-2 expressing cells (C), which is rescued by sole supplementation of oral VEGF (D). E and F are high-powered images of boxed areas in B and D, respectively. (G) Data quantification of the number of VEGFR-2 positive cells per high-power field (HPF) in the palatal wounds. The bar plots represent average ± SEM, n = 4–6, p-values by ANOVA. SMG, submandibular gland; VEGF, vascular endothelial growth factor.
DISCUSSION
Our results show that salivary VEGF plays a critical role in mucosal tissue repair and neovascularization following palatal injury. First, we established a baseline rate of reepithelialization and neovascularization following palatal mucosal wounding in normal mice. Then, in a loss-of-function experiment, we took advantage of the fact that the SMGs produce the majority of salivary VEGF in mice, and performed SMG sialoadenectomies in order to reduce salivary VEGF levels. Lower levels of salivary VEGF were correlated with impaired neovascularization and reepithelialization, suggesting an important role for VEGF in palatal mucosal healing. Further, in a targeted loss-of-function experiment, salivary VEGF was selectively blocked with the protein VEGF-Trap. The selective blockade of VEGF resulted in similar impairment in wound healing and neovascularization in the palate mucosa as the animals that underwent SMG sialoadenectomy. This finding suggests that the VEGF component of the saliva has a major role in palatal wound healing. Lastly, in a gain-of-function experiment, the supplementation of VEGF following SMG sialoadenectomy rescued the impaired wound healing phenotype and restored neovascularization to normal levels. This finding is notable in light of the fact that when all SMG-derived growth factors have been depleted by SMG sialoadenectomy, the sole supplementation of VEGF resulted in the restoration of normal palatal mucosa tissue repair.
In rodents, submandibular salivary glands produce a number of biologically active growth factors. When oral mucosa or other portions of the alimentary tract are damaged, a series of events are rapidly set in motion, including adaptations of the salivary glands through neural, hormonal, and other feedback signaing pathways,16,17 facilitating a release of multiple different growth factors. These salivary-derived growth factors have been implicated in contributing to expedient and efficient wound healing along the alimentary tract. In a murine model, SMG sialoadenectomy has been shown to alter the ability of the liver to regenerate after partial hepatectomy, by transiently increasing the apoptotic hepatocyte death.18 In a different study, Jones et al.19 reported that in a rat partial hepatectomy model, SMG sialoadenectomy prevents the proliferative response and regeneration in the remaining liver. Hepatic tissue repair was restored to levels characteristic of regeneration by administration of exogenous epidermal growth factor. Further, Warner et al. demonstrated that the pattern of salivary EGF levels over the first 2 weeks of life was significantly related to development of necrotizing enterocolitis in very low birthweight infants.20 Taken together with our current study, these findings suggest a significant role for salivary growth factors in gastrointestinal repair. The majority of studies of salivary growth factors and their effects in the gastrointestinal tract have focused on EGF.21–23 However, studies on other salivary growth factors, including VEGF, are limited. Our study shows the importance of salivary growth factors in alimentary tract repair, and the specific importance of salivary-derived VEGF, supported by: (1) selective inhibition of VEGF resulting in impaired palatal mucosal wound healing; (2) supplementation of exogenous VEGF alone corrects the wound healing phenotype after SMG sialoadenectomy; and (3) a previously reported role for salivary VEGF in bowel adaptation and bowel growth following small bowel resection (SBR).9 Our previous work showed that while VEGF increases the adaptation parameters (villus height, crypt depth), the major effect of VEGF on bowel adaptation following SBR is mediated through its angiogenic effects (on submucosal capillary density and vessel-to-villus area ratio). In comparison, in the current study, we used the oral mucosal wound model and demonstrated that VEGF significantly improves wound reepithelialization as well as restores the neovascularization of the repair tissue. Similar to cutaneous wound healing research,24,25 our findings suggest novel non-angiogenic effects of VEGF on normalizing mucosal wound repair process, along with its well-established angiogenic role.
VEGF, as a pleiotropic growth factor, plays an essential role in response to tissue injury, which is mediated through several mechanisms, including neovascularization, reepithelialization, and regulation of collagen deposition.25 In addition, angiogenic growth factors, including VEGF, have been shown to increase recruitment of bone marrow-derived endothelial progenitor cells in the wounds, which may contribute to enhanced wound healing.26,27 Recent data have shown that VEGF is expressed by subsets of neurons, coincident with angiogenesis within the developing cerebral cortex and may serve both paracrine and autocrine functions in the developing central nervous system.28 In addition, salivary-derived VEGF has also been shown to be important in homeostasis, defense from infection, and regenerative mechanisms of the oral mucosa as well as other regions of the alimentary tract.29–32 The functions of VEGF are facilitated through binding to its primary receptors. Although VEGF binds to VEGFR1 (Flt-1), VEGFR2 (Flk-1), neuropilins Nrp-1 and Nrp-2, its main signaling receptor in the endothelium is VEGFR2. VEGFR1 is also expressed by endothelial cells; however, it is believed to act primarily to modulate VEGFR2 signaling.33 Studies in dermal wounds have provided insight into the role of VEGF and its primary receptors. In cutaneous wounds, selective neutralization of VEGF or inhibition of its receptors was associated with wound healing defects.34 Similarly, an important role for VEGF and its receptors has also been shown in disorders of the gastrointestinal tract. In gastrointestinal repair, the receptors facilitate angiogenesis and reepithelialization, as well as the regulation of the epithelial barrier function. In an acute colitis model, inhibition of VEGF worsened inflammation with disruption of healing and exacerbation of injury.35 In other disorders of the gastrointestinal tract, such as in inflammatory bowel disease, a critical role for the balance between the VEGF and its receptor levels has been implicated for successful healing.36,37 Similar to our findings, Ogunshola et al.28 reported that as levels of VEGF increase, the tyrosine phosphorylation and activation of the Flk-1 receptors increase, and vice versa, when agents that sequester VEGF signaling are present in their neuronal cultures, the Flk-1 activation was diminished.
The palatal wound model has been previously described by Bodner et al.38 to provide an opportune environment for delineating the role of saliva as a modulator of mucosal healing. Palatal mucosal wounds may serve as a surrogate marker of gastrointestinal mucosal wound healing and is technically easier to perform compared with other models of the remaining gastrointestinal tract which require laparotomy and mucosal injury.9 Palatal wounds overlying the hard palate were chosen for these experiments as the palatal mucosa is firmly attached to underlying tissues minimizing contraction of the wound edges and permitting the wound to heal by secondary intention. Another advantage of our model is the directed delivery of the vector Ad-VEGF-Trap into the SMG. Our data showed significant reduction in salivary VEGF levels and impairment in wound healing following the administration of VEGF-Trap. These findings were unlikely due to the adenoviral construct as there were no adverse effects on wound healing in the adenoviral control group. Several studies have shown that VEGF-Trap protein selectively blocks VEGF with high affinity.39–41 Our findings of impairment in palatal mucosal wound healing following the administration of VEGF-Trap may be likely due to the depletion of VEGF and not other growth factors. In our experimental design, oral VEGF supplementation was not performed in animals that had VEGF blocked using VEGF-Trap as VEGF-Trap may sequester endogenous as well as supplemented VEGF, confounding restoration of the growth factor.
In summary, our results show a novel role for salivary-VEGF in wound healing of the palate mucosal. The murine palate wound model can facilitate the study of the underlying mechanisms of the vulnerary effects of salivary growth factors not only in the oral cavity but on other alimentary tissue repair sites. Finally, these data provide a basis for the development of novel therapeutics aimed at augmenting wound repair in the oral cavity and potentially other wounds of the alimentary tract.
Acknowledgments
We thank Datis Alaee, Chuck Klanke, and Stephanie Lang for technical help with vector construct, histology, and immunohistochemistry. The generous gift of the Ad-VEGF-Trap construct from John Rudge, PhD, Regeneron is gratefully acknowledged. Current affiliations of authors: Seiichi Yamano is at New York University, College of Dentistry, Prosthodontics, New York, NY 10010; Timothy M. Crombleholme is at Center for Children’s Surgery, Children’s Hospital Colorado and the University of Colorado School of Medicine, Aurora, CO, USA.
Source of Funding: This research was supported by grants from The National Institute of Diabetes and Digestive and Kidney Diseases R01-DK59242, R01-DK074055, and R01-DK072446, Juvenile Diabetes Research Foundation, and American Diabetes Association to T.M. Crombleholme, and National Institutes of General Medical Sciences K08 GM098831-01 and the Wound Healing Society Foundation 3M Award to S.G. Keswani.
Footnotes
Conflicts of Interest: No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Angelov N, Moutsopoulos N, Jeong MJ, Nares S, Ashcroft G, Wahl SM. Aberrant mucosal wound repair in the absence of secretory leukocyte protease inhibitor. Thromb Haemost. 2004;92:288–97. doi: 10.1160/TH03-07-0446. [DOI] [PubMed] [Google Scholar]
- 2.Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res. 2003;82:621–6. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- 3.Mandel ID. The functions of saliva. J Dent Res. 1987;66 (Spec):623–7. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- 4.Zelles T, Purushotham KR, Macauley SP, Oxford GE, Humphreys-Beher MG. Saliva and growth factors: the fountain of youth resides in us all. J Dent Res. 1995;74:1826–32. doi: 10.1177/00220345950740120301. [DOI] [PubMed] [Google Scholar]
- 5.Cho MA, Ko JY, Kim YK, Kho HS. Salivary flow rate and clinical characteristics of patients with xerostomia according to its aetiology. J Oral Rehabil. 2010;37:185–93. doi: 10.1111/j.1365-2842.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 6.Barka T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem. 1980;28:836–59. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- 7.Szpaderska AM, Walsh CG, Steinberg MJ, DiPietro LA. Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res. 2005;84:309–14. doi: 10.1177/154405910508400403. [DOI] [PubMed] [Google Scholar]
- 8.Schrementi ME, Ferreira AM, Zender C, DiPietro LA. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen. 2008;16:80–6. doi: 10.1111/j.1524-475X.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 9.Parvadia JK, Keswani SG, Vaikunth S, Maldonado AR, Marwan A, Stehr W, et al. Role of VEGF in small bowel adaptation after resection: the adaptive response is angiogenesis dependent. Am J Physiol Gastrointest Liver Physiol. 2007;293:G591–8. doi: 10.1152/ajpgi.00572.2006. [DOI] [PubMed] [Google Scholar]
- 10.Booth V, Young S, Cruchley A, Taichman NS, Paleolog E. Vascular endothelial growth factor in human periodontal disease. J Periodontal Res. 1998;33:491–9. doi: 10.1111/j.1600-0765.1998.tb02349.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Takahashi T. Effect of salivarectomy on the growth of gastrointestinal mucosa and urinary secretion of epidermal growth factor in rats. Gastroenterol Jpn. 1989;24:626–31. doi: 10.1007/BF02774160. [DOI] [PubMed] [Google Scholar]
- 12.Helmrath MA, Shin CE, Fox JW, Erwin CR, Warner BW. Adaptation after small bowel resection is attenuated by sialoadenectomy: the role for endogenous epidermal growth factor. Surgery. 1998;124:848–54. [PubMed] [Google Scholar]
- 13.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Baum BJ, Yamano S, Mankani MH, Sun D, Jonsson M, et al. Adenoviral-mediated gene transfer to mouse salivary glands. J Dent Res. 2000;79:701–8. doi: 10.1177/00220345000790020201. [DOI] [PubMed] [Google Scholar]
- 15.Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, et al. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004;12:497–504. doi: 10.1111/j.1067-1927.2004.12501.x. [DOI] [PubMed] [Google Scholar]
- 16.Brzozowski T, Konturek SJ, Pytko-Polonczyk J, Warzecha Z. Gastric adaptation to stress: role of sensory nerves, salivary glands, and adrenal glands. Scand J Gastroenterol. 1995;30:6–16. doi: 10.3109/00365529509093229. [DOI] [PubMed] [Google Scholar]
- 17.Genaro AM, Stranieri GM, Borda E. Involvement of the endogenous nitric oxide signalling system in bradykinin receptor activation in rat submandibular salivary gland. Arch Oral Biol. 2000;45:723–9. doi: 10.1016/s0003-9969(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 18.Buira I, Poch E, Sanchez O, Fernandez-Varo G, Grau M, Tebar F, et al. Sialoadenectomy alters liver cell turn-over and function in mice. J Cell Physiol. 2004;198:12–21. doi: 10.1002/jcp.10402. [DOI] [PubMed] [Google Scholar]
- 19.Jones DE, Jr, Tran-Patterson R, Cui DM, Davin D, Estell KP, Miller DM. Epidermal growth factor secreted from the salivary gland is necessary for liver regeneration. Am J Physiol. 1995;268:G872–8. doi: 10.1152/ajpgi.1995.268.5.G872. [DOI] [PubMed] [Google Scholar]
- 20.Warner BB, Ryan AL, Seeger K, Leonard AC, Erwin CR, Warner BW. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J Pediatr. 2007;150:358–63. doi: 10.1016/j.jpeds.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 21.Noguchi S, Ohba Y, Oka T. Effect of salivary epidermal growth factor on wound healing of tongue in mice. Am J Physiol. 1991;260 (4 Pt 1):E620–5. doi: 10.1152/ajpendo.1991.260.4.E620. [DOI] [PubMed] [Google Scholar]
- 22.Chaet MS, Arya G, Ziegler MM, Warner BW. Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. J Pediatr Surg. 1994;29:1035–8. doi: 10.1016/0022-3468(94)90274-7. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 23.Lambotte L, Saliez A, Triest S, Maiter D, Baranski A, Barker A, et al. Effect of sialoadenectomy and epidermal growth factor administration on liver regeneration after partial hepatectomy. Hepatology. 1997;25:607–12. doi: 10.1002/hep.510250319. [DOI] [PubMed] [Google Scholar]
- 24.Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275–87. doi: 10.1038/jid.2009.26. [DOI] [PubMed] [Google Scholar]
- 25.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–58. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng X, Szabo S, Chen L, Paunovic B, Khomenko T, Tolstanova G, et al. New cell therapy using bone marrow-derived stem cells/endothelial progenitor cells to accelerate neovascularization in healing of experimental ulcerative colitis. Curr Pharm Des. 2011;17:1643–51. doi: 10.2174/138161211796197007. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ, Kim J, Lee KI, Shin JM, Chae JI, Chung HM. Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy. 2011;13:165–78. doi: 10.3109/14653249.2010.512632. [DOI] [PubMed] [Google Scholar]
- 28.Ogunshola OO, Antic A, Donoghue MJ, Fan SY, Kim H, Stewart WB, et al. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem. 2002;277:11410–5. doi: 10.1074/jbc.M111085200. [DOI] [PubMed] [Google Scholar]
- 29.Taichman NS, Cruchley AT, Fletcher LM, Hagi-Pavli EP, Paleolog EM, Abrams WR, et al. Vascular endothelial growth factor in normal human salivary glands and saliva: a possible role in the maintenance of mucosal homeostasis. Lab Invest. 1998;78:869–75. [PubMed] [Google Scholar]
- 30.Pammer J, Weninger W, Mildner M, Burian M, Wojta J, Tschachler E. Vascular endothelial growth factor is constitutively expressed in normal human salivary glands and is secreted in the saliva of healthy individuals. J Pathol. 1998;186:186–91. doi: 10.1002/(SICI)1096-9896(1998100)186:2<186::AID-PATH148>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Brozovic S, Vucicevic-Boras V, Mravak-Stipetic M, Jukic S, Kleinheinz J, Lukac J. Salivary levels of vascular endothelial growth factor (VEGF) in recurrent aphthous ulceration. J Oral Pathol Med. 2002;31:106–8. doi: 10.1034/j.1600-0714.2002.310208.x. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki N, Takahashi S, Okabe S. Relationship between vascular endothelial growth factor and angiogenesis in spontaneous and indomethacin-delayed healing of acetic acid-induced gastric ulcers in rats. J Physiol Pharmacol. 1998;49:515–27. [PubMed] [Google Scholar]
- 33.Maharaj AS, D’Amore PA. Roles for VEGF in the adult. Microvasc Res. 2007;74:100–13. doi: 10.1016/j.mvr.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 35.Chernoguz A, Crawford K, Donovan E, Vandersall A, Berglund C, Cripe TP, et al. EGFR inhibition fails to suppress vascular proliferation and tumor growth in a Ewing’s sarcoma model. J Surg Res. 2012;173:1–9. doi: 10.1016/j.jss.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 36.Kemik O, Kemik SA, Purysa S, Tuzun S. Imbalance of VEGF family serum levels and receptors in patients with inflammatory bowel disease. Bratisl Lek Listy. 2010;111:439–42. [PubMed] [Google Scholar]
- 37.Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585–95. e5. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 38.Bodner L, Dayan D, Pinto Y, Hammel I. Characteristics of palatal wound healing in desalivated rats. Arch Oral Biol. 1993;38:17–21. doi: 10.1016/0003-9969(93)90149-g. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Liang XH, Ferrara N. Comparing protein VEGF inhibitors: in vitro biological studies. Biochem Biophys Res Commun. 2011;408:276–81. doi: 10.1016/j.bbrc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012;87:77–88. doi: 10.1016/j.mayocp.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Munoz A, Perez-Ruiz E, Mendiola Fernandez C, Alba Conejo E, Gonzalez-Martin A. Current status of anti-angiogenic agents in the treatment of ovarian carcinoma. Clin Transl Oncol. 2009;11:589–95. doi: 10.1007/s12094-009-0409-8. [DOI] [PubMed] [Google Scholar]