Abstract
Background
The classical definition of chronic diarrhoea is ≥3 defecations/day, with a stool weight of more than 200 g and duration of ≥4 weeks. However, with this definition many patients with substantial symptoms and pathology will be excluded from further investigations. As a consequence other definitions have been proposed, mainly based on evaluation of the stool form.
Objective
To evaluate the accuracy of the classic criteria for diarrhoea in comparison with a definition based on stool consistency, using the Bristol Stool Form Scale.
Methods
All patients were investigated with laboratory tests, upper and lower gastrointestinal endoscopy with biopsies, and SeHCAT test. They were asked to complete a diary recording stool frequency and consistency during a week, as well as other gastrointestinal symptoms (pain, bloating and gas).
Results
One hundred and thirty-nine subjects were eligible for analysis. Ninety-one had an organic cause of diarrhoea. Fifty-three patients had ≥3 loose stools/day, whereas 86 reported <3 stools/day. Ninety had a median stool consistency that was mushy or loose and 49 had harder stools. A higher proportion of subjects with an organic cause of their diarrhoea compared with subjects with a functional bowel disorder had ≥3 loose stools/day, 43/91 (47%) vs. 10/48 (21%) (p < 0.01). Similarly, more subjects with an organic cause of their diarrhoea versus patients with a functional bowel disorder had a median stool consistency that was mushy or watery, 73/91 (80%) vs. 17/48 (35%), p < 0.0001. When diarrhoea was defined according to stool form, more patients were classified correctly as having a functional disorder or organic disorder, compared with the classical definition (p < 0.05).
Conclusion
Loose stools defined according to the Bristol Stool Form scale seem to be the best predictor of having an organic cause of the diarrhoea.
Keywords: Diarrhoea, definition of diarrhoea, investigation of diarrhoea, bile acid diarrhoe, microscopic colitis
Background
Chronic diarrhoea is a common condition and a key symptom in many disorders, with a reported prevalence of 4–5% in Western populations.1,2 The prevalence varies depending on population and the definition of diarrhoea used. The most common causes of diarrhoea are functional bowel disorders such as irritable bowel syndrome (IBS) and functional diarrhoea.3 Among organic diseases with diarrhoea the most common causes are inflammatory bowel disease, microscopic colitis and bile acid diarrhoea.4 In addition, a large number of other diseases may cause diarrhoea. It is often difficult to make a reliable differentiation between organic and functional causes in patients with chronic diarrhoea based solely on history and physical examination and even more difficult to make a reliable diagnosis. However, the occurrence of alarm symptoms such as anaemia, rectal blood and weight loss are strong indicators of an organic disease.3 The investigation of chronic diarrhoea includes extensive blood sampling and cultures as well as gastroscopy and colonoscopy with biopsies,4–6 and the SeHCAT test to reveal bile acid diarrhoea.4,7,8
It is important that subjects with an organic disorder are properly investigated in order to find a potentially curable disease. On the other hand, patients with a functional bowel disorder should not unnecessarily undergo extensive investigations. Hence, an ideal definition of chronic diarrhoea should be easy to apply and should differentiate subjects with an organic disorder from those with a functional bowel disorder.
The classical definition of chronic diarrhoea is at least three loose stools per day with a faecal weight of more than 200 g/day, lasting for at least four weeks.3,9–11 However, this definition has its limitations. First of all, the precision of this definition has been questioned and many patients with substantial symptoms and pathology on routine investigations could risk being excluded from investigation and specific treatment if this definition is used to guide which patients should undergo further investigations.4,9 Secondly, faecal weight is cumbersome to measure and the test is not popular among patients and staff. In reality, the classical definition is often modified to include only the stool frequency and the duration of diarrhoea. Although stool frequency is easy to assess, the relationship with colonic function is moderate.12 As a consequence of these shortcomings, other definitions have been proposed such as a substantial increase in stool frequency, volume and/or fluidity,4,9 or a stool consistency of type 6 (mushy stools) or 7 (watery stools) as described in the Bristol Stool Form (BSF) scale.12,13 Defining diarrhoea in relation to stool consistency has theoretical advantages since stool form and consistency in previous studies show a better correlation with transit time and faecal output.14–16
Therefore, the aim of this study was to investigate patients referred for diagnostic work-up of chronic diarrhoea and to evaluate the outcome of two different definitions of diarrhoea: the classic criteria for diarrhoea of ≥3 loose stools/day compared with diarrhoea defined as a median stool consistency of at least type 6 (mushy stools), as defined in the BSF scale.
Methods
Consecutive patients referred to our outpatient clinic for investigation of diarrhoea from primary care and non-gastroenterological hospital clinics were included. All patients were subjected to a standardized investigation including laboratory tests for haemoglobin concentration, sedimentation rate, C-reactive protein, vitamin B12, folic acid, iron saturation, albumin, thyroid hormones and alkaline phosphatase. Stool cultures and microscopy for parasites were performed, as well as upper and lower gastrointestinal endoscopy with biopsies and a SeHCAT test for bile acid diarrhoea. Endoscopies were performed according to the clinical routine including biopsies from the descending part of the duodenum and biopsies from both the right and the left part of the colon. Further investigation was performed when needed at the discretion of the physician. Calprotectin was not measured since the study partly was performed before calprotectin was a part of the clinical routine. A diary was also completed to assess stool frequency and consistency and other gastrointestinal symptoms (pain, gas or bloating; see below).
Patients already diagnosed with a disease known to cause chronic diarrhoea were excluded. Informed consent was obtained from the participants, and the study was approved by the Regional Ethical Review Board in Gothenburg and by the radiation safety committee of the University of Gothenburg.
Diary
All subjects were asked to report all stools in a one-week diary to assess stool frequency and consistency. A modified BSF12 was used for grading of stool consistency. Only four grades were used in the questionnaire: consistency of BSF types 1–4 were regarded as non-diarrhoea, not differentiated thus and classified as normal for the purpose of this study. Looser stool consistency was divided according to the BSF as soft blobs with clear cut edges (type 5), mushy stool (type 6) and watery stool (type 7) (Figure 1).
Figure 1.
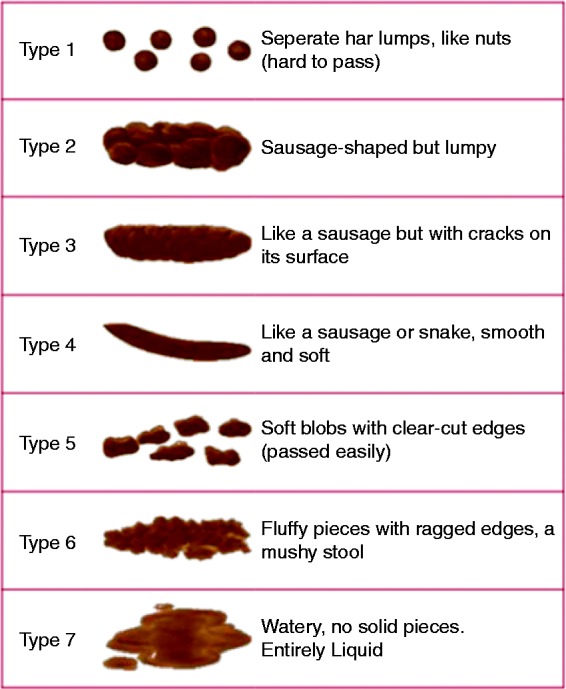
The Bristol Stool Form (BSF) scale (© 2006 The Rome Foundation. All rights reserved.). Only four grades were used in the questionnaire: consistency of BSF types 1–4 were regarded as non-diarrhoea, not differentiated thus and classified as normal for the purpose of this study. Looser stool consistency was divided according to the BSF as soft blobs with clear cut edges (type 5), mushy stool (type 6) and watery stool (type 7).
Diarrhoea was defined as a median stool consistency of at least BSF 6 (mushy or watery stools) with duration of at least four weeks, as proposed in the work by Spiller.13
For grading of pain, gas or bloating symptoms, a scale of 0–3 was used (0 = no symptoms at all, 1 = mild symptoms, 2 = moderate symptoms and 3 = severe symptoms).
75SeHCAT
The test was carried out according to the method described by Thaysen and Pedersen.7 After an overnight fast a capsule containing 0.3 MBq 75Se-labelled homocholic acid taurine (75SeHCAT) was swallowed with a glass of water. Measurements were performed with an uncollimated camera. The patient was in a supine position with the camera positioned at a distance of 60 cm. A measurement over the abdomen 3 h after ingestion of the capsule gave the basal value (100%). A repeated measurement was performed after seven days, and the cut-off value for diagnosing bile acid diarrhoea was <10% retention on day 7.8
Statistical analysis
Continuous numerical variables are presented as mean + SD. Other results are presented as median and range. Categorical variables are presented as percentages. Student’s t-test was used to compare continuous variables and the chi-squared test was used to compare categorical variables. The statistical analyses were carried out by using SPSS Statistics 17.0.
Results
Subjects
Two hundred and seven subjects were referred because of diarrhoea (mean age 45 (range 17–84) years, 122 women, 85 men). Sixty-eight subjects were excluded: 37 did not meet the inclusion criteria, six of whom had known gastrointestinal disease, and 31 withdrew their consent; 31 did not complete the questionnaire without giving any specific reason for this, leaving 139 subjects eligible for analysis (mean age 46 (range 17–84) years, 88 women, 51 men) (Figure 2).
Figure 2.
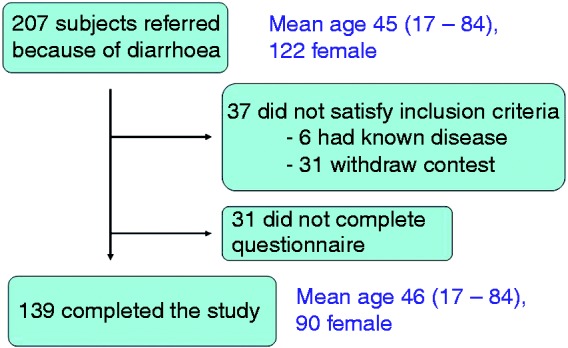
Study flow chart.
Final diagnoses
Forty-eight of 139 patients (35%) had no objective findings during the diagnostic work-up that could explain their diarrhoea and these patients fulfilled the diagnostic criteria for a functional bowel disorder (functional diarrhoea or diarrhoea-predominant IBS) according to the Rome II criteria.17 In 91 patients (65%) an explanation for their diarrhoea was found on investigation or through their medical history. The most common organic bowel diseases found were bile acid diarrhoea and microscopic colitis. For gastrointestinal diagnoses see Table 1.
Table 1.
Diagnoses after work-up
| Functional diarrhoea | n = 48 |
|---|---|
| Bile acid diarrhoea | n = 48 |
| Microscopic colitis | n = 17 |
| Inflammatory bowel disease | n = 9 |
| Microscopic colitis and bile acid diarrhoea | n = 7 |
| Lactose intolerance | n = 3 |
| Coeliac disease | n = 2 |
| Drugs | n = 2 |
| Pancreatic disease | n = 2 |
| Bacterial overgrowth | n = 1 |
| Total | n = 139 |
Definitions of diarrhoea in relation to final diagnoses
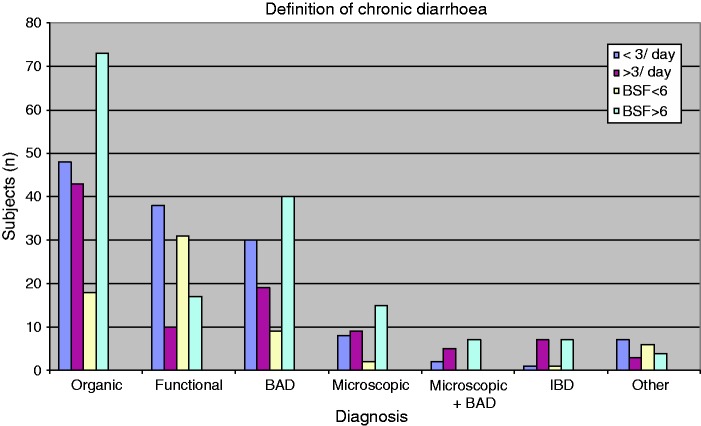
The mean stool frequency was 2.9/day (range 0.4–18.3). Fifty-three patients had ≥3 loose stools/day, whereas 86 reported <3 stools/day. Forty-nine patients had a median stool form of <6/day, whereas 90 had a median stool form of ≥6. A higher proportion of subjects with an organic cause of their diarrhoea compared with subjects with a functional bowel disorder had ≥3 loose stools/day, 43/91 (47%) vs. 10/48 (21%), (p < 0.01). Similarly, more subjects with an organic cause of their diarrhoea compared with subjects with a functional bowel disorder had a median stool form ≥6, 73/91 (80%) vs. 17/48 (35%), p < 0.0001. Specifically, in patients with microscopic colitis 9/17 patients (53%) had ≥3 loose stools/day, whereas 15/17 patients (88%) hade a median stool form ≥6. In patients with bile acid diarrhoea, 18/48 patients (44%) had ≥3 loose stools/day, whereas 39/48 patients (81%) hade a median stool form of ≥6. In patients with functional bowel disorder 10/48 (21%) had ≥3 loose stools/day, whereas 17/48 (35%) had a median stool form of ≥6 (Figure 3). Forty-eight patients with <3 loose stools/day had an organic disease and 18 patients with an organic disease had a median stool form of <6/day. For individual diagnoses see Table 2. When diarrhoea was defined as at least a median stool consistency of mushy stools, that is, at least BSF type 6, significantly more patients were classified correctly as having functional bowel disorder or organic disease (p < 0.05) compared with the classical definition. Sensitivity and specificity as well as positive and negative predictive values for finding organic disease for the two definitions are shown in Table 3.
Figure 3.
Outcome when applying the two different definitions of chronic diarrhoea in relation to diagnosis. <3 and ≥3: less than three diarrhoea defecations per day and three or more diarrhoea defecations per day, respectively. BSF <6 and BSF >6: a median BSF less than 6 and a median BSF more than 6, respectively.
BSF: Bristol Stool Form; BAD: bile acid diarrhoea; IBD: inflammatory bowel disease
Table 2.
Subjects with an organic disease not fulfilling the definitions for diarrhoea, ≥3 diarrhoea defecations/day or median BSF 6
| <3 diarrhoea/day | Median BSF <6 | ||
|---|---|---|---|
| Bile acid diarrhoea | n = 30 | Bile acid diarrhoea | n = 9 |
| Microscopic colitis | n = 8 | Microscopic colitis | n = 2 |
| Microscopic colitis and bile acid diarrohea | n = 2 | Microscopic colitis and bile acid diarrohea | n = 0 |
| Lactose intolerance | n = 3 | Lactose intolerance | n = 3 |
| Celiac disease | n = 2 | Celiac disease | n = 2 |
| Inflammatory bowel disease | n = 1 | Inflammatory bowel disease | n = 1 |
| Drug induced diarrhoea | n = 1 | Drug induced diarrhoea | n = 1 |
| Small intestinal bacterial overgrowth | n = 1 | Small intestinal bacterial overgrowth | n = 0 |
BSF: Bristol Stool Form
Table 3.
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for predicting organic disease for the two definitions, Bristol Stool Form (BSF) 6, and ≥3 diarrhoea defecations/day
| Definition | BSF type 6 | ≥3 loose stools/day |
|---|---|---|
| Sensitivity | 80% (73/91) | 47% (43/91) |
| Specificity | 65% (31/48) | 79% (38/48) |
| PPV | 81% (73/90) | 81% (43/53) |
| NPV | 63% (31/49) | 44% (38/86) |
Other symptoms
No significant differences regarding the median severity of the other gastrointestinal symptoms (pain, flatulence or bloating) were seen between subjects with functional bowel disorder compared with subjects with an organic cause to the diarrhoea. (Data not shown.)
Results of laboratory testing
Compared with patients with functional bowel disorders, patients with an organic disease had lower levels of albumin (40.6 ± 4.7 vs. 42.5 ± 3.8 g/l (mean ± SD); p = 0.01), lower haemoglobin concentration (140 ± 13 vs. 144 ± 11 g/l; p < 0.05) and higher sedimentation rate (13 ± 15 vs. 7.6 ± 6.9 mm; p < 0.01). There were no significant differences between the two groups in levels of vitamin B12, folic acid, iron concentration, thyroid hormones, alkaline phosphatase or C-reactive protein. Two subjects had stool samples positive for Giardia intestinalis. No patient had a positive stool culture for bacteria.
Discussion
In the present study we have demonstrated that the most commonly used definition of diarrhoea, the so-called ‘classical definition’, has a very low sensitivity for organic disease and as many as 46% of subjects with an organic cause of their diarrhoea did not fulfil this definition. Applying the classical definition of diarrhoea in this cohort had the consequence that nearly half of the patients with an organic disorder causing chronic diarrhoea would have been left without a correct diagnosis and, therefore, without proper treatment had this definition been used to determine the diagnostic strategy, that is, performing further investigation or not. When definition of diarrhoea was based on the stool consistency this number was reduced to 19%. The downside of this is reduced specificity, resulting in extensive investigations in a slightly larger proportion of subjects with functional bowel disorders, 35% of the patients when diarrhoea was defined as at least BSF 6, compared with 21% using the classical definition.
An alternative definition of chronic diarrhoea as a substantial increase in stool frequency, volume and/or fluidity has been used4,9 in some previous papers. However this definition is not useful since the result of applying this definition would be an extensive investigation of all patients referred because of diarrhoea, including all subjects with functional bowel disorder.
There are some limitations in the present study. When diarrhoea is defined as ≥3 loose stools/day without including faecal weight, it is a simplification of the classical definition. However, adding faecal weight as a criterion sharpens the definition more and would probably exclude from investigation even more patients with an organic disease.
The proportion of patients with inflammatory bowel disease or cancer is rather low in patients in the present study and differs from a previous study.4 However, the subjects in that study were recruited from an endoscopy unit. Subjects with alarm symptoms such as bloody stools are more likely to be referred directly for endoscopy instead of to the outpatient clinic. In many cases, the patients will then receive a diagnosis at the index endoscopy and have their treatment started and subsequently be excluded due to having a known diagnosis causing chronic diarrhoea when appearing at the outpatient clinic. As a result, the proportion of subjects diagnosed as having, for example, inflammatory bowel disease, is comparably low in the investigated cohort relative to some previous studies.3,4 This approach has probably altered the proportion between functional and organic causes for chronic diarrhoea in the direction of a smaller proportion of subjects having an organic disease in our study.
The study population may not be representative for the general population with chronic diarrhoea. Although subjects already diagnosed with a disease that could explain the diarrhoea were excluded, the population attending our outpatient clinic probably had a tendency to be sicker than the population attending the primary care and non-gastroenterological hospital clinics. This may also have altered the proportion between functional and organic causes for chronic diarrhoea, this time probably in the direction of a larger proportion of organic causes.
There is probably an overlap between bile acid diarrhoea and functional bowel disorder in patients with intermediate levels (5–10%) in the SeHCAT test.8,18 An alternative had been to add response to treatment with bile acid sequestrants as an additional diagnostic tool, demanding a positive response as a prerequisite to fulfil the diagnosis of bile acid diarrhoea. However, doing this should have been of limited value because many subjects with IBS may respond favourably to this treatment as well, since an abnormal SeHCAT is not uncommon in patients with IBS and patients with a normal SeHCAT test sometimes respond to treatment as well. The absolute cut-off to use clinically to differentiate between IBS and bile acid diarrhoea is still debated.18–21
However, it is unlikely that these limitations have affected the results and the conclusions in a major way.
The importance of investigating subjects with <3 loose stools/day or having a stool consistency of less than type 6 could be discussed. However, the mere fact that these subjects seek health care indicates that the symptoms are of some importance. However, an approach could be to symptomatically treat subjects not fulfilling the different definitions, since the risk of a serious disease is low in this group.
Accompanying symptoms such as pain, gas or bloating were unfortunately of limited additional value for discriminating between functional and organic diarrhoea. Signs of inflammation and anaemia in the laboratory testing are more common in organic diarrhoea but normal blood values do not exclude an organic cause of diarrhoea. Faecal cultures seem to be of no value when investigating chronic diarrhoea although tests for parasites may be of some importance.22
In conclusion, none of the investigated definitions of diarrhoea is perfect but defining diarrhoea as at least mushy or watery stools according to the BSF scale will result in fewer patients with an organic disease being excluded from investigation and treatment as compared with the classical definition of diarrhoea.
Funding
This work was supported by the Health and Medical Care Executive Board of Västra Götaland Region (grant 1042) and by the Swedish Society of Medicine (grants SLS-175231).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Fine KD, Schiller LR. AGA technical review on the education and management of chronic diarrhoea. Gastroenterology 1999; 116: 1464–1486. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: A population based study. Gastroenterology 1992; 102: 895–890. [DOI] [PubMed] [Google Scholar]
- 3.Bytzer P, Stokholm M, Andersen L, et al. Aetiology, medical history and faecal weight in adult patients referred for diarrhoea: A prospective study. Scand J Gastroenterol 1990; 25: 572–578. [DOI] [PubMed] [Google Scholar]
- 4.Müller M, Willen R, Stotzer P. Colonoscopy and SeHCAT for investigation of chronic diarrhea. Digestion 2004; 69: 211–218. [DOI] [PubMed] [Google Scholar]
- 5.Marshall JB, Singh R, Diaz-Arias AA. Chronic unexplained diarrhoea: Are biopsies necessary if colonoscopy is normal? Am J Gastroenterol 1995; 90: 372–376. [PubMed] [Google Scholar]
- 6.Lindström CG. Collagenous colitis with watery diarrhoea: A new entity? Pathol Eur 1976; 11: 87–89. [PubMed] [Google Scholar]
- 7.Thaysen EH, Pedersen L. Idiopathic bile acid catharsis. Gut 1975; 17: 965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams A, Merrick M, Eastwood M. Idiopathic bile acid malabsorption – a review of clinical presentation, diagnosis, and response to treatment. Gut 1991; 32: 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper BT. Diarrhoea as a symptom. Clin Gastroenterol 1985; 14: 599–613. [PubMed] [Google Scholar]
- 10.Stanton B, Clemens JD. Chronic diarrhoea: A methodologic basis for its apparent heterogeneity. Trop Geogr Med 1989; 41: 100–107. [PubMed] [Google Scholar]
- 11.Talley NJ, Zinsmeister AR, Van Dyke C, et al. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology 1991; 101: 927–934. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell LJD, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 1990; 300: 439–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiller R. Role of motility in chronic diarrhoea. Neurogastroenterol Motil 2006; 18: 1045–1055. [DOI] [PubMed] [Google Scholar]
- 14.Degen LP, Phillips SF. How well does stool form reflect colonic transit? Gut 1996; 39: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saad RJ, Rao SS, Koch KL, et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol 2010; 105: 403–411. [DOI] [PubMed] [Google Scholar]
- 16.Törnblom H, Van Oudenhove L, Sadik R, et al. Colonic transit time and IBS symptoms: What’s the link? Am J Gastroenterol 2012; 107: 754–760. [DOI] [PubMed] [Google Scholar]
- 17.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut 1999; 45(Suppl. 2): II43–I147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajor A, Törnblom H, Rudling M, et al. Increased colonic bile acid exposure: A relevant factor for symptoms and treatment in IBS. Gut. Jan; 64(1): 84–92. Epub ahead of print 12 April 2014. [DOI] [PubMed]
- 19.Wedlake L, A’Hern R, Russell D, et al. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009; 30: 707–717. [DOI] [PubMed] [Google Scholar]
- 20.Aziz I, Kurien M, Sanders DS, et al. Screening for bile acid diarrhoea in suspected irritable bowel syndrome. Gut. Epub ahead of print 28 May 2014. [DOI] [PubMed]
- 21.Bajor A, Törnblom H, Rudling M, et al. Authors’ response: Bile acids are important in the pathophysiology of IBS. Gut. Epub ahead of print 23 July 2014. [DOI] [PubMed]
- 22.Kaiser L, Surawicz CM. Infectious causes of chronic diarrhoea. Best Pract Res Clin Gastroenterol 2012; 26: 563–571. [DOI] [PubMed] [Google Scholar]