Abstract
Objectives
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) using standard needles has a high diagnostic value in the evaluation of solid pancreatic masses. Fenestrated needles have been developed to improve the quality of EUS-guided tissue sampling by providing core biopsies (FNB).
Methods
Patients with solid pancreatic masses of >2 cm were prospectively included in our study and randomized to receive EUS sampling, using either a standard 22G FNA or a 22G Procore® FNB needle. The main study endpoint was the number of needle passes required to obtain a diagnosis in more than 90% of cases.
Results
We included 100 patients (male = 63, female = 37; mean age = 68.4 years) in our study. We found that 88% of the lesions were malignant, with a mean size of 32 mm. A sample adequate for diagnosis was obtained in more than 90% of cases after the second needle pass in the FNB group, versus the third needle pass in the FNA group. Slide cellularity and presence of tissue microfragments were significantly higher in the FNB group. Sensitivity for the diagnosis of malignancy was 88.4% versus 97.8% for the EUS-FNA and EUS-FNB group, respectively, while specificity for both techniques was 100%. No complications were recorded.
Conclusions
Although the accuracy of both needle types for proving malignancy was similar, a lower number of passes was required with the FNB needles to achieve the same contributive sample rate as with the FNA needles. FNB also improved the histopathological quality of specimens, suggesting an overall superiority of FNB sampling.
Keywords: Biopsy, diagnosis, endosonography, fine needle aspiration, histopathology, needle type, pancreatic cancer, ultrasound guided biopsy
Introduction
Non-resectable pancreatic cancer is associated with a poor prognosis and an overall 5-year survival rate of 5–6%.1 Clinical management of suspected cases is based on an early histological diagnosis that permits tailored therapeutic approaches. Confirmation of malignancy can also be desirable in potentially resectable tumors, in order to avoid misinterpretation of benign masses, such as pseudotumoral autoimmune pancreatitis. Endoscopic ultrasound fine-needle aspiration (EUS-FNA) is currently the standard method for sampling solid pancreatic masses, with a reported sensitivity for malignant cytology of 85–95%, specificity of 95–98% and diagnostic accuracy of 78–95%.2,3 Diagnostic failures of EUS-FNA can be due to inadequate targeting, inexperience of the endoscopist and/or the pathologist, and/or necrotic or fibrotic tumors in which viable cells are difficult to obtain. Selection of needle size and determination of the number of needle passes required during a procedure are also suspected to play a key role in the outcomes of the diagnostic procedure.4,5 The presence of a histopathologist in the endoscopy ward at the time of EUS-FNA can strongly help determine the sample quality after each pass, until sufficient material is obtained, but many endoscopy units are distant from the pathology department and/or the pathologists are too busy to distract enough time out of their unit. Finally, the cellularity and architectural representation of the sample can also be determined by the needle used and its specific features. Recently, needles have become available that are especially designed to promote the collection of core tissue by the shearing of material from target lesions during retrograde motion. Those needles, equipped with a side opening and a reverse bevel, are supposed to allow for EUS-fine needle biopsy (EUS-FNB).
The available literature on FNB needles reports controversial results.6–13 Our objective was to compare the histopathologic quality of the tissue samples and the diagnostic yield, using either the standard FNA or the FNB needle, for the sampling of solid pancreatic tumors under formalized conditions.
Patients and methods
Definitions and study design
We hypothesized that FNB needles would yield a higher content of tissue microfragments, when compared to FNA needles; thus, possibly requiring a lower number of needle passes to achieve diagnosis.
The main study endpoint was the number of needle passes required to achieve a contributive diagnosis in more than 90% of cases with either needle type, FNA or FNB. Other judgment criteria included the sample quality being defined as a composite of cellularity, presence of microfragments with their size and number, digestive or blood contamination, presence of necrotic tissue, clinical and technical complications.
Definitions
Tissue microfragments are understood as small pieces of architecturally preserved pattern, containing intact epithelial cells and connective tissue (Figure 1). A contributive diagnosis was defined as a confirmed malignancy, or a benign tumor confirmed by histology after surgical resection or after 1 year of follow-up. The procedure was also deemed contributive, when the results dictated a change in management of the patient, with no need for repeat EUS-FNA/FNB. Non-contributive samples were those with insufficient cyto-histological material to make a clinical decision, thus requiring further diagnostic procedures.
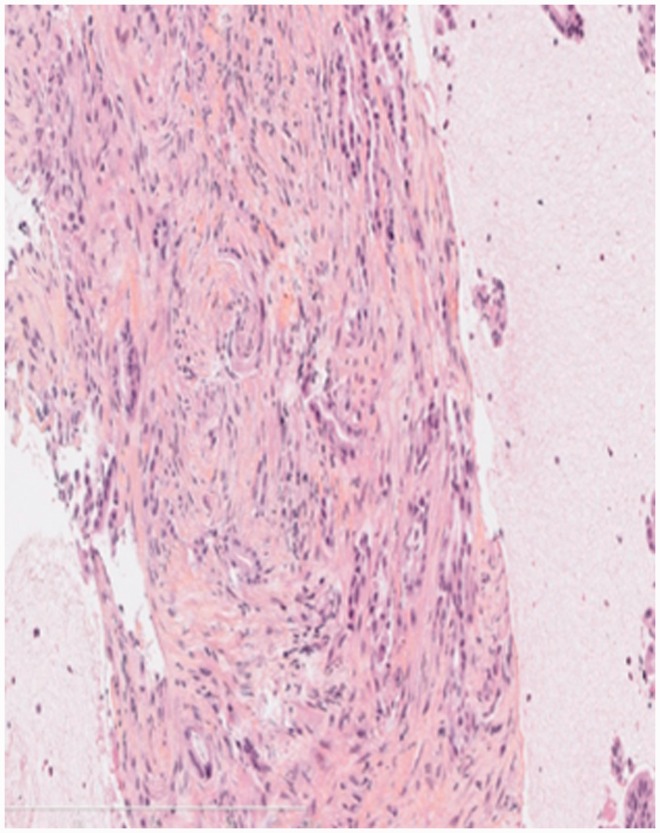
Figure 1.
An EUS-FNB sample adequate for histological analysis: The tissue microfragment with intact tissue architecture showed pancreatic adenocarcinoma.
EUS: endoscopic ultrasound; FNB: fine-needle biopsy; Hematoxylin erythrosin and saffron (HES) staining; 20 × magnification.
Study design
This was a monocentric study in a tertiary referral academic center for bilio-pancreatic pathology, with an annual volume of 1100 diagnostic EUS and more than 300 EUS-guided histological sampling procedures, and a specialized digestive histopathology department with an annual volume exceeding 250 pancreatic tumors. On-site pathologists are not available during EUS sampling in our facility.
The study was prospectively carried out on consecutive patients whom were referred from 1 April 2012 to 30 March 2013. Only 22-Gauge (22G) needles were used for both FNA and FNB, with randomization of needle selection. Only one needle (either FNA or FNB) was used for each patient. All patients were followed up for 24 hours, for any procedure-related complications. Patients with a diagnosis of benign lesion were followed up over a period of 1 year with computed tomography (CT) scan, EUS or magnetic resonance imaging (MRI) if indicated, to get final confirmation of the benign nature of the mass. This study received approval from our institutional review board (CPP Ile de France III).
Inclusion criteria
All patients provided written informed consent. We included only patients with solid pancreatic tumors of at least 2 cm in size showed on CT scanner or MRI, taking into consideration the minimum length required for the needle to fully move back and forth in the target lesion. Considering the endoscopist's experience as an important potential bias, all patients were operated upon by the same senior endoscopist (FP). Patients with biliary metallic stents were excluded.
Technique for endoscopic sampling
With patients in the left lateral position and under deep sedation with propofol, all procedures were performed using a linear array echoendoscope EUS (GF-UCT140 or 180, Olympus, Japan). Pancreatic head and uncinate masses were accessed via the duodenum; and pancreatic body and tail masses via the stomach; whereas tumors located in the pancreatic isthmus were accessed via the most easily available route, either duodenal or gastric. Needles used were 22G Echo-ultra™ (Cook Medical, Cork, Ireland) for FNA, and 22G Echotip Procore (Cook Medical) for FNB. The 22G FNB Procore needle has a 5.2F shaft, a core trap of 2 mm and a reverse-bevel length of 5.9 mm. The same sampling technique was used with both FNA and FNB needles, in order to eliminate technical biases: The needle was advanced into the target with the stylet in place, then the stylet was removed; and a negative pressured vacuum syringe of 10 ml was connected before applying 10 to 20 to-and-fro motions, under endosonographic guidance. Whenever possible, fanning the needle through the target zone was performed by using the elevator and vertical knob of the endoscope. After each pass, collected material was expressed with normal saline into formalin tubes; then immediately name tagged and labeled according to the order of each needle pass. Subsequent passes were carried out and processed in the same way, with no less than two needle passes and as many passes per lesion as needed to obtain macroscopically-visible samples.
Histopathology
All of our study samples were processed with a clear distinction of each needle pass. The visible solid material was fixed in 10% formalin for 12–24 hours, and then centrifuged. The obtained specimen was embedded in paraffin, and cut into 3-µm sections, four cuts producing four slides for each needle pass: Two slides were stained with HES and the other slides were kept aside, for further analysis.
In the absence of visible solid material, a formalin fixation for 12 hours was followed by centrifugation (4000 rpm during 8 min); then the material obtained was embedded in a cytoblock and cut to produce four slides: Two of these were HES and Papanicolaou-stained. When required, immunohistochemistry or specific stainings were performed.
The final diagnosis was provided by a senior histopathologist (BT), blinded to the type of needle used, who analyzed all samples and was informed of the patient's data. For the purpose of the present study, a second senior histopathologist (FB), blinded to both the needle used and the diagnostic result, undertook quantitative and semi-quantitative analyses to assess the histopathological quality of each needle pass, following a four-class grading system described by Wani et al. (A = Absent 0%, B = Minimal surface area (SA) < 25% of slide, C = Moderate 25% < SA < 50% of slide, and D = Significant SA > 50% of the slide), which was applied to the blood content, gastric or duodenal wall contaminant, pancreatic tissue content, necrotic tissue (defined by the loss of cellular integrity) content, and the tumor cell content (Figure 2).14 Cellularity was expressed as a ratio of cell number per slide surface: It was noted as fair (<100), good (100–1000), or excellent (>1000). The presence of microfragments was assessed qualitatively, and their number and size in millimeters were assessed.
Figure 2.
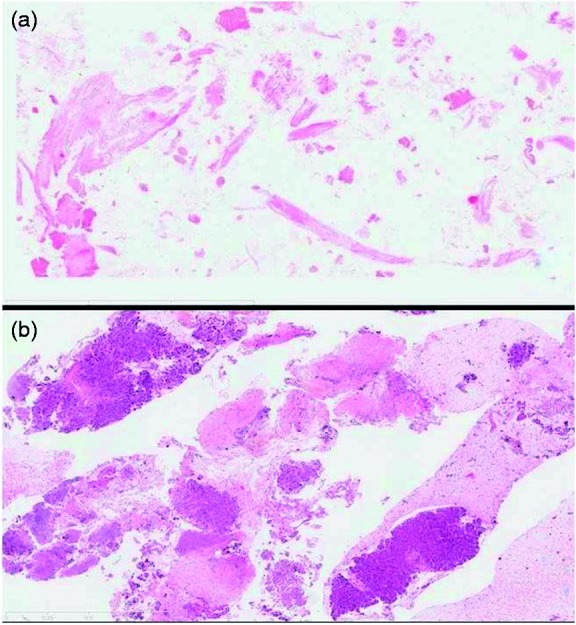
Variation in histological quality of HES-stained tissue samples at 50× magnification, between (a) a hemorrhagic sample inadequate for tissue analysis and (b) a sample with high cellularity and presence of tissue microfragments.
HES: Hematoxylin erythrosin saffron staining.
Statistical analysis
A group of 30 patients (15 patients EUS-FNA and 15 patients EUS-FNB) was retrieved retrospectively from our clinical records, to estimate the number of patients needed to establish a prospective study group of statistical significance, with regard to the main study endpoint. Our study's sample size calculation, based on the analysis of those 30 patients, was estimated at 50 patients per group, in order to detect a difference of 25% in the contributive sample rate between the two needle groups, after the first needle pass. The Type 2 (β) error was set at 0.2 (chi-2 test with corrected continuity) and the Type I error rate (α) was set at 0.05. In so doing, the group size was approximated to detect a difference in the number of needle passes to achieve a contributive diagnosis in more than 90% of patients.
We compared the baseline characteristics and main outcomes between the two groups of patients. For comparison of categorical data, chi-square or Fisher's exact tests were used when appropriate; whereas t-tests were used for the continuous data. Three comparisons were done for both the primary and secondary endpoints:
Comparison of the first pass of FNA versus the first pass of FNB, for all patients: In this category, raw data were used from the findings of the histopathological study;
Comparison of the pooled results of all FNA needle passes, versus the raw data of the first FNB needle pass; and
Comparison of the pooled results of all the FNA needle passes, compared to the pooled data of all the FNB needle passes. To achieve the second and third comparisons, raw data were pooled using the mean value to sum all passes, when necessary.
The time to diagnosis, according to the number of needle passes, was studied using the Kaplan-Meier estimates and we performed a comparison between curves, using a log-rank test.
Statistical significance was set for p values <0.05. Statistical analyses were performed by using SAS software package version 9.2 (SAS Institute Inc., Cary, NC, USA). Patient clinical data and EUS-related complications were registered using a Microsoft™ XL® 2012 program.
Results
We included 100 patients fulfilling the inclusion criteria, equally divided between FNA and FNB groups. More than two out of three tumors were located in the head of the pancreas and adenocarcinoma accounted for 83% of cases. Other diagnoses were neuroendocrine tumors (5%), chronic pancreatitis (7%) and undetermined because of insufficient material (5%). The main patient characteristics, which were not significantly different between the groups, are displayed in Table 1. The diagnosis of cancer was obtained by EUS-FNA of a metastatic lymph node in one patient; and by EUS-FNA of liver metastasis in another one, whereas the primary tumor sample did not contain any malignant cells. In one young male patient with a history of alcoholic chronic pancreatitis, a follow-up MRI 1 year after the EUS and total alcohol suppression showed no remaining pancreatic mass.
Table 1.
Patients' characteristics
| FNA group (n = 50) | FNB group (n = 50) | Overall (n = 100) | p | |
|---|---|---|---|---|
| Age, mean ± SD | 68 ± 11.2 | 67.8 ± 13.1 | 68.4 ± 12.5 | 0.66 |
| Gender, n (%) | 0.14 | |||
| Male | 35 (70) | 28 (56) | 63 (63) | |
| Female | 15 (30) | 22 (44) | 37 (37) | |
| Location of pancreatic mass, n (%) | 0.57 | |||
| Head | 38 (76) | 34 (68) | 72 (72) | |
| Body | 7 (14) | 11 (22) | 18 (18) | |
| Tail | 5 (10) | 5 (10) | 10 (10) | |
| Tumor size in mm, Mean ± SD | 33 ± 2.7 | 32 ± 5.1 | 32 ± 4.6 | |
| Final Diagnosis, n (%) | 0.73 | |||
| Malignancies | 43 (86) | 45 (90) | 88 (88) | |
| Adenocarcinoma | 41 (82) | 42 (84) | 83 (83) | |
| Neuroendocrine | 2 (4) | 3 (6) | 5 (5) | |
| Chronic pancreatitis | 2 (4) | 5 (10) | 7 (7) | |
| Undetermined | 5 (10) | 0 | 5 (5) | |
| After 1 year follow-up | 1 Chronic pancreatitis 2 adenocarcinomas 1 neuroendocrine tumor 1 inflammatory nodule | |||
FNA: Fine needle aspiration; FNB: fine needle biopsy; mm: millimeter; SD: standard deviation
Sampling outcomes for FNA and FNB are presented in Table 2, showing a significantly lower contributive sample rate after FNA than FNB (90% versus 100%, respectively), in spite of a mean (±SD) number of needle passes of 2.59 ± 0.49 for FNB, as against 3.28 ± 1.0 for FNA. After a second EUS-guided FNA sampling, the three patients with suspicious samples (i.e. containing cellular atypia) of the FNA group underwent Whipple's procedure and were proven to bear an adenocarcinoma in two cases and chronic alcoholic pancreatitis in the third case. Breaking down the five cases of FNA patients with inadequate samples showed the following: In one female patient with uncontrolled rheumatoid arthritis, histopathology showed an extensive necrotic inflammatory response with no tumor cells, with the same findings in the pancreatic gland at a 1-year follow-up CT scan. EUS-guided tissue sampling was not repeated. Two patients underwent a second EUS with FNB, showing a pancreatic adenocarcinoma: one patient underwent surgical resection of a tumor in the pancreatic tail that proved to be a neuroendocrine tumor; and one patient had two further EUS-FNA at 6-month intervals, both showing chronic pancreatitis and a steady clinical condition.
Table 2.
EUS sampling outcomes
| FNA group (n = 50) | FNB group (n = 50) | p | |
|---|---|---|---|
| Number of needle passes, median (range) | 3 (2–5) | 2 (2–3) | NS |
| Contributive pancreatic tissue sample, n (%) | 45 (90) | 50 (100) | 0.02 |
| Sensitivity for malignancy, %a | 88.4% (38/43) | 97.8% (44/45) | NS |
| Specificity for malignancy, % | 100% (7/7) | 100% (5/5) | NS |
Includes samplings from the extra-pancreatic sites (lymph nodes and liver metastases).
EUS: endoscopic ultrasound; FNA: fine needle aspiration; FNB: fine needle biopsy; NS: not significant
FNB yielded suspicious or atypical samples from pancreatic masses in five patients: Three patients had chronic pancreatitis that showed no significant changes after 1 year of radiological and EUS follow-up (performed at 3, 6 and 12 months); one patient had a surgical resection of the pancreatic head, revealing a pancreatic adenocarcinoma; and one patient had associated liver metastases of an adenocarcinoma, proven by EUS-FNB during the initial EUS procedure. No procedure-related complication was reported in either of the FNA and FNB patient groups. Sensitivity for the diagnosis of malignancy was 88.4% for EUS-FNA and 97.8% for EUS-FNB (p = 0.7). Specificity for both methods was 100%. Looking only at our results from the pancreatic puncture, the overall diagnostic yield was 84% for EUS-FNA versus 90% for EUS-FNB (p = 0.8), with similar sensitivities for the diagnosis of malignancy at 86% and 90% for FNA and FNB, respectively; as well as for the specific diagnosis of pancreatic adenocarcinoma, at 82% and 84%, respectively. Specificity was 100% in both groups.
Data on histological sample quality, presented as both qualitative and semi-quantitative criteria, are detailed in Table 3. Cellularity and the presence of tissue microfragments were significantly higher in the FNB group, when comparing the first needle passes of each type, as well as all passes of each group. Tumor cell contents were not significantly different between the two types of needles. Furthermore, digestive contamination and presence of necrotic content in the tissue samples were not significantly different between the groups. FNB resulted in a significantly higher blood cell content of the EUS samples; however, we recorded no clinical complication of the EUS procedure, especially gastrointestinal bleeding.
Table 3.
Histopathological findings in FNA and FNB groups according to needle passes
| FNA group: 1st pass only versus FNB group: 1st pass only |
FNA group: all passes versus FNB group: 1st pass only |
FNA group: all passes versus FNB group: all passes |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| FNA | FNB | p | FNA | FNB | p | FNA | FNB | p | |
| N of cells (C/SA) | 0.04 | 0.09 | 0.03 | ||||||
| Fair < 100 | 10 (20%) | 2 (4%) | 6 (12%) | 2 (4%) | 6 (12%) | 0 (0) | |||
| Good 100 to 1000 | 13 (26%) | 14 (28%) | 20 (40%) | 14 (28%) | 20 (40%) | 24 (48%) | |||
| Excellent > 1000 | 27 (54%) | 34 (68%) | 24 (48%) | 34 (68%) | 24 (48%) | 26 (52%) | |||
| Microfragments | |||||||||
| Present | 9 (18%) | 30 (60%) | < 0.001 | 16 (32%) | 30 (60%) | 0.008 | 16 (32%) | 38 (76%) | < 0.001 |
| Mean number ± SD | 1.56 ± 0.73 | 2.53 ± 2.7 | 0.29 | 0.7 ± 1.3 | 1.5 ± 2.4 | 0.57 | 0.7 ± 1.3 | 2.3 ± 2.0 | 0.10 |
| Mean size ± SD | 1.54 ± 0.68 | 1.6 ± 1.0 | 0.88 | 0.5 ± 0.8 | 1.0 ± 1.1 | 0.53 | 0.5 ± 0.8 | 1.2 ± 1.0 | 0.58 |
| Tumoral cells (SA/S) | 0.30 | 0.17 | 0.70 | ||||||
| Absent | 15 (30%) | 9 (18%) | 7 (14%) | 9 (18%) | 7 (14%) | 5 (10%) | |||
| Minimal < 25% | 22 (44%) | 24 (48%) | 32 (64%) | 24 (48%) | 32 (64%) | 33 (66%) | |||
| Moderate 25–50% | 11 (22%) | 11 (22%) | 10 (20%) | 11 (22%) | 10 (20%) | 9 (18%) | |||
| Significant > 50% | 2 (4%) | 6 (12%) | 1 (2%) | 6 (12%) | 1 (2%) | 3 (6%) | |||
| Blood content (SA/S) | 0.17 | 0.001 | 0.06 | ||||||
| Absent | 9 (18%) | 4 (8%) | 2 (4%) | 4 (8%) | 2 (4%) | 0 | |||
| Minimal <25% | 24 (48%) | 19 (38%) | 28 (56%) | 19 (38%) | 28 (56%) | 22 (44%) | |||
| Moderate 25–50% | 8 (16%) | 11 (22%) | 18 (36%) | 11 (22%) | 18 (36%) | 19 (38%) | |||
| Significant >50% | 9 (18%) | 16 (32%) | 2 (4%) | 16 (32%) | 2 (4%) | 9 (18%) | |||
| Digestive contamination (SA/S) | 0.6 | 0.41 | 0.43 | ||||||
| No contamination | 25 (50%) | 23 (46%) | 21 (42%) | 23 (46%) | 21 (42%) | 15 (30%) | |||
| Contamination <25% | 23 (46%) | 22 (44%) | 27 (54%) | 22 (44%) | 27 (54%) | 33 (66%) | |||
| Contamination 25–50% | 2 (4%) | 5 (10%) | 2 (4%) | 5 (10%) | 2 (4%) | 2 (4%) | |||
| Necrotic tissue (SA/S) | 0.42 | 0.005 | 0.13 | ||||||
| Absent | 14 (28%) | 19 (38%) | 5 (10%) | 19 (38%) | 5 (10%) | 9 (18%) | |||
| Minimal < 25% | 23 (46%) | 23 (46%) | 34 (68%) | 23 (46%) | 34 (68%) | 37 (74%) | |||
| Moderate 25–50% | 7 (14%) | 6 (12%) | 10 (20%) | 6 (12%) | 10 (20%) | 3 (6%) | |||
| Significant > 50% | 6 (12%) | 2 (4%) | 1 (2%) | 2 (4%) | 1 (2%) | 1 (2%) | |||
| Inadequate for analysis | 13 (26%) | 3 (6%) | 0.001 | 5 (10%) | 3 (6%) | 0.15 | 5 (10%) | 0 (0%) | 0.02 |
C/SA: cells per surface area; FNA: fine needle aspiration; FNB: fine needle biopsy; NS: not significant; SA/S: surface area per slide; SD: standard deviation
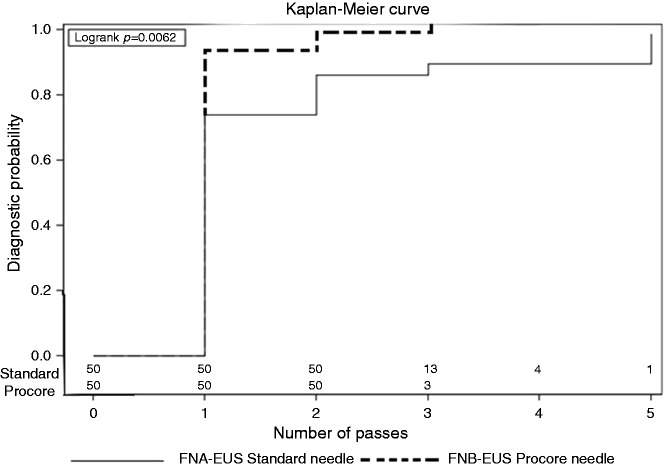
The cumulative diagnostic yield in each group after successive needle passes was different between the groups (p = 0.006), as is shown in Figure 3. A contributive sample was obtained in more than 90% of cases after the second needle pass in the FNB group, and after the third needle pass in the FNB and FNA group, respectively. The maximal diagnostic yield was obtained after the third pass in the FNB group, as compared against the fifth pass, in the FNA group.
Figure 3.
Probability of obtaining diagnostic material, according to the number of needle passes using EUS-FNA versus EUS-FNB needles.
EUS: endoscopic ultrasound; FNA: fine-needle aspiration; fine-needle biopsy
Discussion
The main finding of this study was that use of reverse beveled needles can achieve a high diagnostic performance after two needle passes, and that additional passes do not significantly improve the diagnostic yield. The ‘standard’ FNA needles can achieve the same sensitivity for the cytological diagnosis of pancreatic cancer, at the expense of a higher number of needle passes. This result should be linked to the higher yield of tissue microfragments retrieved with the FNB needles, when compared to standard FNA needles. The acquisition of intact architectural tissue microfragments appeared to increase the histopathological quality of samples, thus potentially facilitating the assessment and subtyping of ductal, intraductal and parenchymal pancreatic lesions; and also potentially providing adequate specimens for further immunohistochemistry investigations of the tumor type.6,7
Because pancreatic ductal adenocarcinomas, which accounted for the majority of pancreatic malignancies diagnosed in our study, often display a low proportion of epithelial cells and an important fibrotic stroma, the higher cellularity and proportion of microfragments did not result in significantly higher rates of tumoral cells in the FNB, as compared to the FNA samples. Indeed, tumor cells were absent or scarce in the majority of tissue samples, whatever needle type was used; however, sensitivity for malignancy was higher with FNB. This underlines the importance of obtaining tissue microfragments, as changes in stromal tissue architecture (typically stromal tissue invasion by epithelial cells) can help the pathologist in diagnosing malignancy. Moreover, our study showed that FNB needles acquired significantly more microfragments than FNA needles after a first pass. The number of microfragments increased along with the number of passes, leading to an increased diagnostic yield in both FNA and FNB groups. It must be noted that the contributive sample rate (95% overall), as defined previously, was higher than the sensitivity of pancreatic sampling, because the diagnosis of malignancy had been obtained in three cases by sampling of metastatic locations, rather than the pancreatic mass.
No specific recommendation of practice had been defined as to the number of needle passes required to optimize procedures. A prospective study by Leblanc et al.15 showed that the optimal number of EUS-FNA needle passes ranges from two to six, to achieve a diagnosis. Our study confirmed the previously-reported relationship existing between the number of FNA needle passes and diagnostic performance: FNA needles reached a sensitivity of 90% after a median of three passes, not significantly different from the 98% rate obtained with FNB needles after a median of two passes.
It has been reported that EUS-FNA in the absence of an on-site histopathologist can result in a 10–15% reduction in the rate of definitive cytological diagnosis.15,16 Immediate cytological evaluation not only improves the diagnostic yield, but can potentially reduce the number of needle passes, procedure time and patient risk; however, the presence of an experienced pathologist in the endoscopy room during EUS is a logistical and financial burden that only a few centers can afford, leading many endoscopists to multiply needle passes, in order to increment cytological yield. A study by Hucl et al.8 where FNB needles were evaluated for feasibility, efficiency and diagnostic yield, in comparison to FNA needles, in the puncture of deep mediastinal, pancreatic and peripancreatic lesions shows that fewer number of needle passes were required for FNB needles to achieve diagnosis, when compared to FNA needles. A recent Korean study also found that fewer passes were needed with FNB needles, with a median of only one needle pass versus two for FNA to establish a diagnosis; however, this study was designed with the presence of an onsite pathologist.13 Our results enhance these findings and are important for endoscopists working in centers with no pathologist available on-site, because they can consider performing only two FNB needle passes and with macroscopically visible material collected, and expect the best possible result under such conditions. Thus, this practice may be considered as a valid alternative to the on-site presence of the pathologist to optimize histopathological results. Also importantly, and in accordance with previous studies,8,9,15 this improvement in histological sample quality was not obtained at the expense of safety, because no complication was noted with either needle type.
Four recently-published studies found the diagnostic yield of the 22G FNB system to be similar to that of a FNA assembly and show that the technical performance and safety profile of both needles are comparable; however, in the first study by Bang et al.,9 the main endpoint was different from ours, with the power calculated to detect a median effect size of one pass between the two types of needles, and a smaller sample size as a result. Two potential biases could also be raised: One was the use of needles from different manufacturers for the standard and FNB needles; the second was a different method of sampling with both needles (no stylet and no suction for FNA, suction and stylet for the first pass only for FNB). Our method consisted in using needles from the same manufacturer and only one sampling method throughout the study, for both types of devices.
The second study by Strand et al.12 reports a dramatic decrease in the diagnostic yield, when using FNB as compared to FNA (93.8% versus 28.1%, p < 0.01); however, the study included only 32 patients in each group, and there are concerns about technical quality of the procedures, because over 15% of the FNB and 25% of FNB specimens failed tissue processing. The third study by Vanbiervliet et al.10 compares the effect of only one FNB needle pass versus two FNA needle passes, thus biasing the study in favor of FNA needles. Contrary to the comparison between different methods or procedures, where multiple centers and operators enhance the validity of findings, procedural homogeneity is paramount to reliably compare two different devices. Because important differences can be found in the diagnostic performance of EUS sampling between expert and novice endosonographers, one strength of our study was that all procedures were performed by the same endoscopist, in contrast to most previous works. Lastly, the above-mentioned study from Korea on 116 patients found a higher, although not statistically significant, accuracy rate for the diagnosis of malignancy with FNB, as compared with FNA (98.3% versus 94.8%; p = 0.671).13
The present study did not compare FNA and FNB needles in the same patient in a randomized order, as did other authors;10,12 however, although improving the statistical quality of results, such a ‘head-to-head’ comparison makes the effect of the first and subsequent needle passes difficult to compare: Because our primary endpoint meant to specifically assess the effects of each pass, and most passes more or less follow the same pathway as the first one, it was not acceptable to have a pass from one group influenced by former passes from the other needle group. It must be added that such a methodology can generate confusion in the routine of an EUS schedule and would lead to doubling of the intra-procedural costs.
Many technicalities and economic issues require further discussion and investigation, which this study is unable to address completely. Among others are the following:
Can larger (19G) or slimmer (25G) FNB needles, which are also available, exhibit similar differences with standard needles of the same caliber? However, although flexible needles have been developed for pancreatic access through the duodenum17 and 25G needles were shown in some studies to be at least as efficient as 22G,11 it is still uncertain whether or not the 22G standard size can be easily replaced for solid pancreatic masses;
Could recently introduced methods of sampling, such as the so-called ‘capillary’ or ‘slow-pull’ method, exhibit the same differences between FNB and FNA needles? Another study will be necessary to answer this question, but it was important to compare needle types using the most standard and widespread sampling method;
Do beveled needles exhibit more fragility and subsequently a higher dysfunction rate, thus dwarfing some advantages of FNB? Although some authors report up to 5–10% dysfunctional FNB needles, we experienced a lower rate of <2%, with no dysfunctional needle (either FNA or FNB) occurrence during the present study;
Is the extra spending induced by FNB needles economically relevant, if the proof of cancer can be obtained equally with FNA needles? This point exceeds the scope of this study and can only be addressed with knowledge of the local market and reimbursement conditions (e.g. FNB and FNA needles may be sold at the same price, as is the case in our center and other high-volume EUS centers in France).
In conclusion, our study showed that when compared to ‘standard’ FNA needles of the same gauge, the 22G reverse-beveled ‘FNB’ needles yielded samples of significantly higher histological quality, required a lower number of needle passes to achieve a diagnosis and a lower increment to achieve the maximal diagnostic contribution, despite a similar overall diagnostic accuracy. We consider that our findings can mitigate recently published views on the limited contribution of FNB needles in pancreatic cancer workup:18 Although histology is not necessary in most patients at the moment, the rapid advancement of personalized therapies and the associated need for molecular studies could soon make this statement obsolete and the need for EUS-sampled tissue microfragments more urgent. More immediately important is the evidence obtained from this and other studies that FNB allows for a better ratio of accuracy to number of passes, regardless of the availability of onsite pathology.
Funding statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare there are no conflicts of interest.
References
- 1.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB 2008; 10: 58–62. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ngamruengphong S, Li F, Zhou Y, et al. EUS and survival in patients with pancreatic cancer: A population-based study. Gastrointest Endosc 2010; 72: 78–83. [DOI] [PubMed] [Google Scholar]
- 3.Othman MO, Wallace MB. The role of endoscopic ultrasonography in the diagnosis and management of pancreatic cancer. Gastroenterol Clin North Am 2012; 41: 179–188. [DOI] [PubMed] [Google Scholar]
- 4.Itoi T, Sofuni A, Itokawa F, et al. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc 2011; 23: S17–21. [DOI] [PubMed] [Google Scholar]
- 5.Hewitt MJ, McPhail MJ, Possamai L. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc 2012; 75: 319–331. [DOI] [PubMed] [Google Scholar]
- 6.Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy 2004; 36: 397–401. [DOI] [PubMed] [Google Scholar]
- 7.Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc 2003; 57: 101–106. [DOI] [PubMed] [Google Scholar]
- 8.Hucl T, Wee E, Anuradha S, et al. Feasibility and efficiency of a new 22G core needle: A prospective comparison study. Endoscopy 2013; 45: 792–798. [DOI] [PubMed] [Google Scholar]
- 9.Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc 2012; 321: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanbiervliet G, Napoléon B, Paul MC, et al. Core needle versus standard needle for endoscopic ultrasound-guided biopsy of solid pancreatic masses: A randomized crossover study. Endoscopy 2014; 46: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 11.Iwashita T, Nakai Y, Samarasena JB, et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc 2013; 77: 909–915. [DOI] [PubMed] [Google Scholar]
- 12.Strand DS, Jeffus SK, Sauer BG, et al. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn Cytopathol 2014; 42: 751–758. [DOI] [PubMed] [Google Scholar]
- 13.Lee YN, Moon JH, Kim HK, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized parallel-group study. Endoscopy 2014; 46: 1056–1062. [DOI] [PubMed] [Google Scholar]
- 14.Wani S, Gupta N, Gaddam S, et al. A comparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Dig Dis Sci 2011; 56: 2409–2414. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc 2004; 59: 475–481. [DOI] [PubMed] [Google Scholar]
- 16.Jhala NC, Jhala DN, Chhieng DC, et al. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist's perspective. Am J Clin Pathol 2003; 120: 351–367. [DOI] [PubMed] [Google Scholar]
- 17.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc 2012; 76: 336–343. [DOI] [PubMed] [Google Scholar]
- 18.Holt B, Varadarajulu S. Endoscopic ultrasound-guided fine needle aspiration or fine needle biopsy: The beauty is in the eye of the beholder. Endoscopy 2014; 46: 1046–1048. [DOI] [PubMed] [Google Scholar]