Abstract
In this paper, a new kind of oil-soluble ultrathin MoS2 nanosheets is prepared through a one-pot process. A superior extreme pressure property, which has not been attained with other nano-additives, is discovered when the nanosheets are used as lubricant additives. The as-synthesized MoS2 nanosheet is only a few atomic layers thick and tens of nanometers wide, and it is surface-modified with oleylamine so it can be well dispersed in oil or lubricant without adscititious dispersants or surfactants. By adding 1 wt% ultrathin MoS2 nanosheets, at the temperature of 120 °C, the highest load liquid paraffin can bear is tremendously improved from less than 50 N to more than 2000 N. Based on the tribological tests and analysis of the wear scar, a lubrication mechanism is proposed. It is believed that the good dispersion and the ultrathin shape of the nanosheets ensure that they can enter the contact area of the opposite sliding surfaces and act like a protective film to prevent direct contact and seizure between them. This work enriches the investigation of ultrathin MoS2 and has potential application in the mechanical industry.
Ultrathin MoS2 has attracted great interest in the past few years due to its application in the fields of catalysis for hydrogen evolution reaction, new generation of semiconductors and high rate lithium battery1,2,3. Moreover, MoS2 is an excellent solid lubricant due to the easy interlayer sliding4,5. Recently, with the development of nanotechnology, MoS2 nanoparticles with various sizes and morphologies were prepared and they are proved to be good lubricant additives6,7,8,9. As is acknowledged, the lubrication property of MoS2 largely depends on its structure10,11. Although many kinds of multilayer MoS2 nanoparticles have been investigated, there have been only a few studies of the tribological property of ultrathin MoS2 nanosheets with single or few layers. However, it is a challenge to prepare appropriate ultrathin MoS2 nanosheets for use as lubricant additives, because ultrathin MoS2 nanosheets synthesized with existing methods have relatively large size and poor dispersion in lubricants12,13,14,15. Aiming at this problem, here we report a one-pot wet chemistry route to prepare a new kind of oil-soluble ultrathin MoS2 nanosheets. The as-synthesized sample is analysed with X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), Raman spectroscopy, fourier transform infrared spectroscopy (FTIR) and the technique of dynamic light scattering (DLS). Then the extreme pressure property of the lubricant containing the as-synthesized ultrathin MoS2 nanosheets is evaluated with a load-climbing tribological test. Through the comparision with lubricants containing other nano-additives or organic additives, it is found that the ultrathin MoS2 nanosheets have superior ability of improving the extreme pressure property of the lubricant. The wear scar is analysed with scanning electron microscope (SEM), energy dispersive spectrometer (EDS) and XPS. Finally, a lubrication mechanism is proposed based on the tribological tests and surface analysis.
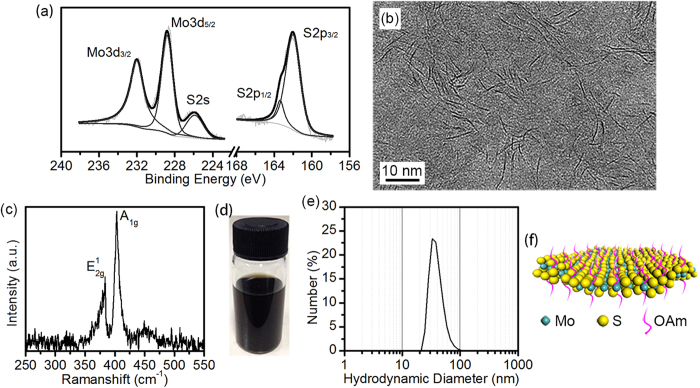
The ultrathin MoS2 nanosheets were prepared as described below (Methods). XPS results, which are shown in Fig. 1a, prove that the as-synthesized material is MoS2 and other compounds such as Mo2S5, MoS3 or MoO3, whose Mo3d peaks have higher binding energies, can be excluded16,17. The UV-vis spectrum of the as-synthesized MoS2 is presented in Supplementary Fig. S118,19. The TEM image is displayed in Fig. 1b and there are many dark lines in the image. It looks very similar with the images obtained by Altavilla et al. and Grossiord et al.15,20 Both of them regarded the dark lines as standing parts of MoS2 layers. The details of the dark line (see Supplementary Fig. S2) and an observation of natural MoS2 through TEM (see Supplementary Fig. S3) both support this conclusion. Given that there is no parallel dark lines observed in the TEM image, one preliminary conclusion is drawn that the as-synthesized MoS2 is of single layer structure. In addition, it can be found through TEM that the as-synthesized MoS2 is in the morphology of nanosheets (see Supplementary Fig. S4). To further confirm the layer number of the as-synthesized MoS2 nanosheets, the sample was analysed with Raman spectroscopy. In the Raman spectrum of MoS2, the relative shift between the two peaks of 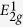 and
and 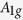 will shrink as the number of MoS2 layers decreases21,22. Although previous related works do not provide a uniform value of the relative peak shift for certain layered MoS2 and some work found that the substrate will slightly affect both the peak shift and the relative peak shift, the difference between few layer and multi layer can be distinguished21,22,23. For the Raman spectrum of the as-synthesized MoS2 nanosheets, as depicted in Fig. 1c, the relative shift between the two peaks is 19.9 ± 0.6 cm−1, which confirms that the as-synthesized MoS2 nanosheets are of ultrathin shape with only single or few layers. Moreover, it was found that the as-synthesized MoS2 nanosheets can be well dispersed in cyclohexane forming stable and black colored translucent solution (see Fig. 1d). Considering the fact that clean or unmodified MoS2 will sink in cyclohexane, it can be deduced that the MoS2 nanosheets have been surface-modified. And the modifier is proved to be oleylamine with the help of XPS and FTIR (see Supplementary Fig. S5)16,24. Moreover, it is believed that the amine groups attach on the MoS2 nanosheets through electrostatic force, because the MoS2 surface is negatively charged (see Supplementary Fig. S6)25,26,27,28, while the amine group is positively charged29. Because of the good dispersibility in cyclohexane, the particle size distribution of the as-synthesized MoS2 nanosheets can be obtained by the technique of DLS after adequate ultrasonic treatment and it is shown in Fig. 1e. It tells that the hydrodynamic diameters of the MoS2 nanosheets are in the range from about 20 nm to 150 nm and concentrated at 37.4 ± 2.0 nm. After all the analysis above, it can be concluded that the MoS2 nanosheets we prepared have only single or few layers with its lateral size at tens of nanometers and its surface is modified with oleylamine, as illustrated in Fig. 1f.
will shrink as the number of MoS2 layers decreases21,22. Although previous related works do not provide a uniform value of the relative peak shift for certain layered MoS2 and some work found that the substrate will slightly affect both the peak shift and the relative peak shift, the difference between few layer and multi layer can be distinguished21,22,23. For the Raman spectrum of the as-synthesized MoS2 nanosheets, as depicted in Fig. 1c, the relative shift between the two peaks is 19.9 ± 0.6 cm−1, which confirms that the as-synthesized MoS2 nanosheets are of ultrathin shape with only single or few layers. Moreover, it was found that the as-synthesized MoS2 nanosheets can be well dispersed in cyclohexane forming stable and black colored translucent solution (see Fig. 1d). Considering the fact that clean or unmodified MoS2 will sink in cyclohexane, it can be deduced that the MoS2 nanosheets have been surface-modified. And the modifier is proved to be oleylamine with the help of XPS and FTIR (see Supplementary Fig. S5)16,24. Moreover, it is believed that the amine groups attach on the MoS2 nanosheets through electrostatic force, because the MoS2 surface is negatively charged (see Supplementary Fig. S6)25,26,27,28, while the amine group is positively charged29. Because of the good dispersibility in cyclohexane, the particle size distribution of the as-synthesized MoS2 nanosheets can be obtained by the technique of DLS after adequate ultrasonic treatment and it is shown in Fig. 1e. It tells that the hydrodynamic diameters of the MoS2 nanosheets are in the range from about 20 nm to 150 nm and concentrated at 37.4 ± 2.0 nm. After all the analysis above, it can be concluded that the MoS2 nanosheets we prepared have only single or few layers with its lateral size at tens of nanometers and its surface is modified with oleylamine, as illustrated in Fig. 1f.
Figure 1. The characterizations and thus speculative structure of the as-synthesized ultrathin MoS2 nanosheets.
(a) The Mo3d XPS spectrum and S2p XPS spectrum of the as-synthesized MoS2 nanosheets with Mo3d3/2 at 232.0 eV, Mo3d5/2 at 228.8 eV, S2s at 226.0 eV, S2p1/2 at 163.3 eV and S2p3/2 at 162.0 eV. (b) The TEM image of the as-synthesized MoS2 nanosheets and each of these dark lines is a standing part of one MoS2 layer. (c) The Raman spectrum of the as-synthesized MoS2 nanosheets with peak 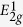 at 383.6 ± 0.4 cm−1 and peak
at 383.6 ± 0.4 cm−1 and peak 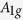 at 403.2 ± 0.5 cm−1. (d) The black colored translucent solution with the as-synthesized MoS2 nanosheets dispersed in cyclohexane. (e) The particle size distribution of the as-synthesized MoS2 nanosheets. (f) The atomic illustration of the as-synthesized MoS2 nanosheet.
at 403.2 ± 0.5 cm−1. (d) The black colored translucent solution with the as-synthesized MoS2 nanosheets dispersed in cyclohexane. (e) The particle size distribution of the as-synthesized MoS2 nanosheets. (f) The atomic illustration of the as-synthesized MoS2 nanosheet.
To investigate the lubrication property of the as-synthesized ultrathin MoS2 nanosheets as lubricant additives, they were dispersed in liquid paraffin (LP), which is a common base oil and whose dynamic viscosity is only 9.94 ± 0.31 cP at 50 °C and 2.28 ± 0.29 cP at 120 °C. A load-climbing test, whose details are described below (Methods), was adopted to fully evaluate the ability of the as-synthesized ultrathin MoS2 nanosheets to enter the contact area under high load. Together with the nanosheets, molybdenum dialkyldithiophosphate (MoDDP), inorganic fullerene-like MoS2 nanoparticles (IF MoS2) and micro-sized MoS2 powder (micro MoS2) were tested for comparision. MoDDP is a commonly used commercial lubricant additive. Under high pressure and high temperature, MoDDP will decompose forming nano-sized multilayer MoS2 (see Supplementary Fig. S7), but the synthesis of MoDDP is rather complex and the cost is high30,31,32. Since there were four different kinds of Mo-contained substance and the weight fraction of Mo in each of these substances are different, the final weight fraction of the element of Mo in the lubricant was controlled at 6 wt‰, meaning that the corresponding weight fraction of MoS2 was 1 wt%, which is a common concentration reported in similar researches9,33,34. Besides those lubricants, oleylamine was tested as well. The test temperature was set at 120 °C, which is very normal in automobile engines. The COF and load as functions of time are displayed in Fig. 2a and the highest load with no seizure of each sample is plotted in Fig. 2b.
Figure 2. The results of the load-climbing tribological tests.
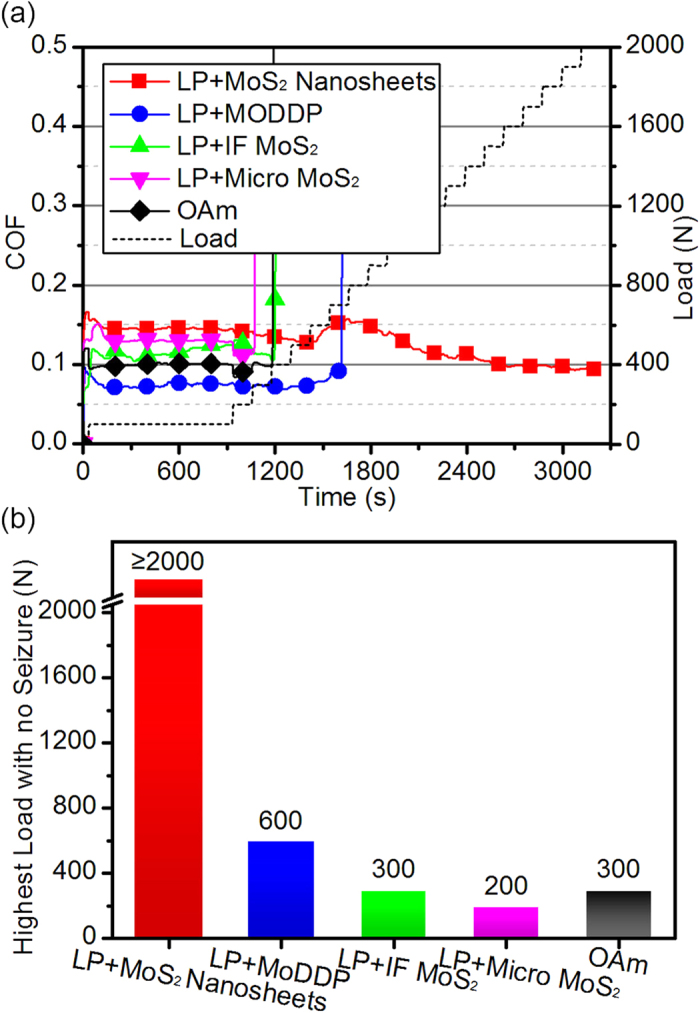
The tested samples include four lubricants, which contain the as-synthesized ultrathin MoS2 nanosheets, MoDDP, IF MoS2 and micro MoS2 respectively, and oleylamine. The weight fraction of element of Mo in each lubricant was controlled at 6 wt‰. (a) COF and load as functions of time of the tests. The COF of repeated tests fluctuates within 0.01. (b) Highest load with no seizure of these lubricants and oleylamine (OAm).
It is quite interesting that, the tribological test with the lubricant containing the as-synthesized ultrathin MoS2 nanosheets did not stop until the load reached 2000 N, which is the highest load the tribotester can provide, which means that the highest load with no seizure of the lubricant is no less than 2000 N. The COF of the lubricant containing MoDDP rose abruptly when the load reached 700 N, indicating that the highest load with no seizure is 600 N. The highest load of the lubricant containing IF MoS2 is only 300 N. The performance of the lubricant containing micro MoS2 is the worst and 200 N is its limit. The lubricant containing the as-synthesized ultrathin MoS2 nanosheets distinguishes itself from other tested lubricants that its highest load with no seizure is at least three times bigger than that of others. Besides, the highest load with no seizure of oleylamine is only 300 N, whichs rule out any possibility that oleylamine is the reason of the superior lubrication property. Furthermore, tribological tests with various Mo weight fraction at different temperatures (see Supplementary Fig. S8) showes that the as-synthesized MoS2 nanosheets performed better than MoDDP under all the tested conditions. In addition, graphene, triphenyl phosphorothionate (TPPT) and zinc dithiophosphate (ZDDP), the later two of which are commonly used organic lubricant additives and are introduced in Supplementary Fig. S9, were evaluated under the same conditions. None of the lubricants containing these additives can bear more than 400 N (see Supplementary Fig. S10).
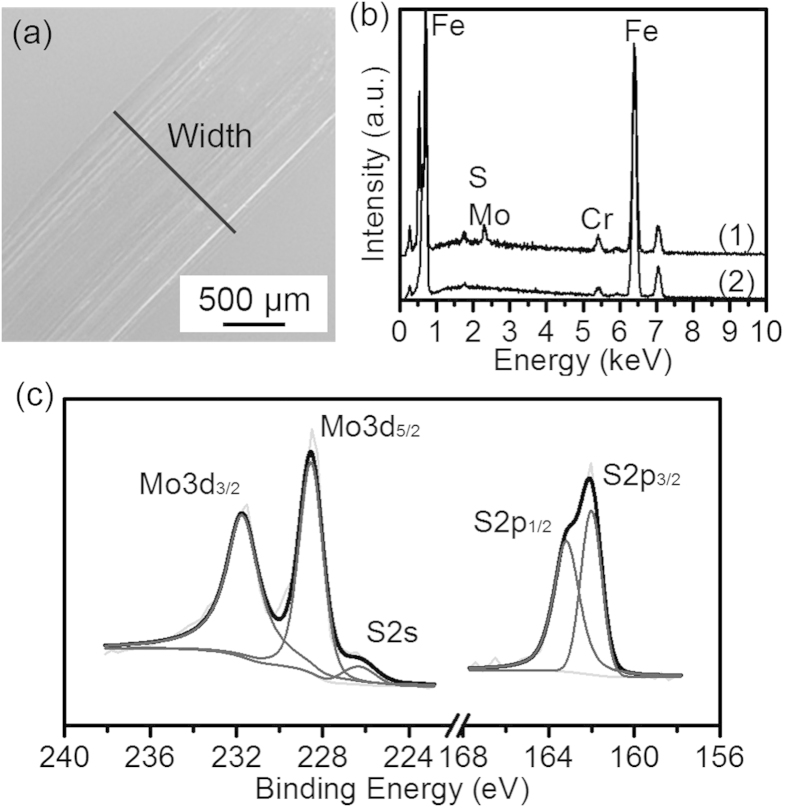
After the tribological test lubricated with the lubricant containing the as-synthesized MoS2 nanosheets stopped at 2000 N, the wear scar was ultrasonicly cleaned and analysed. The SEM image is provided in Fig. 3a. The width of the wear scar is about 1.39 ± 0.06 mm, indicating that the final pressure is about 1.32 GPa, which is 37.5% bigger than that of MoDDP (see Supplementary Fig. S11). EDS analysis (see Fig. 3b) shows that the elements of Mo and S can be detected only inside the wear scar and XPS analysis (see Fig. 3c) further proves that the elements of Mo and S are in the chemical state of MoS216. Therefore, it can be concluded that a tribofilm made of MoS2 was formed on the wear scar.
Figure 3. The analysis of the wear scar of the test of the lubricant containing the as-synthesized MoS2 nanosheets stopped at 2000 N.
(a) The SEM image of the wear scar and the width is about 1.39 ± 0.06 mm. (b) The EDS spectra (1) inside and (2) outside the wear scar, and the elements of Mo and S can only be detected inside the wear scar. (c) XPS analysis of the elenents of Mo and S in the wear scar, with Mo3d3/2 at 231.7 eV, Mo3d5/2 at 228.6 eV, S2s at 226.3 eV, S2p1/2 at 163.2 eV and S2p3/2 at 162.0 eV.
From the absolute value of COF, it can be deduced that the contribution of interlayer sliding for the lubrication is limited. It is consistent with the work of Lee et al. and Deng et al. that the friction force will increase as the layer number decreases10,35. And it is due to the local distortion of the single- or few-layered nanosheets. Although in this condition, the ultrathin MoS2 nanosheets will increase the interlocking friction between the sliding surfaces as the ‘third bodies’36,37, it is believed that they act more like a protective film to seperate the opposite peaks from direct contact. As is reported, the Young’s modulus and breaking strength of monolayer MoS2 is much larger than those of steel38. In addition, the simulation from Klemenz et al. provides further evidence that 2D-material can be an excellent coating for low friction and wear as long as the 2D-material itself is not damaged39. According to the analysis, the ability for 2D-material nano-additives to enter and stay in the contact area is the core problem. Fortunately, the as-synthesized MoS2 nanosheets have this ability due to its unique characters. Firstly, the befitting surface modification makes them floated seperately and freely in the lubricant without agglomeration. Secondly, the extreme tiny thicknness makes them easy to enter the contact area. As shown in Fig. 4a, when one micro peak is moving to the other one, the ultrathin MoS2 nanosheets will enter the contact area instead of being pushed away. When the two opposite peaks touch each other, because of the ultrathin MoS2 nanosheet between them, they will not contact with each other directly. They may be deformed due to pressure but little wear will be made because of the protection from the ultrathin MoS2 nanosheet. After the two peaks leave each other, the nanosheet may adhere to one of the peaks or floate back into the lubricant. This process is taking place countless times at the same moment during the test, because countless micro peaks from different sliding surfaces are meeting and seperating. As long as most of the contact peaks do not contact directly, the lubrication will not fail and the operation of the test will not stop. As for the ordinary 3D nanoparticles, such as the IF MoS2 or other multi-layer MoS2 nanoparticles, because of the relatively big size, they are more likely to be pushed away and opposite peaks will contact directly resulting in wear or seizure under high load (see Fig. 4b).
Figure 4. Schematics of the lubrication mechanism of.
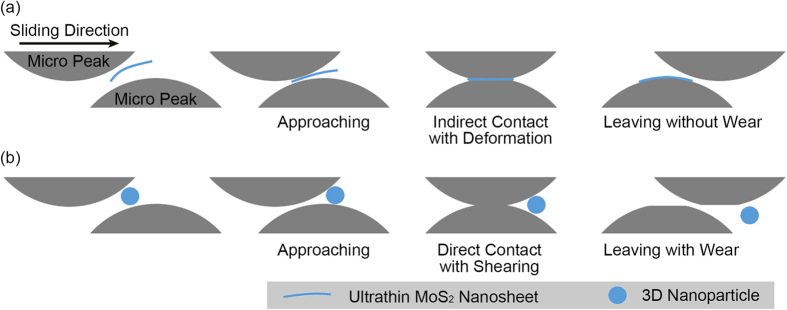
(a) ultrathin MoS2 nanosheets and (b) 3D nanoparticles. (a) The nanosheet can enter the contact area of the opposite micro peaks easily and prevent them from direct contact. The peaks may deform but little wear will take place. (b) As for the 3D nanoparticle, it will be pushed away due to its relatively big size and opposite peaks will directly contact, resulting in wear.
In summary, we have prepared a novel kind of oil-soluble ultrathin MoS2 nanosheets, which have only single or few atomic layers with its lateral size of tens of nanometers. Befitting surface modification was made through the synthesis process, so the ultrathin MoS2 nanosheets can be well dispersed in oil or lubricant without any extra surfactants or dispersants. The ultrathin shape of the nanosheets, together with the good dispersion in lubricant, makes them very easy to enter the contact area and prevent direct contact of the sliding surfaces under high load. As a result, the as-synthesized MoS2 nanosheets, when dispersed in lubricant, can largely enhance the extreme pressure property of the lubricant and have potential application for mechanical equipment operated in harsh conditions to avoid seizure or jam.
Methods
Synthesis
In a typical synthesis route of the ultrathin MoS2 nanosheets, 0.05 mmol of ammonium molybdate and 0.1 mmol of thiourea were added into the solution with 15 ml 1-octadecene and 15 ml oleylamine. After 15 minutes with vigorous stirring, the mixture was transferred to a teflon-lined stainless autoclave. The reaction lasted for 12 hours at 230 °C. After that, the autoclave was taken out to cool down slowly to the ambient temperature. The products were collected and washed with 30 ml ethanol for 3 times. Then the samples were dried in vacuum at 40 °C for 2 hours.
Tribological Tests
All the lubrication tests were performed with a commercial tribotester (Optimal SRV4) and the mode of the test is reciprocating ball-on-disk. The disk is fixed and the ball is pressed on the disk doing reciprocating motion with the stroke of 2 mm and the frequency of 50 Hz. During the load-climbing test, the normal load is firstly kept at 50 N for 30 seconds for running-in, and then the load rises to 100 N and keeps for 15 minutes. After that, the load is increased by 100 N every 2 minutes and the test will stop when the COF increases abruptly over 0.3, which indicates that the lubrication has failed and the ball and the disk have contacted with each other directly and seizure between them has taken place. The load before the lubrication fails is referred as the highest load with no seizure of the lubricant. For each test, a new ball and an un-rubbed position of the disk were put into use. All the tribological tests were performed several times with at least three tests having the same result to ensure that the value of the highest load is correct. The material of both balls and disks are bearing steel (AISI 52100) and the diameter of the ball is 10 mm. The ball is a commercial product and the surface roughness (Ra) is about 18.5 nm. The surface of the disk was polished and the roughness (Ra) is about 20.4 nm. The surface roughness of both ball and disk was measured by a commercial surface mapping microscope (ADE PHASE SHIFT MicroXAM). The viscosity of liquid paraffin was measured three times with a standard rheometer (Anton Paar Physica MCR301).
Characterization and Analysis
Chemical configurations were determined by an X-ray photoelectron spectroscope (Thermo Fisher ESCALAB 250Xi). XPS measurements were performed with an Al K α X-ray source on the samples. The energy calibrations were made against the C1s peak. A silicon chip was used to hold the sample. UV-vis spectrum was obtained with a commercial ultraviolet-visible spectrophotometer (Hitachi U-3010). Transmission electron microscope (JEOL JEM-2010, operated at 120 kV) was used to obtain the morphology of as-synthesized samples. Raman spectra with the resolution of 0.7 cm−1 were obtained after all the processes introduced in the Synthesis section. On that occasion, the as-synthesized MoS2 nanosheets were aggregate forming small black solid blocks. Laser was focused on the surface of these blocks. Three different locations, which were chosen randomly, were analysed. A confocal Raman microscopic systems (HORIBA Jobin Yvon HR800) was used. The spectrometer used the 514 nm line of an argon ion laser and it was calibrated with the Si peak at 520.7 cm−1. Particle size distribution and zeta potential was obtained with a commercial zetasizer (Malvern Nano-ZS). The infrared spectra were obtained from a commercial FTIR Spectrometer (Thermo Scientific Nicolet iS10). The wear scar on the disk was analysed with scanning electron microscope (FEI Quanta 200 FEG) equipped with an energy dispersive spectrometer (EDAX Genesis).
Additional Information
How to cite this article: Chen, Z. et al. Ultrathin MoS2 Nanosheets with Superior Extreme Pressure Property as Boundary Lubricants. Sci. Rep. 5, 12869; doi: 10.1038/srep12869 (2015).
Supplementary Material
Acknowledgments
This research was financially supported by Shell Global Solutions (US) Inc, the Tsinghua University Initiative Scientific Research Program, Innovative Research Groups of the National Natural Science Foundation of China (51321092) and the Foundation for the Supervisor of Beijing Excellent Doctoral Dissertation (20111000305).
Footnotes
Author Contributions J.B.L. and S.G. initiated the idea to prepare the ultrathin MoS2 nanosheets as boundary lubricants. J.B.L., Y.H.L., Z.C. and X.W.L. designed the experiments. Z.C. and X.W.L. performed the experiments, wrote the main manuscript text and prepared all the figures. All authors contributed to the analysis and discussion of the data and reviewed the manuscript.
References
- Li Y. et al. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011). [DOI] [PubMed] [Google Scholar]
- Radisavljevic B., Radenovic A., Brivio J., Giacometti V. & Kis A. Single-layer MoS2 transistors. Nat. Nanotechnol. 6, 147–150 (2011). [DOI] [PubMed] [Google Scholar]
- Hwang H., Kim H. & Cho J. MoS2 nanoplates consisting of disordered graphene-like layers for high rate lithium battery anode materials. Nano Lett. 11, 4826–4830 (2011). [DOI] [PubMed] [Google Scholar]
- Levita G., Cavaleiro A., Molinari E., Polcar T. & Righi M. C. Sliding properties of MoS2 layers: Load and interlayer orientation effects. J. Phys. Chem. C 118, 13809–13816 (2014). [Google Scholar]
- Oviedo J. P. et al. In situ TEM characterization of shear-stress-induced interlayer sliding in the cross section view of molybdenum disulfide. ACS Nano 9, 1543–1551 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang Z. J., Xue Q. J. & Zhang J. Synthesis, structure and lubricating properties of dialkyldithiophosphate-modified Mo-S compound nanoclusters. Wear 209, 8–12 (1997). [Google Scholar]
- Hu K. H., Hu X. G., Xu Y. F., Huang F. & Liu J. S. The effect of morphology on the tribological properties of MoS2 in liquid paraffin. Tribol. Lett. 40, 155–165 (2010). [Google Scholar]
- Feldman Y., Wasserman E., Srolovitz D. J. & Tenne R. High-rate, gas-phase growth of MoS2 nested inorganic fullerenes and nanotubes. Science 267, 222–225 (1995). [DOI] [PubMed] [Google Scholar]
- Rosentsveig R. et al. Fullerene-like MoS2 nanoparticles and their tribological behavior. Tribol. Lett. 36, 175–182 (2009). [Google Scholar]
- Lee C. et al. Frictional characteristics of atomically thin sheets. Science 328, 76–80 (2010). [DOI] [PubMed] [Google Scholar]
- Martin J. M., Donnet C., Lemogne T. & Epicier T. Superlubricity of molybdenum-disulfide. Phys. Rev. B 48, 10583–10586 (1993). [DOI] [PubMed] [Google Scholar]
- Novoselov K. S. et al. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. 102, 10451–10453 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. N. et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331, 568–571 (2011). [DOI] [PubMed] [Google Scholar]
- Zhan Y., Liu Z., Najmaei S., Ajayan P. M. & Lou J. Large-area vapor-phase growth and characterization of MoS2 atomic layers on a SiO2 substrate. Small 8, 966–971 (2012). [DOI] [PubMed] [Google Scholar]
- Altavilla C., Sarno M. & Ciambelli P. A novel wet chemistry approach for the synthesis of hybrid 2D free-floating single or multilayer nanosheets of MS2@oleylamine (M = Mo, W). Chem. Mater. 23, 3879–3885 (2011). [Google Scholar]
- Moulder J. F., Stickle W. F., Sobol P. E. & Bomben K. D. Handbook of X-Ray Photoelectron Spectroscopy (Perkin-Elmer, Boca Raton, 1992). [Google Scholar]
- Wong K. C. et al. Surface and friction characterization of MoS2 and WS2 third body thin films under simulated wheel/rail rolling-sliding contact. Wear 264, 526–534 (2008). [Google Scholar]
- Wilson J. A. & Yoffe A. D. Transition metal dichalcogenides discussion and interpretation of observed optical, electrical and structural properties. Adv. Phys. 18, 193–335 (1969). [Google Scholar]
- Eda G. et al. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 11, 5111–5116 (2011). [DOI] [PubMed] [Google Scholar]
- Grossiord C. et al. MoS2 single sheet lubrication by molybdenum dithiocarbamate. Tribol. Int. 31, 737–743 (1998). [Google Scholar]
- Lee C. et al. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 4, 2695–2700 (2010). [DOI] [PubMed] [Google Scholar]
- Li H. et al. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 22, 1385–1390 (2012). [Google Scholar]
- Buscema M., Steele G. A., van der Zant H. S. J. & Castellanos-Gomez A. The effect of the substrate on the Raman and photoluminescence emission of single-layer MoS2. Nano Res. 7, 561–571 (2014). [Google Scholar]
- Stuart B. Infrared spectroscopy (John Wiley & Sons, Inc., 2005). [Google Scholar]
- Onodera T. et al. A computational chemistry study on friction of h-MoS2. Part I. Mechanism of single sheet lubrication. J. Phys. Chem. B 113, 16526–16536 (2009). [DOI] [PubMed] [Google Scholar]
- Rodriguez J. A. Interaction of hydrogen and thiophene with Ni/MoS2 and Zn/MoS2 surfaces: A molecular orbital study. J. Phys. Chem. B 101, 7524–7534 (1997). [Google Scholar]
- Lemmon J. P. & Lerner M. M. Preparation and characterization of nanocomposites of polyethers and molybdenum-disulfide. Chem. Mater. 6, 207–210 (1994). [Google Scholar]
- Ataca C. & Ciraci S. Functionalization of single-layer MoS2 honeycomb structures. J. Phys. Chem. C 115, 13303–13311 (2011). [Google Scholar]
- Peng E. et al. Synthesis of manganese ferrite/graphene oxide nanocomposites for biomedical applications. Small 8, 3620–3630 (2012). [DOI] [PubMed] [Google Scholar]
- Martin J. M., Le Mogne T., Grossiord C. & Palermo, T. Tribochemistry of ZDDP and MoDDP chemisorbed films. Tribol. Lett. 2, 313–326 (1996). [Google Scholar]
- Yamamoto Y. & Gondo S. Friction and wear characteristics of molybdenum dithiocarbamate and molybdenum dithiophosphate. Tribol. Trans. 32, 251–257 (1989). [Google Scholar]
- Yamamoto Y., Gondo S., Kamakura T. & Tanaka N. Frictional characteristics of molybdenum dithiophosphates. Wear 112, 79–87 (1986). [Google Scholar]
- Cizaire L. et al. Mechanisms of ultra-low friction by hollow inorganic fullerene-like MoS2 nanoparticles. Surf. Coat. Tech. 160, 282–287 (2002). [Google Scholar]
- Lahouij I., Vacher B., Martin J. & Dassenoy F. IF-MoS2 based lubricants: Influence of size, shape and crystal structure. Wear 296, 558–567 (2012). [Google Scholar]
- Deng Z., Smolyanitsky A., Li Q., Feng X. & Cannara R. J. Adhesion-dependent negative friction coefficient on chemically modified graphite at the nanoscale. Nat. Mater. 11, 1032–1037 (2012). [DOI] [PubMed] [Google Scholar]
- Muser M. H. & Robbins M. O. Conditions for static friction between flat crystalline surfaces. Phys. Rev. B 61, 2335–2342 (2000). [Google Scholar]
- He G., Muser M. H. & Robbins M. O. Adsorbed layers and the origin of static friction. Science 284, 1650–1652 (1999). [DOI] [PubMed] [Google Scholar]
- Bertolazzi S., Brivio J. & Kis A. Stretching and breaking of ultrathin MoS2. ACS Nano 5, 9703–9709 (2011). [DOI] [PubMed] [Google Scholar]
- Klemenz A. et al. Atomic scale mechanisms of friction reduction and wear protection by graphene. Nano Lett. 14, 7145–7152 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.