Abstract
Purpose of review
The mid-gestation fetus is capable of regenerative healing with wound healing indistinguishable from surrounding skin. This review aims to evaluate the current knowledge of how the mid-gestation fetus heals without scar and the implications of these findings in efforts to recapitulate the fetal regenerative phenotype in the postnatal environment.
Recent findings
It has been over 30 years since the empirical observation that the fetus heals without scar; yet, the underlying mechanisms of this phenomenon have not been elucidated. Fetal wound healing is characterized by a distinct growth factor profile, an attenuated inflammatory response with an anti-inflammatory cytokine profile, an extracellular matrix rich in type III collagen and hyaluronan, attenuated biomechanical stress, and a potential role for stem cells. Current therapies to minimize scarring in postnatal wounds have attempted to recapitulate singular aspects of the fetal regenerative phenotype and have met with varying degrees of clinical success. We now have the molecular tools to more completely comprehend the fundamental mechanisms of fetal regenerative wound repair, which has the potential to provide insights into the identification of therapeutic targets to minimize the scar formation.
Summary
Successful therapies that help minimize postnatal scar formation can be realized through understanding the cellular and molecular mechanisms of fetal regenerative wound healing. These insights will have implications not only for cutaneous wound healing, but also potentially for any disease process characterized by excessive fibroplasia.
Keywords: fetal wound healing, hyaluronan, interleukin-10, scar formation
INTRODUCTION
The economic and social impact of scarring is significant. Each year over 100 million patients acquire scars, some of which cause considerable functional or psychosocial morbidity [1, 2]. Additionally, there are an estimated 11 million keloid scars and 4 million burn scars, 70% of which occur in children [3]. An effective therapy resulting in scarless wound healing would have an extraordinary impact across many fields of medicine.
Wound healing is a complex process that normally occurs in the postnatal setting through the formation of scar tissue, with regenerative healing limited to the liver and bone [4]. In contrast, the fetus is known to heal cutaneous wounds without a scar by regeneration of normal dermal architecture including restoration of dermal appendages and neurovasculature [5, 6▪]. This regenerative capability occurs in the mid-gestational period and has been demonstrated in all mammalian species studied to date, including humans, mice, rats, sheep, and monkeys. In each fetal model, this period of regenerative healing is followed by a transition period in which wounds heal with an extracellular matrix (ECM) indistinguishable from unwounded tissue, but fail to regenerate its dermal appendages [7]. Near the end of gestation, there is a progression to the postnatal phenotype with wounds healing with an excess of collagen in the ECM, a loss of dermal appendages, and a flattened epidermis in what we classify as a scar.
Early studies in the biology of fetal wound healing have focused on the intrauterine wound environment. The sterile, nutrient-rich amniotic fluid, which continuously bathes the fetal skin, was believed to have an important role in fetal wound phenotype. A number of animal studies suggest that fetal regenerative repair is independent of the intrauterine environment. For example, wounds in the marsupial model, in absence of amniotic fluid, heal without scar formation during the period of development within the maternal pouch [8]. Furthermore, studies in wounds created in human mid-gestation fetal skin following xeno-grafting to immunocompromised adult mice showed scarless wound healing [9]. Conversely, incisional wounds in adult sheep skin engrafted to fetal lambs and returned to the in-utero environment were noted to heal with scar formation [10]. These findings suggest an intrinsic property of fetal skin that is permissive of scarless wound healing and independent of the in-utero environment.
A more complete understanding of the fundamental mechanisms of fetal wound healing will identify the therapeutic targets with the capacity to minimize scar formation. This goal has obvious implications for cutaneous wound healing, but also has potential for any disease characterized by increased fibroplasia, such as intra-abdominal adhesions, keloids, scleroderma, pulmonary and renal fibrosis, and hepatic cirrhosis. Current studies have focused on the cellular and molecular aspects of fetal wound healing to elucidate the mechanisms of fetal regenerative wound healing and can be divided into five major categories: growth factors; inflammatory response and cytokines; ECM; mechanical stress; and stem cells (Table 1). The purpose of this review is to describe the biologic basis of the fetal phenotype in order to apply these physiologic principles to recapitulate the scarless wound healing phenotype in postnatal tissue repair.
Table 1.
Comparison of fetal regenerative wound healing profile with postnatal wound healing
| Fetal | Postnatal | |
|---|---|---|
| Phenotype | Regenerative | Scar formation |
| Growth factors | ||
| bFGF | Lower | Higher |
| PDGF | Lower | Higher |
| VEGF | Higher | Lower |
| TGF-β | ||
| TGF-β1 | Low levels | High levels |
| TGF-β2 | Low levels | High levels |
| TGF-β3 | High levels | Low levels |
| Inflammatory response | ||
| Inflammatory cell | Minimal | High levels leukocytes, macrophages, mast cells infiltrate |
| Cytokines | ||
| Proinflammatory: IL-6, IL-8 | Low levels | High levels |
| Anti-inflammatory: IL-10 | High levels | Low levels |
| Extracellular matrix | ||
| Collagen | ||
| Histology | Fine, reticular weave | Thick, rope-like bundles |
| Type III collagen | High levels | Low levels |
| Deposition | Immediate | Delayed |
| Cross-linking | Low levels | High levels |
| TGF-β1-stimulated deposition | Absent | Present |
| Hyaluronan | ||
| Expression | High levels | Low levels |
| Persistent expression | Transient expression | |
| Molecular weight | High | Low |
| HA receptors (fibroblast) | High levels | Low levels |
| Mechanical force | ||
| Myofibroblast (day 14) | Absent | Present |
| Stem cells | ||
| MSC | High levels | Lower levels |
| Dot cells | Present | Absent |
bFGF, basic fibroblast growth factor; HA, hyaluronan; MSC, mesenchymal stem cell; PDGF, platelet-derived growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
GROWTH FACTORS
A variety of growth factor families are believed to play a role in fetal wound healing. Much of this research has been centered on transforming growth factor (TGF)-β and its three mammalian isoforms (TGF-β1, TGF-β2, and TGF-β3). TGF-β is secreted by many cell types involved in tissue repair [11]. In adult wounds, there is a relative increase in the expression of TGF-β1 and TGF-β2 compared with TGF-β3. In contrast, fetal wounds express more TGF-β3 and decreased levels of TGF-β1 and TGF-β2. Pathologic hypertrophic scars in adults have been noted to have even higher levels of TGF-β1. Further, functional inhibition of TGF-β1 in adult wounds significantly reduces scarring. Conversely, addition of recombinant TGF-β1 to mid-gestation fetal wounds results in the formation of scar tissue [12]. These data suggest low levels of TGF-β1 are associated with decreased scarring. Additional studies have suggested that, beyond the relative paucity of TGF-β1, elevated levels of TGF-β3 and a higher ratio of TGF-β3 to TGF-β1 may also be critical to decreasing scar formation [13]. Studies in postnatal wounds have demonstrated reduced scarring with addition of recombinant TGF-β3 and increased scarring with attenuation of TGF-β3 levels [14]. In addition, treatment of adult fibroblasts with TGF-β3 has demonstrated increased migration, a characteristic of fetal fibroblasts [15].
These promising studies in animal models prompted the development of Avotermin, human recombinant TGF-β3, as a potential antiscarring therapy. Avotermin was evaluated in multiple randomized, double-blinded, placebo-controlled, phase II clinical trials with administration immediately prior to and 24h following wounding. It was found to improve scar appearance compared with placebo [16▪▪]. Unfortunately, phase III trials of Avotermin were terminated as the therapy failed to meet its primary endpoint of decreasing scar formation [17]. Interestingly, the recombinant TGF-β3 was administered in the immediate perioperative period, with effects on scarring noted months later. This highlights a potential critical early window in postnatal wounds that may be amenable to manipulation that will provide a permissive environment for scarless wound healing to proceed.
Another TGF-β inhibitor, mannose-6-phosphate (M6P), has also been evaluated as a scar-reducing therapy. Phase II trials have demonstrated accelerated cutaneous wound closure and decreased erythema [18]. Evaluation of M6P as a scar-reducing therapy in applications beyond cutaneous wound repair, such as following nerve repair, is currently being evaluated [19].
INFLAMMATORY RESPONSE AND CYTOKINES
A hallmark of the fetal regenerative phenotype is an attenuated inflammatory response. This is observed in decreased inflammatory cellular infiltrate, as well as a characteristic expression of cytokines, the mediators of the inflammatory response.
Inflammatory cells
The infiltration of neutrophils which characterizes the early postnatal wound is greatly diminished in fetal wounds [5]. The number of tissue macrophages in the subsequent stages of wound healing is also decreased. However, it has been shown that these fetal neutrophils and macrophages respond to appropriate signals in vitro. These data suggest that the attenuated inflammatory response is a result of decreased recruitment and not an intrinsic deficiency of the cells [20]. In addition, fetal platelets are shown to aggregate poorly and produce lower levels of profibrotic growth factors, such as TGF-β1 and platelet-derived growth factor (PDGF) [21]. Recent studies have demonstrated a paucity of mast cells in the fetal scarless phenotype. Mast cells in mid-gestation fetal wounds are less mature, decreased in number, and fail to degranulate in response to injury compared with scar forming wounds in late gestation [22].
Cytokines
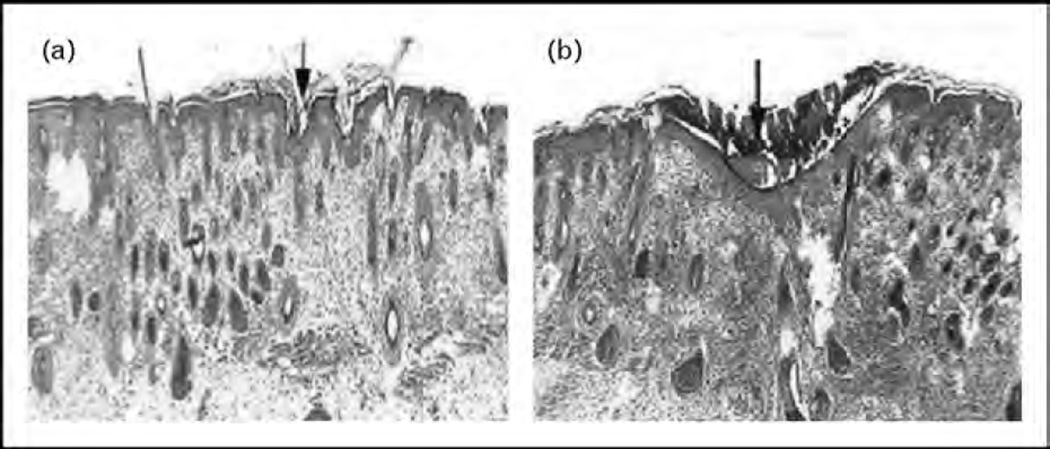
The attenuated inflammatory response observed in fetal wound repair suggests that cytokines, which regulate inflammation, may have a characteristic profile associated with the scarless phenotype. Production of proinflammatory cytokines such as interleukin-6 (IL-6) and interleukin-8 (IL-8) are decreased in fetal skin compared with postnatal skin [23, 24]. Additionally, fetal fibroblasts stimulated by lipopolysaccharide or PDGF have an attenuated IL-6 and IL-8 response compared with adult fibroblasts [25]. This relative paucity of proinflammatory cytokines led to the investigation of the role of the anti-inflammatory cytokine interleukin-10 (IL-10) in fetal wound healing. IL-10 has been shown to deactivate macrophages and neutrophils, as well as diminishing the production of proinflammatory cytokines. Early gestation fetal skin, serum, and amniotic fluid express high levels of IL-10, whereas neonatal skin demonstrates minimal IL-10 expression [26]. Studies in mid-gestation fetal wounds created in transgenic IL-10 knockout mice have elevated levels of IL-6 and IL-8, and heal with a scar at a gestational age which normally heals scarlessly (Fig. 1) [27].
FIGURE 1.
Trichrome staining of normal (a) and IL-10 knockout (b) fetal mouse skin 7 days following incisional wound. Normal fetal skin results in minimal cellular infiltrate, normal distribution of dermal appendages, and reconstitution of reticular collagen deposition. In contrast, IL-10 knockout fetal mouse skin grafts from mice of the same gestational age demonstrate increased, dense collagen deposition (blue staining) with loss of dermal appendages consistent with scar formation. Site of incisional wound is indicated by the arrow. Data from [23]. IL-10, interleukin-10.
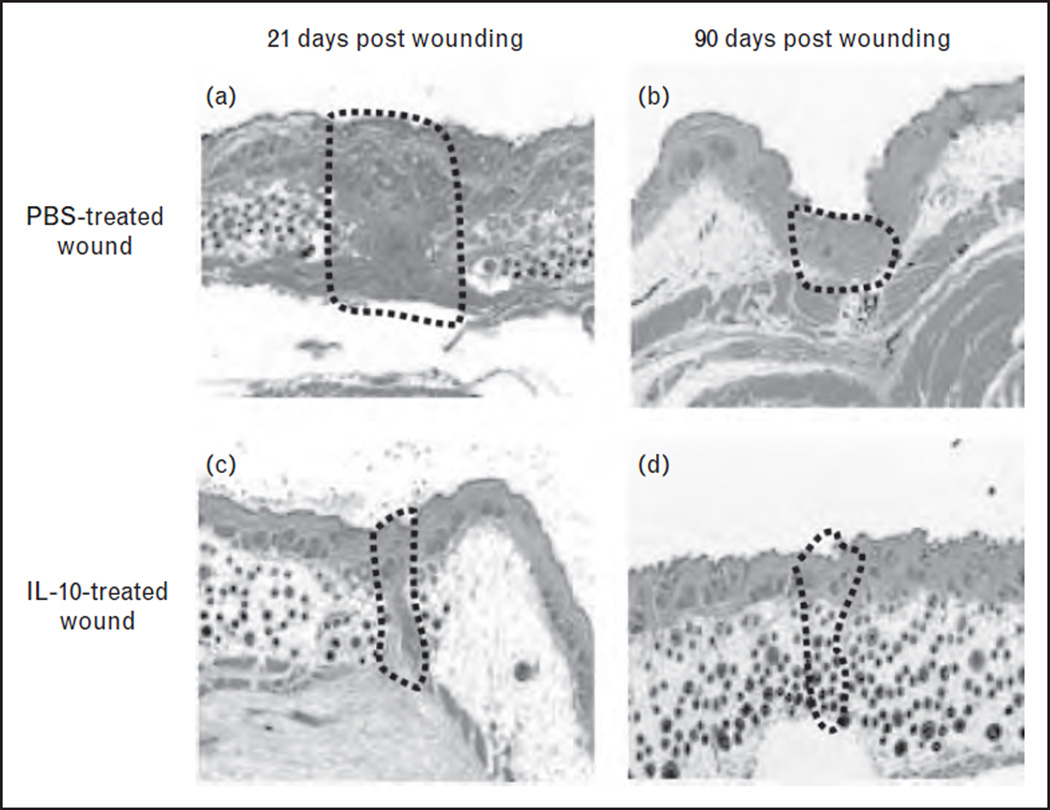
Most compellingly, independent laboratories have demonstrated that overexpression of IL-10 using viral vectors results in a dose-dependent recapitulation of fetal regenerative wound healing (Fig. 2) [25, 26]. This includes a decreased inflammatory cellular infiltrate, a characteristic fetal pattern of matrix deposition, a regeneration of dermal appendages, and overall wound healing that is indistinguishable from the surrounding skin. Wound repair in IL-10-treated postnatal incisional wounds occurred with restoration of normal skin integrity by biomechanical testing, which suggests that application of IL-10 may have benefits long after the vector and transgene are cleared from the wound[26]. These results suggest that an early expression of IL-10 initiates a cascade of events which results in the scarless wound healing phenotype. Further, these data support a cytokine hypothesis in which the shift toward increased anti-inflammatory cytokine expression relative to corresponding proinflammatory cytokine expression is permissive of the recapitulation of the fetal regenerative tissue repair.
FIGURE 2.
Adenoviral-mediated overexpression of IL-10 results in minimal scar formation. At day 21, phosphate buffer solution (PBS)-treated skin shows a well-demarcated scar with dense, haphazardly arranged collagen fibers with a loss of dermal appendages (a). In contrast, in wounds treated with 1 × 109 plaque forming units Ad-IL-10, no well defined scar is seen, collagen is organized in a fine reticular pattern, and there is a normal distribution of dermal appendages (c). At day 90, the PBS-treated group shows a remodeled scar that is well organized and flattened with the dropout of dermal appendages (b). Ad-IL-10-treated wounds exhibit a reconstitution of a normal dermal reticular pattern of collagen deposition and dermal elements including hair follicles, with the wound being indistinguishable from surrounding unwounded skin (d, 5×).
These promising findings have led to the development of IL-10 as a potential therapeutic antiscarring agent, Prevascar, human recombinant IL-10. Following intradermal injection immediately preceding incision and an additional injection 24 h later, Prevascar was found to improve scar formation at 12 months in a randomized, double-blinded, placebo-controlled trial [28]. Further Prevascar trials are ongoing.
EXTRACELLULAR MATRIX
The characteristic type III collagen and hyaluronan-rich ECM are key components of the fetal scarless wound healing phenotype. This distinct ECM facilitates the migration of cellular components and also mediates the wound repair process via the interaction of cell-surface receptors.
Collagen
Both fetal and adult wounds heal with collagen deposition. In fetal wounds, collagen is deposited in a fine reticular pattern indistinguishable from the surrounding uninjured tissue. In postnatal wounds, collagen is deposited in densely arranged parallel bundles [29]. Although all wounds heal with predominantly type I collagen, there is a greater ratio of type III collagen to type I collagen deposited in fetal wounds compared with postnatal wounds. Type III collagen fibers are smaller compared with type I fibers and may allow a more reticular deposition of fibers in the wound [30, 31]. Also, type I collagen is found in more rigid tissues, whereas type III is found in more elastic tissue. This association with decreased amount of cross-linked collagen fibers may lead to a more flexible wound which may contribute to scarless repair. Additionally, in response to profibrotic growth factors such as TGF-β1, fetal fibroblasts have a characteristic response in collagen deposition compared with postnatal fibroblasts. This suggests fetal fibroblasts are programmed to form a more regenerative ECM [15].
Multiple efforts to minimize scarring have been made through the manipulation of collagen. Therapeutic efforts have been focused on inhibiting cross-linking in order to render the wound more susceptible to remodeling [32]. Unfortunately, these agents have been clinically ineffective thus far. There are, however, some reports of small molecular inhibitors of prolyl hydroxylase that may have therapeutic value but raise concern over skin integrity once healing is complete [33].
Hyaluronan
Fetal wounds have an increased level and prolonged elevation of high molecular weight (HMW) hyaluronan. Levels of HMW hyaluronan are increased in the fetus. Fetal tissues have increased levels for up to 3 weeks following injury, whereas in adult wounds hyaluronan levels are only transiently elevated and low molecular weight hyaluronan predominates [7, 34]. Hyaluronan also upregulates both TGF-β3 and type III collagen. Additionally, fetal fibroblasts have two-fold to four-fold greater hyaluronan-receptor expression, suggesting hyaluronan helps facilitate more rapid fibroblast migration [35]. Hyaluronan is also shown to suppress platelet aggregation and their release of growth factors in a dose-dependent fashion [36]. In a murine fetal organ culture model, the addition of hyaluronan to the late gestation wound resulted in the recapitulation of the scarless phenotype of mid-gestation [37]. In the past, hyaluronan has been considered to be a passive structural component; however, over the last decade it has been suggested that hyaluronan may have a significant role in wound healing by stimulating cell migration, differentiation, and proliferation in addition to its role as an integral component of the ECM [38].
Multiple applications of hyaluronan have been developed to minimize scarring. A hyaluronan-rich film, Seprafilm, has been developed to decrease intra-abdominal scarring and has been somewhat effective in decreasing intra-abdominal adhesions [39]. Topical applications of HMW hyaluronan have also been investigated with promising results [40].
BIOMECHANICAL FORCES
Not all mid-gestation fetal wounds heal without a scar. In contrast to small incisional wounds, larger excisional wounds heal with a characteristic scar at gestational ages that heal smaller wounds scarlessly. This suggests that biomechanical forces may be in part an underlying mechanism of the fetal regenerative phenotype [41]. A part of this explanation may come from the myofibroblast, a specialized cell that is known to produce profibrotic growth factors and promote rapid wound closure. The larger, scar-forming fetal wound may have increased shear forces and mechanical stress. Increased shear force results in mechanotransduction, which induces local production of profibrotic growth factors, such as TGF-β1 and TGF-β2 [42]. The mechanical stress induces the fibroblasts within the wound bed to differentiate into myofibroblasts, acquiring contractile properties through the expression of alpha-smooth muscle actin. Myofibroblasts are present in most fetal wounds, as early as day 1 following injury. In wounds that heal scarlessly, they are notably absent at day 14. In contrast, in wounds of the same gestational age that heal with a scar, myofibroblasts are present in abundance [41]. Further, mechanical forces affect scar formation through the regulation of cell–matrix interactions via intracellular focal adhesion components, such as focal adhesion kinase (FAK). In a recent study, FAK inhibition resulted in successful attenuation of inflammatory cell recruitment and decreased resulting scar formation [43▪].
The implications of decreasing transduction of profibrogenic mechanical stress are readily translated to postnatal wounds in the effort to minimalize scarring. A recent study demonstrated the value of offloading strain from the wound using a silicone mechanomodulating device applied to the peri-wound area, which resulted in significant decrease in fibrosis. Recent reports of phase I trials in patients using this strategy following abdominoplasty procedures have shown promise in ameliorating scar formation [44].
STEM CELLS
Multiple pluripotent stem cells have been proposed to play a role in fetal wound healing, including epithelial stem cells (ESCs), mesenchymal stem cells (MSCs), and ‘small dot’ cells. ESCs are interspersed throughout the basal layers. The slowly proliferating ESCs are surrounded by more quickly proliferating basal cells and their suprabasal progeny to form epidermal proliferative units. ESCs are also found within the bulge area of hair follicles. The stem cells within the bulge area migrate to the epidermis following injury and proceed to differentiate into dermal, vascular, and neural components [45]. MSCs have been shown to have a role in regenerative healing, including immunomodulation, anti-fibrosis, antiapoptosis, and angiogenesis [46▪▪]. Recent studies have shown MSCs to be a potent gatekeeper in preventing excessive inflammation. They have demonstrated the ability to immunoregulate through multiple independent pathways, including the induction of IL-10 secretion by macrophages [47▪]. Further evaluation of MSCs and scarless wound healing continues, with the need for more complete characterization of the cell population. ‘Small dot’ cells have also been identified to play a role in fetal wound healing. There is a 20-fold greater increase of these cells in fetal blood than postnatal blood. A recent study transplanting fluorescence-labeled ‘small dot’ cells in a postnatal murine incisional wound model showed migration of these cells to the wound bed with a decrease in scarring [48, 49▪▪]. Further study will help to elucidate the importance of these stem cell populations.
CONCLUSION
Wound healing is a complex process, requiring an orchestrated response of growth factors, cytokines, ECM components, modulation of biomechanical stress, and stem cells. The components in fetal regenerative healing are tightly regulated, favoring a pro-migratory, anti-inflammatory milieu in a characteristic ECM. The evidence suggests that there may be an early critical window in postnatal wound healing that may be amenable to manipulation to provide a permissive environment for scarless wound healing to proceed. The implications for scarless healing go far beyond cutaneous wound healing, with nearly every field of medicine affected by excessive scarring. Further research on the molecular mechanisms of fetal scarless wound healing has the potential to have therapeutic benefit for a wide array of diseases.
KEY POINTS.
The fetal regenerative phenotype is characterized by a characteristic growth factor profile, anti-inflammatory response, extracellular matrix, and distinct biomechanical forces.
Recent studies suggest an early critical window in postnatal wound healing that may be amenable to manipulation to provide a permissive environment for scarless wound healing to proceed.
Further studies of pluripotent stem cells are needed to better understand their physiologic role and therapeutic potential.
Understanding the underlying mechanism of fetal regenerative healing has the potential to lead to novel therapies to minimize scarring in any pathology characterized by excessive fibroplasia.
Acknowledgements
This study was supported in part by the grants from the National Institutes of Health (1K08GM098831) and the Wound Healing Society Foundation (SGK).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 433).
- 1.Brown BC, McKenna SP, Solomon M, et al. The patient-reported impact of scars measure: development and validation. Plast Reconstr Surg. 2010;125:1439–1449. doi: 10.1097/PRS.0b013e3181d4fd89. [DOI] [PubMed] [Google Scholar]
- 2.Islam S, Ahmed M, Walton GM, et al. The association between depression and anxiety disorders following facial trauma - a comparative study. Injury. 2010;41:92–96. doi: 10.1016/j.injury.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ. 2003;326:88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adzick NS, Harrison MR, Glick PL, et al. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985;20:315–319. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- 6. Henderson J, Terenghi G, Ferguson MW. The reinnervation and revascularisation pattern of scarless murine fetal wounds. J Anat. 2011;218:660–667. doi: 10.1111/j.1469-7580.2011.01366.x. This study further evaluates the fetal wound healing phenotype characterizing the regeneration of neurovasculature beyond the previously known dermal appendages and ECM.
- 7.Longaker MT, Whitby DJ, Adzick NS, et al. Studies in fetal wound healing. VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–68. doi: 10.1016/s0022-3468(05)80165-4. discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong JR, Ferguson MW. Ontogeny of the skin and the transition from scar-free to scarring phenotype during wound healing in the pouch young of a marsupial, Monodelphis domestica. Dev Biol. 1995;169:242–260. doi: 10.1006/dbio.1995.1141. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz HP, Longaker MT, Perkocha LA, et al. Scarless wound repair: a human fetal skin model. Development. 1992;114:253–259. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]
- 10.Longaker MT, Whitby DJ, Ferguson MW, et al. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg. 1994;219:65–72. doi: 10.1097/00000658-199401000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991;147:207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- 12.Lin RY, Sullivan KM, Argenta PA, et al. Exogenous transforming growth factorbeta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg. 1995;222:146–154. doi: 10.1097/00000658-199508000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- 14.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 15.Ellis IR, Schor SL. Differential motogenic and biosynthetic response of fetal and adult skin fibroblasts to TGF-beta isoforms. Cytokine. 1998;10:281–289. doi: 10.1006/cyto.1997.0294. [DOI] [PubMed] [Google Scholar]
- 16. So K, McGrouther DA, Bush JA, et al. Avotermin for scar improvement following scar revision surgery: a randomized, double-blind, within-patient, placebo-controlled, phase II clinical trial. Plast Reconstr Surg. 2011;128:163–172. doi: 10.1097/PRS.0b013e318217429b. This study describes the successful clinical trial of recombinant human IL-10 in efforts to recapitulate the fetal anti-inflammatory cytokine profile. The therapeutic strategy demonstrates the potential to intervene in the permissive window surrounding injury in order to successfully minimize scarring in the long term.
- 17.Renovo. [Accessed 4 January 2012];Juvista. 2012 http://www.renovo.com/en/products/juvista.
- 18.Renovo. [Accessed 4 January 2012];Juvidex. 2012 http://www.renovo.com/en/products/juvidex.
- 19.Ngeow WC, Atkins S, Morgan CR, et al. The effect of mannose-6-phosphate on recovery after sciatic nerve repair. Brain Res. 2011;1394:40–48. doi: 10.1016/j.brainres.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Mast BA, Albanese CT, Kapadia S. Tissue repair in the fetal intestinal tract occurs with adhesions, fibrosis, and neovascularization. Ann Plast Surg. 1998;41:140–144. doi: 10.1097/00000637-199808000-00005. discussion 144–147. [DOI] [PubMed] [Google Scholar]
- 21.Olutoye OO, Alaish SM, Carr ME, et al. Aggregatory characteristics and expression of the collagen adhesion receptor in fetal porcine platelets. J Pediatr Surg. 1995;30:1649–1653. doi: 10.1016/0022-3468(95)90443-3. [DOI] [PubMed] [Google Scholar]
- 22.Wulff BC, Parent AE, Meleski MA, et al. Mast cells contribute to scar formation during fetal wound healing. J Invest Dermatol. 2011;132:458–465. doi: 10.1038/jid.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12:671–676. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- 24.Liechty KW, Crombleholme TM, Cass DL, et al. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77:80–84. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- 25.Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 26.Gordon A, Kozin ED, Keswani SG, et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 2008;16:70–79. doi: 10.1111/j.1524-475X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 27.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–872. doi: 10.1053/jpsu.2000.6868. discussion 872–873. [DOI] [PubMed] [Google Scholar]
- 28.Renovo. [Accessed 4 January 2012];Prevascar. 2012 http://www.renovo.com/en/products/prevascar.
- 29.Cuttle L, Nataatmadja M, Fraser JF, et al. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. 2005;13:198–204. doi: 10.1111/j.1067-1927.2005.130211.x. [DOI] [PubMed] [Google Scholar]
- 30.Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988;187:493–497. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- 31.Burd DA, Longaker MT, Adzick NS, et al. Foetal wound healing in a large animal model: the deposition of collagen is confirmed. Br J Plast Surg. 1990;43:571–577. doi: 10.1016/0007-1226(90)90122-g. [DOI] [PubMed] [Google Scholar]
- 32.Clark RA, McCoy GA, Folkvord JM, McPherson JM. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol. 1997;170:69–80. doi: 10.1002/(SICI)1097-4652(199701)170:1<69::AID-JCP8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Kim I, Mogford JE, Witschi C, et al. Inhibition of prolyl 4-hydroxylase reduces scar hypertrophy in a rabbit model of cutaneous scarring. Wound Repair Regen. 2003;11:368–372. doi: 10.1046/j.1524-475x.2003.11509.x. [DOI] [PubMed] [Google Scholar]
- 34.Krummel TM, Nelson JM, Diegelmann RF, et al. Fetal response to injury in the rabbit. J Pediatr Surg. 1987;22:640–644. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- 35.Alaish SM, Yager D, Diegelmann RF, Cohen IK. Biology of fetal wound healing: hyaluronate receptor expression in fetal fibroblasts. J Pediatr Surg. 1994;29:1040–1043. doi: 10.1016/0022-3468(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 36.Olutoye OO, Barone EJ, Yager DR, et al. Hyaluronic acid inhibits fetal platelet function: implications in scarless healing. J Pediatr Surg. 1997;32:1037–1040. doi: 10.1016/s0022-3468(97)90394-8. [DOI] [PubMed] [Google Scholar]
- 37.Iocono JA, Ehrlich HP, Keefer KA, Krummel TM. Hyaluronan induces scarless repair in mouse limb organ culture. J Pediatr Surg. 1998;33:564–567. doi: 10.1016/s0022-3468(98)90317-7. [DOI] [PubMed] [Google Scholar]
- 38.Mast BA, Diegelmann RF, Krummel TM, Cohen IK. Hyaluronic acid modulates proliferation, collagen and protein synthesis of cultured fetal fibroblasts. Matrix. 1993;13:441–446. doi: 10.1016/s0934-8832(11)80110-1. [DOI] [PubMed] [Google Scholar]
- 39.Van der Wal JB, Iordens GI, Vrijland WW, et al. Adhesion prevention during laparotomy: long-term follow-up of a randomized clinical trial. Ann Surg. 2011;253:1118–1121. doi: 10.1097/SLA.0b013e318217e99c. [DOI] [PubMed] [Google Scholar]
- 40.Manuskiatti W, Maibach HI. Hyaluronic acid and skin: wound healing and aging. Int J Dermatol. 1996;35:539–544. doi: 10.1111/j.1365-4362.1996.tb03650.x. [DOI] [PubMed] [Google Scholar]
- 41.Cass DL, Bullard KM, Sylvester KG, et al. Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg. 1997;32:411–415. doi: 10.1016/s0022-3468(97)90593-5. [DOI] [PubMed] [Google Scholar]
- 42.Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95:1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2011;18:148–152. doi: 10.1038/nm.2574. This study demonstrates the complex interplay of multiple factors, with mechanomodulation affecting the inflammatory profile in the wound healing process.
- 44.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217–225. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 45.Levy V, Lindon C, Zheng Y, et al. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 46. Wang S, Qu X, Zhao RC. Mesenchymal stem cells hold promise for regenerative medicine. Front Med. 2011;5:372–378. doi: 10.1007/s11684-011-0164-4. This review evaluates the therapeutic potential of mesenchymal stem cells, with description of their ability to differentiate to multiple cell types, their ability to secrete various growth factors, and the lack of immunogenicity.
- 47. Prockop DJ, Youn Oh J. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2011;20:14–20. doi: 10.1038/mt.2011.211. This study evaluates the multiple molecular mechanisms mesenchymal stem cells use to modulate the inflammatory response.
- 48.Kong W, Li S, Longaker MT, Lorenz HP. Blood-derived small dot cells reduce scar in wound healing. Exp Cell Res. 2008;314:1529–1539. doi: 10.1016/j.yexcr.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kong W, Li S, Lorenz HP. Germ plasm-like dot cells maintain their wound regenerative function after in vitro expansion. Clin Exp Pharmacol Physiol. 2010;37:e136–e144. doi: 10.1111/j.1440-1681.2010.05343.x. This study describes the ability of Dot cells to maintain their wound regenerative ability with in-vitro expansion. This observation has great therapeutic potential in the development of clinical applications in the role of Dot cells in regenerative healing.