Abstract
Biomimetic scaffolds hold great promise for therapeutic repair of cartilage, but although most scaffolds are tested with cells in vitro, there are very few ex vivo models (EVMs) where adult cartilage and scaffolds are co-cultured to optimize their interaction prior to in vivo studies. This study describes a simple, non-compressive method that is applicable to mammalian or human cartilage and provides a reasonable throughput of samples. Rings of full-depth articular cartilage slices were derived from human donors undergoing knee replacement for osteoarthritis and a 3 mm core of a collagen/glycosaminoglycan biomimetic scaffold (Tigenix, UK) inserted to create the EVM. Adult osteoarthritis chondrocytes were seeded into the scaffold and cultures maintained for up to 30 days. Ex vivo models were stable throughout experiments, and cells remained viable. Chondrocytes seeded into the EVM attached throughout the scaffold and in contact with the cartilage explants. Cell migration and deposition of extracellular matrix proteins in the scaffold was enhanced by growth factors particularly if the scaffold was preloaded with growth factors. This study demonstrates that the EVM represents a suitable model that has potential for testing a range of therapeutic parameters such as numbers/types of cell, growth factors or therapeutic drugs before progressing to costly pre-clinical trials. © 2015 The Authors. Cell Biochemistry and Function Published by John Wiley & Sons Ltd.
Significance
Pre-clinical trials of biomaterials for cartilage repair are very costly, and all too often, studies progress directly from in vitro studies using isolated cells to in vivo studies without investigating the interaction between the target tissue and the scaffold. Our study uses viable cartilage from adult human donors with osteoarthritis and therefore represents the exact scenario that the scaffold is designed for. The system is cheap and simple to set up and is suitable for a 48-well plate format, meaning a reasonable throughput is obtainable. This lends the model to therapeutic drug testing.
Keywords: regenerative medicine, cartilage, osteoarthritis, scaffold
Introduction
Articular cartilage is a form of hyaline cartilage that covers the bony articulating ends of synovial joints. This stiff load-bearing tissue resists tensile forces, compression and shearing, while maintaining some resilience and elasticity. The unique biomechanical properties of articular cartilage are attributed to the complex zonal arrangement of its constituent macromolecules, collagen and proteoglycan, which are maintained by cells known as chondrocytes.1 Injuries to articular cartilage yield poor intrinsic repair and present a major risk factor in the development of osteoarthritis (OA) in later life.2 Partial thickness (chondral) defects that only affect the cartilage are the most difficult to repair as neighbouring chondrocytes are incapable of repairing adjacent defects, and as these injuries do not penetrate the bone, the body does not initiate a spontaneous repair response.3 The key factors required for cartilage repair are the recruitment, retention and proliferation of cells at the defect site,4 and improving cartilage repair has become a major goal in the tissue engineering field.
In the last 20 years, the related fields of tissue engineering, regenerative medicine and cell therapy have emerged as major research areas leading to new developments in orthopaedic surgery.5,6 These approaches aim to repair diseased/damaged tissue and promote new tissue regeneration. One particularly active research area is the use of biomimetic scaffolds [either used alone or as vehicles to deliver growth factors (GFs)] to enhance the repair process.7,8
While bone regeneration is relatively easy to influence with tissue engineering, scaffolds designed to promote the repair of other joint tissues, such as articular and meniscal cartilage, and tendon have met with limited success when used to treat human patients in the clinic.9,10 However, the development of scaffolds+/−GF remains a key goal in musculo-skeletal research and is likely to continue to be a growth area for research and development. Although many in vitro and in vivo studies are carried out each year searching for the ‘perfect’ scaffold, a huge gulf remains in making the transition from the production of the scaffold to success in the clinic. This is partly due to the technical difficulties in generating effectively engineered scaffolds but is also due to a lack of efficacy of the pre-clinical animal models that are used to predict human tissue repair responses.11 Often, scaffolds are simply tested in small laboratory-based projects quantifying cytotoxicity and cell proliferation prior to progressing to animal trials. The major limitation of these simple studies is that they fail to address the complexity of the tissue that is being targeted for repair, i.e. the three-dimensional structure, the mixture of cell types, the cellular organization of the tissue and the local autocrine environment.
In this study, we have produced an ex vivo model (EVM) system that replicates, as far as possible in vitro, the interaction between scaffold and articular cartilage. These EVMs will allow both the testing of scaffolds as biomaterials and cell supports but also as delivery devices for GF. In addition to evaluating the ability of scaffolds and GF to influence repair, the EVM would also be ideal to test therapeutic drugs. As such, it has the potential to provide valuable experimental tools to the medical, veterinary and biological research communities. Following characterization of the EVM, we then used it to test its suitability to deliver biologically active GFs [insulin-like growth factor-1 (IGF-1) and transforming growth factor beta-1 (TGF-β1)] to sites of repair.
To date, the collagen–glycosaminoglycan (CG) scaffold has been incubated with IGF-1 to load this growth factor via physical sorption. Previous work has demonstrated that the IGF-1 released from CG scaffolds had a therapeutic effect on OA chondrocytes seeded within the CG scaffold to synthesis cartilaginous extracellular matrix (ECM).9 However, the effects of this system on a host tissue in vivo is unknown, and therefore, it is desirable to have an EVM in which to test the interactions of growth factor-loaded scaffold in close proximity to living cartilage as the interaction between chondrocytes and their microenvironment plays a key role in cell behaviour.
Towards this goal, this research describes loading a CG scaffold with bioactive molecules and cells to enhance tissue regeneration within a novel EVM of articular cartilage repair. The aims of this study were twofold. Firstly, we aim to develop a reasonable throughput EVM of cartilage repair that enables scaffold and cartilage explants to be incubated in close contact. Secondly, we aim to incorporate bioactive molecules into the CG scaffold with aim to yield de novo hyaline articular cartilage repair tissue. In this EVM, the CG scaffold will be in constant contact with an OA cartilage explant, which allows a more realistic evaluation of the potential of this cell-seeded scaffold to induce repair in a model that includes catabolic enzymes and cytokines released from chondrocytes from the cartilage explant.
Materials and Methods
Materials
Type I acid-insoluble collagen was prepared as described.8 Human recombinant IGF-1 and TGF-β1 were purchased from R&D Systems. Dulbecco's modified eagle medium was purchased from Invitrogen and Collagenase A from Roche. Articular cartilage was obtained from patients undergoing total knee replacement surgery with full ethical consent (NREC 06/Q0108/213) and used to prepare explants, and primary chondrocytes were derived by collagenase digestion.
Primary antibodies: collagen type I (Rockland), collagen type II: AVT6E3 (kind gift from Anne Vaughan-Thomas, University of Cardiff), decorin (R&D Systems), fibronectin (Santa Cruz). Peroxidase or fluorescein isothiocyanate-conjugated secondary antibodies were all from Sigma. Collagen types I and II standards were prepared in-house from bovine skin or human articular cartilage, respectively. Recombinant human decorin was from R&D systems. Immobilon Polyvinylidene fluoride (PVDF) (Millipore) membranes and ECL plus (GE Healthcare) were used for Western blotting. 1,9-Dimethyl-methylene blue (DMMB) was purchased from Sigma-Aldrich.
Methods
Scaffold preparation
Collagen/glycosaminoglycan scaffolds were prepared as described previously.8
EVM preparation
Full-depth articular cartilage was removed from OA femoral condyles using a scalpel and 5 mm discs created using a biopsy punch (Kai Medical). A 3 mm diameter biopsy punch was then used to punch out a hole in the centre of each disc to form ring-shaped explants prior to inserting 3 mm CG scaffold discs.
Cell culture
Incubated in media were 3 mm CG scaffold discs [Dulbecco's modified eagle medium containing 10% foetal calf serum, 100 IU/100 µg/ml penicillin/streptomycin and 20μg/ml ascorbate-2-phosphate (Sigma) containing IGF-1 (25 µg/ml), TGF-β1 (10 µg/ml), IGF-1 plus TGF-β1 or media only for 24 h at 37 °C]. Unbound GF was removed, and each scaffold seeded with 1 × 105 primary OA chondrocytes as described previously8 before insertion into each explant ring (Figure1A). EVM were cultured in 0.5 ml of complete media or complete media supplemented with exogenous growth factors for 28 days. Media were collected every 3–4 days and replaced with fresh media and fresh exogenous growth factors if applicable. Table 1 lists the experimental groups investigated.
Figure 1.
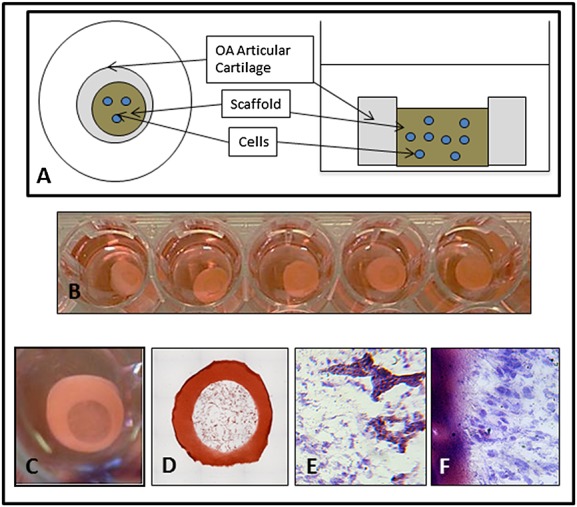
Characteristics of human articular cartilage ex vivo model (EVM). (A) EVM construction. (B) EVMs in 48-well plate. (C) EVM after 14 days in vitro. (D) EVM stained with safranin O. (E) and (F) EVM stained with toluidine blue after 14 days in vitro showing cells added at day 0 populating the scaffold (E) and in contact with the cartilage (F). Scale bar = 100 µm. OA, osteoarthritis
Table 1.
Treatment groups
| Scaffold model | Growth factor (media) | Concentration (ng/ml) |
|---|---|---|
| Control | 0 | 0 |
| Adsorbed IGF-1 | 0 | 0 |
| IGF | IGF-1 | 100 |
| TGF | TGF-β1 | 10 |
| IGF + TGF | IGF-1 + TGF-β1 | 100, 10 |
IGF-1, insulin-like growth factor-1; TGF-β1, transforming growth factor beta-1.
DMMB assay
The total amount of proteoglycan synthesized by the cells was measured using the DMMB assay.8 Media samples (40 µl) and chondroitin sulphate (CS) standards (40 µl) were added to a 96-well plate, and 250 µl of DMMB was added to each well. The absorbance was measured immediately at 544 nm in a FLUOstar OPTIMA plate reader. The average amount of accumulated sulphated glycosaminoglycan (GAG) and standard error of the mean were reported and data compared using a two-tailed Student's t-test.
Western blotting
After 28 days, the EVMs were dismantled, the explants discarded and the amount of extracellular matrix deposited in the scaffold analysed. Added to each scaffold was 300 µl of cell extraction buffer (Invitrogen). The samples were rotated overnight at 4 °C, centrifuged and the supernatant mixed with sodium dodecyl sulphate (SDS) sample buffer before running on SDS electrophoresis and Western blotting. Standard curves for decorin and type II collagen were prepared using purified proteins and used to convert the relative optical density (OD) values from the scaffold samples into protein concentrations. All error bars represent the standard error of the mean, and each experiment was carried out for at least N = 3 donors.
Immunohistochemistry
After 28 days, whole EVMs were placed in Tissue-Tek (Sakara) embedding medium, snap-frozen in liquid nitrogen and 8 µm sections made using a cryostat. Sections were fixed using a 1:1 solution of acetone and methanol before probing with primary antibodies and fluorescein isothiocyanate-conjugated secondary antibodies and mounting in Vectashield (Vector Laboratories) containing 4′,6-diamidino-2-phenylindole.
Results
EVM histology
A single human OA knee joint can yield enough intact cartilage to create up to 50 EVMs allowing multiple treatment groups on the same donor tissue. These proved stable enough in culture to remain intact for many weeks throughout media changes and other manipulations (Figure1B and C). Histological staining demonstrated that the scaffold was in close contact with the cartilage in the model (Figure1D) and that seeded cells populated the scaffold void (Figure1E) and grew in close contact with the cartilage after 4 weeks in culture (Figure1F).
Release of sulphated GAG from the CG scaffold
Ex vivo models pre-treated with adsorbed IGF-1 (AI) and those treated with IGF-1 plus TGF-β1 released a significantly higher amount of sulphated GAG into the media compared with the no-GF control group after 28 days (Figure2), but there were no significant differences between the GF groups.
Figure 2.
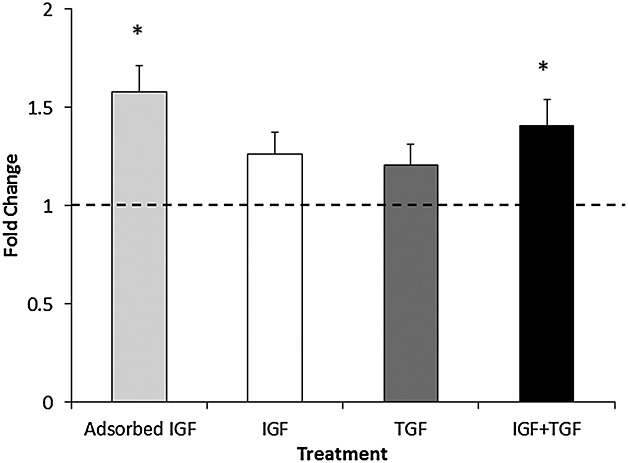
Amount of sulphated GAG released into the media expressed as fold change compared with the no-growth factors control group. Concentration of growth factors as in Table 1. N = 3 donors, N = 3 replicates. * represents a statistically significant difference between groups versus control group (p < 0.05). IGF, insulin-like growth factor; TGF, transforming growth factor
Decorin deposition within scaffolds
Decorin was detected in all scaffold groups as shown in a representative Western blot [Figure3(top)]. However, when combined with analysis of replicate experiments using OA chondrocytes derived from other patients (not shown), only the AI group yielded statistically significant (p < 0.05) higher amounts of decorin deposition in the scaffold when compared with the control and the other GF groups [Figure3(bottom)].
Figure 3.
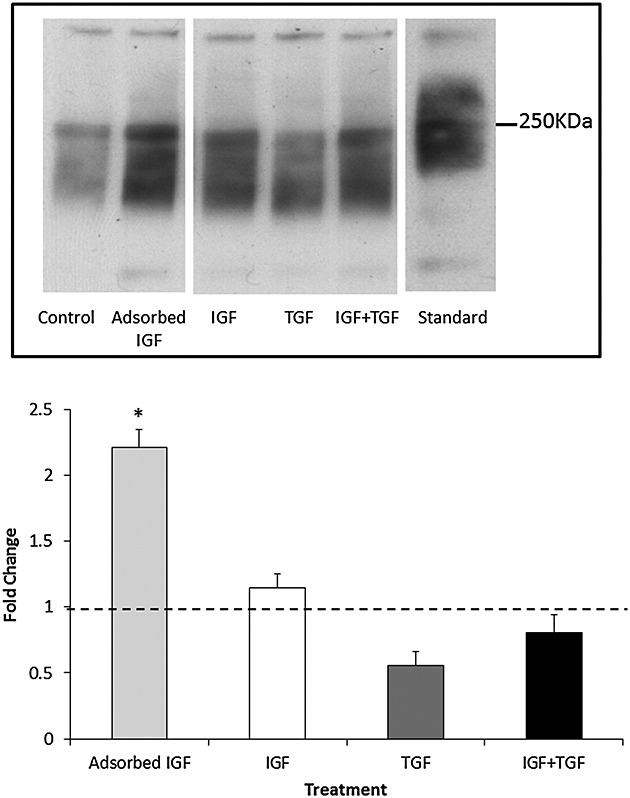
Decorin deposition within the scaffold. (Top) Representative Western blot demonstrating the amount of decorin deposited by osteoarthritis chondrocytes within each scaffold group. Concentration of growth factors as in Table 1. Standard = extract of whole human articular cartilage. (Bottom) Decorin deposition in scaffolds quantified by densitometry of Western blot (top) expressed as fold change compared with the no-growth factor control group. N = 3 donors, N = 3 replicates. * represents a statistically significant difference between groups (p < 0.05) compared with control. IGF, insulin-like growth factor; TGF, transforming growth factor
Type I collagen deposition within scaffolds
Type I collagen deposition was detected in all scaffold groups [Figure4(top)] and although there was a slight overall increase in type I collagen with both IGF-1 (I) and IGF-1 plus TGF-β1 compared with control, neither was statistically significant when combined with data from further donors [Figure4(bottom)]. However, OA chondrocytes from the AI group deposited statistically significantly less (p < 0.01 vs control) type I collagen onto the scaffold compared with the control or any other of the GF conditions [Figure4(bottom)]. Scaffolds incubated without cells for comparable times did not release any detectable type I collagen when Western blotted (data not shown).
Figure 4.
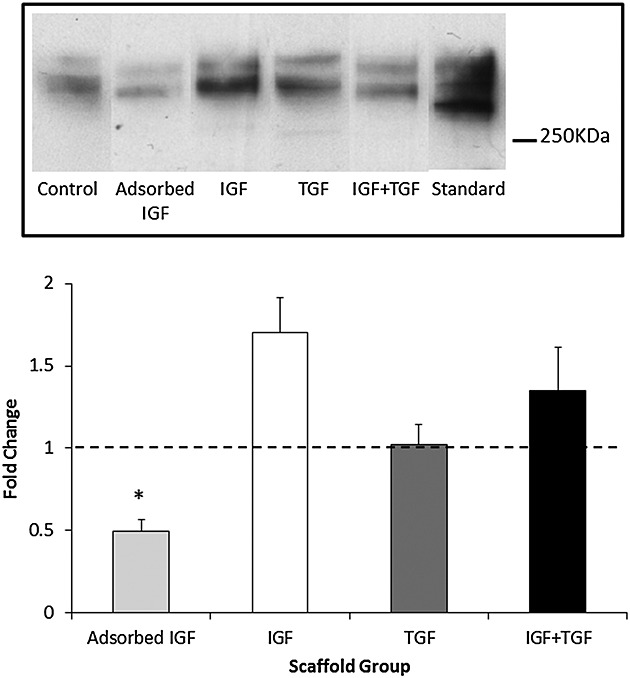
Type I collagen deposition within the Scaffold. (Top) Representative Western blot demonstrating the amount of type I collagen deposited by osteoarthritis chondrocytes within each scaffold group. Concentration of growth factors as in Table 1. Standard = type I collagen standard. (Bottom) Type I collagen deposition in scaffolds quantified by densitometry of Western blot (top) expressed as fold change compared with the no-growth factor control group. N = 3 donors, N = 3 replicates. * represents a statistically significant difference between groups (p < 0.01) compared with control. IGF, insulin-like growth factor; TGF, transforming growth factor
Type II collagen deposition within scaffolds
Low levels of type II collagen were detected in all scaffold groups [Figure5(top)], but chondrocytes seeded within the AI group deposited significantly more type II collagen in the scaffold compared with the control or any other GF groups when data from all donors were combined [Figure5(bottom)]. This result was found to be statistically significant (p < 0.01 vs control).
Figure 5.
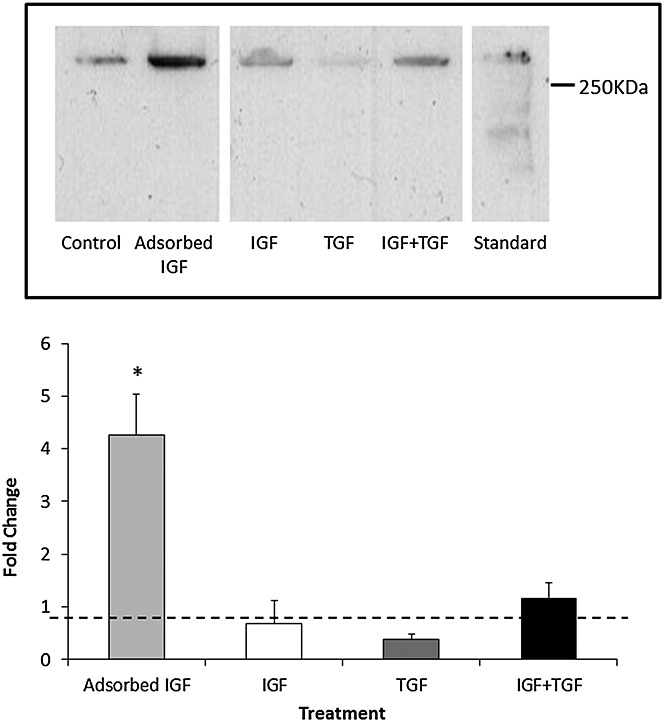
Type II collagen deposition within the scaffold. (Top) Representative Western blot demonstrating the amount of type II collagen deposited by osteoarthritis chondrocytes within each scaffold group. Concentration of growth factors as in Table 1. Standard = extract of articular cartilage (Bottom) Type II collagen deposition in scaffolds quantified by densitometry of Western blot (top) expressed as fold change compared with the no-growth factor control group. N = 3 donors, N = 3 replicates. * represents a statistically significant difference between groups (p < 0.05) compared with control. IGF, insulin-like growth factor; TGF, transforming growth factor
Immunohistochemistry
A qualitative visualization of the ECM was made by cryo-sectioning whole EVMs at the final time point and staining for various ECM proteins. In all treatment groups, cells had deposited a substantial amount of ECM within the scaffold and between the scaffold and the cartilage explants (Figure6). Chondrocytes were observed throughout the new ECM.
Figure 6.
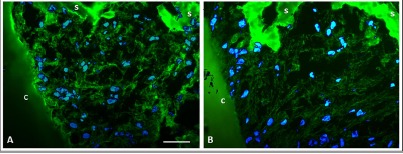
Localization of extracellular matrix proteins in the ex vivo model. (A) Immunostaining for fibronectin. (B) Immunostaining for type I collagen. c: articular cartilage explant; s: scaffold. Scale bar = 50 µm
Discussion
An optimal EVM of cartilage repair needs to meet several criteria:
The model needs to maintain the scaffold at a pressure that will not deform the scaffold but will allow close contact with the cartilage in a similar fashion to that which will be experienced in vivo.
The model needs to be applicable to multiple species, scaffold types and tissue types.
The model needs to be of reasonable throughput in order to test a range of compounds or growth factors.
Scaffold design has centred on developing ECM analogues. For cartilage tissue engineering, highly porous scaffolds with large surface areas and adequate mechanical strength are desirable, while a pore size of 100–500 µm has been reported to be optimal for this repair.11,10,12–16 A plethora of different porous CG scaffolds have been investigated for soft tissue regeneration (see17,18 for detailed reviews). Collagen is a key component of many biomedical devices, while the addition of chondroitin sulphate and a freeze-drying step creates a porous scaffold that closely resembles the native ECM. CG scaffolds are promising regeneration templates for different tissues including conjunctiva, heart valves, tendon and ligaments.19–22 The most extensive research has focused on skin and peripheral nerve regeneration and has yielded substantial success, as demonstrated by Federal Drugs Authority (FDA) approval.17 The CG scaffold used in this EVM is composed of type I collagen and chondroitin sulphate that was freeze-dried to create a porous microstructure and chemically cross-linked to enhance its mechanical properties. CG scaffolds possessed highly interconnected porous architectures with an average pore size of 216 +/− 39 µm.8
Growth factor-directed repair has been reported to produce a better-quality repair tissue.23 Growth factors are soluble proteins that stimulate cell proliferation and differentiation and may be used to aid cell migration, and to increase matrix production.4,13 In vivo cell–ECM interactions provide adequate signals to cells via growth factors to induce or maintain a desired state of cell differentiation; thus, these proteins play an important role in in vitro tissue engineering.24
Insulin-like growth factor-1, an anabolic growth factor involved in cartilage development and homeostasis,25,26 has been extensively investigated for use in articular cartilage repair.25–29 Fortier et al. demonstrated that the addition of 10–100 ng/ml IGF-1 enhanced the levels of proteoglycan and type II collagen synthesized by chondrocytes seeded in fibrin matrices and that the cells maintained their phenotype in vitro.28 In addition, IGF-1 also protects the ECM from interleukin-1 and tumour necrosis factor α-mediated degradation during cartilage injury.30–32 In vivo, IGF-1-loaded fibrin matrices enhanced the formation of hyaline-like repair tissue compared with controls in full-thickness articular cartilage defects in horses.25 In addition, Tuncel et al. reported that collagen sponges loaded with 5 µg IGF-1 enhanced the tissue response and produced significantly better gross, histological and histochemical neocartilage compared with collagen sponge controls in a rabbit osteochondral defect model.26
A number of different studies have reported that the addition of TGF-β1 enhances the production of cartilage ECM proteins such as proteoglycan and type II collagen under in vitro conditions.33–35 Yaeger et al. added exogenous IGF-1 and TGF-β1 both singly and in combination to dedifferentiated human articular chondrocytes and studied the effect of adding growth factors to serum-free media on cell proteoglycan and type II collagen synthesis.36 Neither IGF-1 nor TGF-β1 alone stimulated these cells to produce aggrecan or type II collagen; however, the combination of both growth factors induced mRNA expression of these proteins. The results of their study imply that the addition of both growth factors yielded a synergistic response that induced chondrogenesis in this cell type.
The balance between catabolic and anabolic factors is required to maintain homeostasis of tissue turnover. Anabolic growth factors include IGF-1, TGF-β and bone morphogenic proteins (BMPs), whereas tumour necrosis factor (TNF)-α and interleukin (IL)-1β are catabolic factors. In OA, highly catabolic factors dominate matrix turnover; therefore, despite the initial increase in matrix synthesis in early OA, a net loss of proteoglycans is a key feature of all stages of osteoarthritic cartilage degeneration.37,38 Cells within a scaffold that is in contact with a cartilage explant in vitro or implanted into a cartilage defect in vivo will be affected by a combination of both catabolic and anabolic signals from those cells in the host tissue. Thus, creating an EVM using cartilage explants allows the effect of adding growth factors to seeded cells to be assessed in a more natural environment.
Pabbruwe et al. investigated an ex vivo cartilage model to enhance the integration of implanted cartilage with host tissue.39 Bovine nasal chondrocytes were seeded within a collagen scaffold implant that was then sandwiched between two bovine cartilage explants to induce the cells to migrate between the explants and to integrate with the cartilage tissue. After 40 days, the cartilage–implant–cartilage construct appeared macroscopically as a continuous tissue section and full integration was also observed via histological analysis. The implanted chondrocytes migrated into the mature tissue of each explant and induced ECM remodelling, which resulted in integration. Furthermore, the cell-seeded implants yielded a higher cartilage repair index and tensile strength than cell-free control, which indicated the potential of this model for achieving integration of repair cartilage in vivo.
An alternative cartilage EVM involved implanting chondrocytes into a cartilage explant adjacent to a decellularized cartilage matrix and measuring cell adhesion, migration and ECM production after 28 days.40 The results showed that chondrocytes adhered to the cartilage explant but did not migrate into the acellular cartilage region, indicating that low chondrocyte migration into host cartilage occurs in vivo.
The scaffold/explant EVM described in this study provides a valuable model for studying the interactions between a biomimetic scaffold and the tissue that it is designed to be repairing. The model is applicable to most mammalian articular cartilages with the only restriction being the area of cartilage available. This study used a 5 mm disc as the starting point in order to maximize the signal generated from ECM production, but we have also created 3 mm discs with 1 mm scaffolds successfully. The system also allows a reasonable throughput as 5 mm EVMs will fit in a 96-well plate format if necessary. Integration of the scaffold with the explants can be measured by simple ‘push out’41 studies, and the scaffold and explants are easily separated at the end of the experiment in order to analyse the relative amounts of ECM deposition or degradation in the scaffold or the tissue. Similarly, protease activity in the explants, scaffold and media can also be measured. In addition, the distribution of cells within the scaffold or explants can also be analysed by simple histology or immunohistocytochemistry.
In our study, the DMMB assay demonstrated that cells seeded within the AI group produced the highest level of sulphated GAG release into the media (Figure2). The DMMB assay was utilized to monitor GAG levels at every media change, but the assay is primarily a measure of degradation, e.g. proteoglycan released by proteolysis of cartilage, rather than synthesis. Hence, Western blots using decorin as a marker of cartilage proteoglycan were also carried out in order to get a better estimation of proteoglycan deposition in the scaffold (Figure3). Both assays demonstrated that the chondrocytes from the endogenous IGF-1 group yielded a significantly higher amount of GAG release from or decorin deposition into the scaffold when compared with the control group. We consider that the combination of these two assays points to an overall increase in proteoglycan synthesis. Although decorin is a relatively minor component of the cartilage when compared with aggrecan, it is much easier to measure accurately, and therefore, we use it as a surrogate marker of proteoglycan content. It should be noted that the molecular weight of decorin deposited in the scaffolds is between 200–250 KDa and that in the articular cartilage control is slightly higher [Figure3(top)]. Decorin undergoes considerable glycosylation both in vitro and in vivo, and therefore, its molecular weight is considerably higher than that often quoted in the literature (48 kDa), which represents the unglycosylated core protein.42
Type I collagen was detected in all groups analysed by Western blotting (Figure4). Type I collagen is synthesized by OA chondrocytes and is generally considered to be a marker of cells that have dedifferentiated into a fibroblastic or hypertrophic cell phenotype.43,44 There was significantly less type I collagen in the AI group than the control group or any of the other added GF groups. Although values in the added GF groups were slightly elevated compared with those in the control group, none of these were significant. Although a desirable result, it is not clear why the AI group would down-regulate collagen type I deposition compared with all other groups, but it is possible that IGF-1 bound to the scaffold enhances then chondrogenic phenotype more than when it is free in solution. This hypothesis is borne out by the data in (Figure5) where chondrocytes in the AI group produced significantly more type II collagen deposition on the scaffold than any of the other groups.
There are a number of possible explanations as to why IGF-1 bound to the scaffold should be more effective than IGF-1 added to the media. The growth factor may be protected from proteolytic digestion or from IGF-binding proteins allowing a more sustained release of IGF-1 over time compared with adding multiple doses of exogenous IGF-1. The stimulation of type II collagen and decorin production implies that the AI scaffolds release a therapeutic dosage of IGF-1, which aids maintenance of the cell phenotype and stimulates an anabolic response.
In each case, samples for Western blotting represent the extraction of the contents of a whole scaffold with no normalization for total DNA or protein. Previous data in our laboratory has demonstrated that there are no significant differences in cell number between treatment groups during the timecouse nor in total protein content of the scaffolds. The latter is due possibly to the contribution of serum albumin from the calf serum in the media, which appears to bind to the scaffold despite washing prior to extraction (not shown). This protein masks any small changes in the total protein levels that may have been caused by the growth factors. Therefore, we believe that any changes that we detect by Western blot represent a change of a specific ECM protein relative to the total protein content of the scaffold.
It will now be necessary to analyse the cartilage explants from the EVM to determine if there are measurable increases in protein content or whether these will be too small to detect above normal cartilage metabolism. Preliminary data have also been acquired (not shown) relating to the catabolic processes in both the cartilage explant and seeded cells, and this will be the subject of a later publication.
Conclusions
This study has demonstrated that the EVM described in this study is a suitable model for studying anabolic stimuli that have been applied specifically to enhance chondrogenesis and repair in a biomimetic scaffold.
Acknowledgments
The authors would like to kindly acknowledge funding from the EPSRC, Tigenix Ltd (LM), Technology Strategy Board and Tigenix Ltd (JW) and the NIHR (DH). The authors would also like to thank Professor Serena Best, Professor Ruth Cameron and Dr Roger Brooks for valuable contributions and discussions.
References
- 1.Buckwalter JA, Mankin HJ. Articular cartilage. 1. Tissue design and chondrocyte-matrix interactions. Journal of Bone and Joint Surgery-American. 1997;79A(4):600–11. [Google Scholar]
- 2.Reddi AH. Symbiosis of biotechnology and biomaterials: applications in tissue engineering of bone and cartilage. J Cell Biochem. 1994;56(2):192–5. doi: 10.1002/jcb.240560213. [DOI] [PubMed] [Google Scholar]
- 3.Hunziker EB. Biologic repair of articular cartilage. Defect models in experimental animals and matrix requirements. Clin Orthop Relat Res. 1999;(367 Suppl):S135–46. [PubMed] [Google Scholar]
- 4.Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage. 2006;14(5):403–12. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Ivkovic A, Marijanovic I, Hudetz D, Porter R, Pecina M, Evans C. Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci. 2011;3:923–44. doi: 10.2741/e299. [DOI] [PubMed] [Google Scholar]
- 6.Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:20. doi: 10.1186/1758-2555-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maehara H, Sotome S, Yoshii T, Torigoe I, Kawasaki Y, Sugata Y, et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2) J Othop Res. 2010;28(5):677–86. doi: 10.1002/jor.21032. [DOI] [PubMed] [Google Scholar]
- 8.Mullen LM, Best SM, Brooks RA, Ghose S, Gwynne JH, Wardale J, et al. Binding and release characteristics of insulin-like growth factor-1 from a collagen-glycosaminoglycan scaffold. Tissue Eng Part C Methods. 2010;16(6):1439–48. doi: 10.1089/ten.tec.2009.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullen LM, Best SM, Ghose S, Wardale J, Rushton N, Cameron R. Bioactive IGF-1 release from collagen–GAG scaffold to enhance cartilage repair in vitro. J Mater Sci Mater Med. 2015;26(1):5325. doi: 10.1007/s10856-014-5325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sgaglione NA. Mensical repair update: current concepts and new techniques. Orthopaedics. 2005;28(3):280–6. doi: 10.3928/0147-7447-20050301-13. [DOI] [PubMed] [Google Scholar]
- 11.de Vries-van Melle ML, Mandl EW, Kops N, Koevoet WJ, Verhaar JA, van Osch GJ. An osteochondral culture model to study mechanisms involved in articular cartilage repair. Tissue Eng Part C Methods. 2012;18(1):45–53. doi: 10.1089/ten.tec.2011.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutts RD, Healey RM, Ostrander R, Sah RL, Goomer R, Amiel D. Matrices for cartilage repair. Clin Orthop Relat Res. 2001;391:S271–9. doi: 10.1097/00003086-200110001-00025. [DOI] [PubMed] [Google Scholar]
- 13.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3(10):589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects osteoarthritis and cartilage. 2002;10(6):432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 15.Frenkel SR, Di Cesare PE. Scaffolds for articular cartilage repair. Ann Biomed Eng. 2004;32(1):26–34. doi: 10.1023/b:abme.0000007788.41804.0d. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26(4):433–41. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 17.Yannas IV, Tzeranis DS, Harley BA, So PT. Biologically active collagen-based scaffolds: advances in processing and characterization. Philos Transact A Math Phys Eng Sci. 2010;368(1917):2123–39. doi: 10.1098/rsta.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harley BAC, Gibson LJ. In vivo and in vitro applications of collagen-GAG scaffolds. Chem Eng J. 2008;137(1):102–21. [Google Scholar]
- 19.Hsu WC, Spilker MH, Yannas IV, Rubin PA. Inhibition of conjunctival scarring and contraction by a porous collagen-glycosaminoglycan implant. Invest Ophthalmol Vis Sci. 2000;41(9):2404–11. [PubMed] [Google Scholar]
- 20.Rabkin-Aikawa E, Mayer JE, Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol. 2005;94:141–79. doi: 10.1007/b100003. [DOI] [PubMed] [Google Scholar]
- 21.Bhavsar D, Shettko D, Tenenhaus M. Encircling the tendon repair site with collagen-GAG reduces the formation of postoperative tendon adhesions in a chicken flexor tendon model. J Surg Res. 2010;159(2):765–71. doi: 10.1016/j.jss.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Meaney Murray M, Rice K, Wright RJ, Spector M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21(2):238–44. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 23.Ueda H, Hong L, Yamamoto M, Shigeno K, Inoue M, Toba T, et al. Use of collagen sponge incorporating transforming growth factor-beta1 to promote bone repair in skull defects in rabbits. Biomaterials. 2002;23(4):1003–10. doi: 10.1016/s0142-9612(01)00211-3. [DOI] [PubMed] [Google Scholar]
- 24.van der Kraan PM, Buma P, van Kuppevelt T, van den Berg WB. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthritis Cartilage. 2002;10(8):631–7. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 25.Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17(4):475–87. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 26.Tuncel M, Halici M, Canoz O, Yildirim Turk C, Oner M, Ozturk F, et al. Role of insulin like growth factor-I in repair response in immature cartilage. Knee. 2005;12(2):113–9. doi: 10.1016/j.knee.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Elisseeff J, McIntosh W, Fu K, Blunk BT, Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19(6):1098–104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 28.Fortier LA, Lust G, Mohammed HO, Nixon AJ. Coordinate upregulation of cartilage matrix synthesis in fibrin cultures supplemented with exogenous insulin-like growth factor-I. J Orthop Res. 1999;17(4):467–74. doi: 10.1002/jor.1100170403. [DOI] [PubMed] [Google Scholar]
- 29.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101(1-3):111–25. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Tyler JA. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989;260(2):543–8. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fosang AJ, Tyler JA, Hardingham TE. Effect of interleukin-1 and insulin like growth factor-1 on the release of proteoglycan components and hyaluronan from pig articular cartilage in explant culture. Matrix. 1991;11(1):17–24. doi: 10.1016/s0934-8832(11)80223-4. [DOI] [PubMed] [Google Scholar]
- 32.Frisbie DD, Nixon AJ. Insulin-like growth factor 1 and corticosteroid modulation of chondrocyte metabolic and mitogenic activities in interleukin 1-conditioned equine cartilage. Am J Vet Res. 1997;58(5):524–30. [PubMed] [Google Scholar]
- 33.Redini F, Galera P, Mauviel A, Loyau G, Pujol JP. Transforming growth factor beta stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 1988;234(1):172–6. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- 34.Zimber MP, Tong B, Dunkelman N, Pavelec R, Grande D, New L, Purchio AF. TGF-beta promotes the growth of bovine chondrocytes in monolayer culture and the formation of cartilage tissue on three-dimensional scaffolds. Tissue Eng. 1995;1(3):289–300. doi: 10.1089/ten.1995.1.289. [DOI] [PubMed] [Google Scholar]
- 35.van Susante JL, Buma P, van Beuningen HM, van den Berg WB, Veth RP. Responsiveness of bovine chondrocytes to growth factors in medium with different serum concentrations. J Orthop Res. 2000;18(1):68–77. doi: 10.1002/jor.1100180111. [DOI] [PubMed] [Google Scholar]
- 36.Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237(2):318–25. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 37.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hipsII. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523–37. [PubMed] [Google Scholar]
- 38.Aigner T, Sachse A, Gebhard PM, Roach HI. Osteoarthritis: pathobiology-targets and ways for therapeutic intervention. Adv Drug Deliv Rev. 2006;58(2):128–49. doi: 10.1016/j.addr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Pabbruwe MB, Esfandiari E, Kafienah W, Tarlton JF, Hollander AP. Induction of cartilage integration by a chondrocyte/collagen-scaffold implant. Biomaterials. 2009;30(26):4277–86. doi: 10.1016/j.biomaterials.2009.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Secretan C, Bagnall KM, Jomha NM. Effects of introducing cultured human chondrocytes into a human articular cartilage explant model. Cell Tissue Res. 2010;339(2):421–7. doi: 10.1007/s00441-009-0901-z. [DOI] [PubMed] [Google Scholar]
- 41.Theodoropoulos JS, De Croos JN, Park SS, Pilliar R, Kandel RA. Integration of tissue-engineered cartilage with host cartilage: an in vitro model. Clin Orthop Relat Res. 2011;469(10):2785–95. doi: 10.1007/s11999-011-1856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramamurthy P, Hocking AM, McQuillan DJ. Recombinant decorin glycoforms – purification and structure. Journal of Biological Chemistry. 1996;271(32):19578–84. doi: 10.1074/jbc.271.32.19578. [DOI] [PubMed] [Google Scholar]
- 43.Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15(4):1313–21. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 44.Gouttenoire J, Valcourt U, Ronzière MC, Aubert-Foucher E, Mallein-Gerin F, Herbage D. Modulation of collagen synthesis in normal and osteoarthritic cartilage. Biorheology. 2004;41(3-4):535–42. [PubMed] [Google Scholar]


